Back to Journals » OncoTargets and Therapy » Volume 12
The efficacy and safety of anlotinib treatment for advanced lung cancer
Authors Shao L, Wang W , Song Z, Zhang Y
Received 16 February 2019
Accepted for publication 21 July 2019
Published 15 August 2019 Volume 2019:12 Pages 6549—6554
DOI https://doi.org/10.2147/OTT.S205674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Lan Shao,1,2 Wenxian Wang,1 Zhengbo Song,1,2 Yiping Zhang1,2
1Department of Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, People’s Republic of China; 2Key Laboratory Diagnosis and Treatment Technology on Thoracic Oncology, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, People’s Republic of China
Objective: Anlotinib is an oral novel multi-target tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, and stem cell factor receptor (c-Kit). The aim of this study was to evaluate the efficacy and safety of anlotinib treatment in advanced lung cancer in the real world.
Methods: We evaluated the efficacy and toxicity of apatinib in patients with previously treated advanced lung cancer from 2018 to 2019 in Zhejiang Cancer Hospital. Survival analysis was performed by the Kaplan–Meier method.
Results: Fifty-eight patients were included in the present study. Thirty-one of these patients received anlotinib treatment as a third line and 27 patients received further therapy. All 58 patients had therapeutic evaluation and 46 patients acquired progression-free survival evaluation. Ten patients achieved partial response (PR), and 36 achieved stable disease (SD), representing a response rate of 17.2% and a disease control rate of 77.6%. Median progression-free survival was 3.3 months (95% CI 1.595–5.071). The toxicities associated with anlotinib were generally acceptable with a total grade 3/4 toxicity of 5.2%. The toxicities of anlotinib were generally tolerated and the common toxicities were hand–foot syndrome and hypertension.
Conclusion: In the third-line or more-line treatment of advanced lung cancer, anlotinib appears to have some activity when utilized as a salvage treatment. Adverse reactions are controllable.
Keywords: lung cancer, anlotinib, VEGF, efficacy, safety
Introduction
Lung cancer is one of the leading causes of cancer-related death worldwide.1 For patients with advanced non-small cell lung cancer (NSCLC), targeted therapy, immunotherapy, and chemotherapy can prolong survival time and are recommended as standard treatment in advanced NSCLC.2–4 However, there is difficult to choose therapeutic regimen in patients who failed second-line or third-line treatment. Many patients receive mono-chemotherapy in practice. Some patients cannot tolerate chemotherapy. According to the available reports, for the EGFR unselected or EGFR wild-type patients, erlotinib as third-line treatment has been considered.5 There is still a lack of treatment. Therefore, new therapies are clearly needed. Nowadays, antiangiogenic drugs may be alternative drugs.
Antiangiogenic drugs show some efficacy in the treatment of advanced lung cancer.6 Bevacizumab as an anti–vascular endothelial growth factor (anti-VEGF) humanized monoclonal antibody prevents VEGF ligand‑receptor binding has been shown to significantly improve efficacy and overall survival (OS) in advanced NSCLC.7 In addition, apatinib, an oral VEGFR inhibitor, has potential as a therapeutic agent for lung cancer.8,9 Anlotinib is a novel oral multi-target tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor (VEGFR) type 2 and 3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR) α and β, stem cell factor receptor (c-Kit), and Ret.10 A phase I study has reported that anlotinib was generally well tolerated at a daily oral dose of 12 mg or lower in patients with advanced refractory solid tumors.10 Then, in the phase 3 trial, anlotinib improved the overall survival compared with placebo with manageable toxic effects in refractory advanced NSCLC.11 Therefore, anlotinib was approved as a third-line treatment for refractory advanced NSCLC by the China Food and Drug Administration (CFDA) on May 9, 2018. For small cell lung cancer (SCLC), in the phase II study of ALTER1202, anlotinib appeared to provide significant progression-free survival (PFS) and disease control rate (DCR) benefit for patients with SCLC who progressed after two lines of chemotherapy.12 However, no clinical studies with detailed data have investigated the efficacy and safety of anlotinib in lung cancer as third-line or more-line treatment in the real world.
In the present study, we conducted a retrospective evaluation of the efficacy and toxicity of anlotinib after failure of prior treatment in advanced lung cancer in clinical practice.
Methods
Patient eligibility
Patients with advanced lung cancer who received anlotinib as third-line or further treatment between 2018 and 2019 were included. Histological or cytological diagnosis of lung cancer according to histopathological criteria (World Health Organization, 2015). All patients were confirmed to have advanced or recurrent stage IIIB/IV NSCLC according to the TNM classification (version 7) with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2. During the duration of anlotinib therapy, patients did not receive local treatment such as radiotherapy or interventional therapy. All patients were confirmed recurrence or metastases identified in chest, abdominal, or brain computed tomography (CT) scans and/or bone scans after at least two treatment strategies. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by Ethics Committee of Zhejiang Cancer Hospital (Zhejiang, China) and written informed consent was obtained from each patient involved in the study.
Treatment methods
Anlotinib was given orally, once daily (12 mg, 10 mg, 8 mg) on days 1–14 of a 21-day cycle. The starting drug dose of anlotinib was judged by the clinicians based on the patients’ conditions. One dose reduction (12 mg to 10 mg or 8 mg; 10 mg to 8 mg) or stopped for drug-related toxicity was allowed.
Responses and toxicity
Tumor responses were assessed every two cycles or were evaluated early when significant signs of progression appeared. Objective tumor responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The DCR was defined as the addition of objective response and stabilization rates (CR+PR+SD). Toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 4.0 (CTC4.0).
Follow-up
All the patients who were evaluated for tumor response had PFS and OS. PFS encompassed the time from the first day of anlotinib to documented progression or death from any cause. OS was defined as the time from the first day of anlotinib treatment to death or last follow-up. Follow-up extended till February 8, 2019.
Statistical analysis
Survival analysis was conducted by the Kaplan–Meier analysis and comparison by the log-rank test. The survival curves were calculated according to the Kaplan–Meier method. Statistical analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was regarded as statistically significant.
Results
Patients’ characteristics
A total of 58 patients were included; of these, 34 had adenocarcinoma, 16 had squamous cell carcinoma, 6 had small cell lung cancer, 1 was adenosquamous carcinoma, and 1 was undifferentiated carcinoma. Forty-two had smoking history and 16 were never-smokers. Forty-three patients were male and 15 were female, and they had a median age of 62 years (range 38–78 years). Tumor samples for gene mutation analysis were available for 32 patients (31 were adenocarcinoma and one was adenosquamous carcinoma). EGFR mutations were identified in 17 patients (5 with deletion in exon 19, 10 with L858R in exon 21, and 2 was EGFR 20 insert mutation). All patients received palliative chemotherapy and 15 had targeted therapy in prior treatment. Thirty-one (53.4%) patients received anlotinib as third-line therapy and 27 (46.6%) as further-line treatment. Forty-five (77.6%) patients had performance status (PS) of 0–1, 10 (17.2%) had PS of 2, and 3 (5.2%) patients were PS 3. There were 28 patients who received anti-vascular drug therapy including bevacizumab, endostar, and apatinib. Fifty-one patients received 12 mg anlotinib treatment and 7 received 10 mg anlotinib treatment as starting dose. The patient characteristics are listed in Table 1.
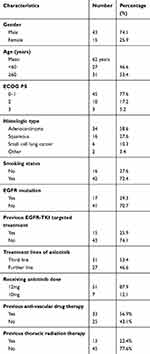 |
Table 1 Clinicopathologic features among 58 advanced lung cancer patients |
Clinical efficacy
Response data for anlotinib are as follows: CR (n=0), PR (n=10), SD (n=35), and PD (n=13). The ORR and DCR were 17.2 and 77.6%, respectively. The median PFS was 3.3 months (95% CI, 1.595–5.071; Figure 1). The median OS was not achieved. There was no significant association of PFS with gender (P=0.674), age (P=0.595), smoking history (P=0.262), pathological subtype (P=0.694), line of therapy (P=0.540), and PS (P=0.688). The EGFR mutation status was not found to be statistical difference (P=0.873). The details of univariate analysis are shown in Table 2.
 |
Table 2 Univariate analysis of PFS |
Toxicity evaluation
All patients were assessed for toxicity. The rate of grade 3 toxicity was 15.5% (7/58). Grade 4 toxicity was not observed in all the patients. No patients discontinued the anlotinib treatment. Two patients required dosage reductions (both 12 mg and10 mg) because they developed hand–foot syndrome and hypertension. The common grade 3 adverse events were hand–foot syndrome (n=2), hypertension (n=1). The grade 1–2 toxicities are listed in Table 3.
 |
Table 3 Main toxicities of anlotinib treatment |
Discussion
This is a study to assess the clinical benefit of anlotinib as third-line or more-line treatment in patients with advanced lung cancer in the real world. The results demonstrated that anlotinib had efficacy against advanced lung cancer in later-line treatment and patients can tolerate it.
For patients with known gene mutations, such as EGFR, ALK, or ROSI, targeted therapies have been identified that can prolong survival and preserve quality of life.2,3 In addition, approximately 50% advanced NSCLC patients are without gene mutations, platinum-based chemotherapy is still used in routine NSCLC treatment. However, almost all patients develop drug resistance to previous treatments. Many patients did not tolerate later chemotherapy because of the adverse reactions. Current treatments are still inadequate and patients who fail second-line or third-line treatments require additional treatments that are usually based on each physician’s experience and patients’ status. Therefore, highly effective and low toxicity drugs are needed. Nowadays, anlotinib was approved as a third-line treatment for refractory advanced NSCLC by the CFDA on May 9, 2018. Then, for SCLC, anlotinib also showed good response in third-line or more-line treatment.12
Anlotinib is a novel oral multi-target tyrosine kinase inhibitor and in the phase 3 trial of anlotinib compared with the placebo, the results showed that anlotinib improved the overall survival compared with placebo (9.6 vs 6.3 months; P=0.002), with manageable toxic effects.11 The primary PFS was longer in the anlotinib group (4.8 months; 95% CI, 3.5–6.4) compared with the placebo group (1.2 months, 95% CI, 0.7–1.6). The ORR was 10.0% and the DCR was 83.3% in the anlotinib group. In the study of ALTER1202, the primary PFS was longer in the anlotinib group compared with the placebo group (3.8 m vs 0.8 m, P<0.001) in SCLC.12 In the present study, the DCR and ORR were 77.6% and 17.2%, respectively, which indicates similar efficacy comparing with the phase 3 trial of anlotinib. The median PFS was 3.3 months. Comparing with the data, the mPFS seemed to be shorter than clinical research of phase 3. We considered our study was conducted in the real world and nearly half of patients (46.6%) were more than third lines of anlotinib treatment. The ECOG PS of patients in our report was worse. The PFS was 2.8 months and 4.0 months in third-line and further-line treatment, respectively. Although there is no difference in statistics, we believe that anlotinib may also be an option for posterior treatment. In the ALTER 0303 phase III trial, no patients were with PS≥2 (three patients with ECOG score of 2 were enrolled by mistake). However, in the real world, we should take into account the general condition of the patient including height, weight, PS score, and tolerance. In our seven patients, five patients were PS 2–3. Therefore, we chose 10 mg as the starting dose to be safer. This also suggests that we can study the correlation between dose and efficacy in the future.
The most common grade 3 AEs during anlotinib treatment were hypertension.13 Hypertension is a common adverse effect in patients treated with VEGF-targeted agents. However, in the present study, the most common AE was hand-foot syndrome. No patients need to interrupt treatment. Anlotinib is an oral anti-vascular targeted drug. According to the ALTER 0303 study, 53 patients with squamous cell carcinoma were included. The incidence of hemoptysis was 20.4% in all patients (60/294) and grade 3/4 was 3.1% (9/294). No death was found to be associated with anlotinib in the study of ALTER 0303. In our study, no serious bleeding was observed. Hence, anlotinib is relatively well tolerated, and adverse reactions are controllable in patients receiving therapy. Of course, in clinical treatment, we must closely monitor adverse reactions and make drug dose adjustments in a timely manner.
The limitations of our study should be recognized. The major limitation is its retrospective study. Because of different histologies, there was also some heterogeneity in the study. Then, the number of people was still small. Finally, the dose of 10 mg was used in the present study, which should be confirmed in future trials and future research could explore how the initial dose is chosen.
Conclusion
Our research results demonstrate that anlotinib is an effective regimen for third-line or more-line treatment of advanced lung cancer and tolerance to anlotinib therapy is acceptable. In the future, prospective studies with larger numbers of patients can be conducted out to explore a dose selection mode and efficacy in different pathological types.
Acknowledgment
The study was funded by the Medical Scientific Research Foundation of Zhejiang Province (No. 2019RC027).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957.
3. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394.
4. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;8:JCO1800149. doi:10.1200/JCO.18.00149.
5. Yoshioka H, Hotta K, Kiura K, et al. A phase II trial of erlotinib monotherapy in pretreated patients with advanced non-small cell lung cancer who do not possess active EGFR mutations: Okayama Lung Cancer Study Group trial 0705. J Thorac Oncol. 2010;5(1):99–104.
6. Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2015;4(5):515–523.
7. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, Phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204.
8. Wu F, Zhang S, Xiong A, et al. A Phase II clinical trial of apatinib in pretreated advanced non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e831–e842.
9. Song Z, Yu X, Lou G, et al. Salvage treatment with apatinib for advanced non-small-cell lung cancer. Onco Targets Ther. 2017;10:1821–1825. doi:10.2147/OTT.S113435
10. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105.
11. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661.
12. Cheng Y, Wang Q, Li K, et al. Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicentre, randomized, double-blind Phase 2 trial. J Thorac Oncol. 2018;13(10):S351–S352.
13. Si X, Zhang L, Wang H, et al. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: experiences in ALTER-0303. Thorac Cancer. 2019. doi:10.1111/1759-7714.12977.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

