Back to Journals » Clinical Ophthalmology » Volume 8
The efficacy and safety of add-on 0.1% brimonidine tartrate preserved with sodium chlorite in on-treatment Japanese normal-tension glaucoma patients
Authors Tsumura T, Yoshikawa K , Kimura T , Suzumura H , Kawashima M, Nanno M, Ishijima K, Takeda R
Received 7 May 2014
Accepted for publication 24 June 2014
Published 1 September 2014 Volume 2014:8 Pages 1681—1687
DOI https://doi.org/10.2147/OPTH.S67366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Toyoaki Tsumura,1 Keiji Yoshikawa,2 Tairo Kimura,3 Hirotaka Suzumura,4 Miwako Kawashima,5 Mami Nanno,6 Kiyotaka Ishijima,7 Ryuji Takeda8
1Fussa Hospital, Tokyo, Japan; 2Yoshikawa Eye Clinic, Tokyo, Japan; 3Ueno Eye Clinic, Tokyo, Japan; 4Suzumura Eye Clinic, Tokyo, Japan; 5Nakano General Hospital, Tokyo, Japan; 6Kagurazaka Minamino Eye Clinic, Tokyo, Japan; 7Irumagawa Hospital, Saitama, Japan; 8Department of Biological Chemistry, Faculty of Agriculture, Kinki University, Nara, Japan
Background: To evaluate the efficacy and safety of newly formulated brimonidine (0.1% brimonidine tartrate preserved with sodium chlorite: brimonidine) as add-on therapy in on-treatment Japanese normal-tension glaucoma (NTG) patients.
Methods: Brimonidine was added to on-treatment NTG patients with intraocular pressures (IOP) of between 13 mmHg and 16 mmHg after three consecutive IOP measurements. The time courses of IOP, conjunctival hyperemia, superficial punctate keratitis, and adverse events were examined at 4, 8, and 12 weeks after brimonidine instillation.
Results: Though 75 of 83 patients (31 males and 52 females; mean age: 63.4±11.6 years) completed the study, six patients discontinued because of side effects and two patients withdrew. The mean IOP after brimonidine addition at week 4 (12.6±1.8 mmHg, P<0.001), week 8 (12.4±1.7 mmHg, P<0.001), and week 12 (12.6±1.8 mmHg, P<0.001) was significantly decreased compared with that before the addition of brimonidine (13.9±1.2 mmHg). No significant changes in superficial punctate keratitis or conjunctival hyperemia scores were observed throughout the study. Dizziness, sleepiness, eye pain, and itching (mild to moderate) were noted in five, four, three, and three patients, respectively.
Conclusions: The addition of newly formulated brimonidine to on-treatment Japanese NTG patients with IOP of 13–16 mmHg further reduced the levels of IOP with minimal side effects and adverse events.
Keywords: normal-tension glaucoma, 0.1% brimonidine tartrate with sodium chlorite, additive intraocular pressure reduction, side effect, adverse event
Corrigendum for this paper has been published
Further corrigendum has been published
Introduction
Normal-tension glaucoma (NTG) is the most common type of glaucoma in Japanese individuals.1,2 Although monotherapy with prostaglandin (PG) analogs is mainly applied as the first-line treatment for NTG,3,4 additional treatment with second-line glaucoma ophthalmic solutions is frequently needed, as the target intraocular pressure (IOP) for NTG is relatively low compared with that in primary open-angle glaucoma (POAG).5
Brimonidine, an α2-adrenergic agonist, is postulated to be clinically useful for glaucoma treatment, in particular for add-on therapy, because it has a unique IOP-lowering mechanism of action.6
The concentration of brimonidine in ophthalmic solution was decreased from 0.2% to 0.1%, and the preservative was reformulated from benzalkonium chloride (BAK) to sodium chlorite (SC) in an effort to enhance tolerability in brimonidine usage.7 At present, this new formulation of brimonidine (0.1% brimonidine tartrate ophthalmic solution with SC: Aiphagan®; Senju Pharmaceutical Co., Ltd, Osaka, Japan) has been marketed in Japan since 2012.
To the best of the authors’ knowledge, however, previous studies of the efficacy and safety of brimonidine in NTG treatment evaluated 0.2% brimonidine with BAK.8,9 No study has been conducted for the treatment of NTG using 0.1% brimonidine with SC.10
We therefore evaluated the IOP-lowering efficacy and safety of newly formulated 0.1% brimonidine preserved with SC as an add-on therapy in on-treatment Japanese NTG patients with IOP of 13–16 mmHg.
Materials and methods
Study design
This prospective, open-label, multicenter study was conducted over a period of 12 weeks at Fussa Hospital, Yoshikawa Eye Clinic, Ueno Eye Clinic, Nakano General Hospital, Kagurazaka Minamino Eye Clinic, and Irumagawa Hospital in Japan from June 2012 to January 2013. The study was carried out according to the ethical principles of the Declaration of Helsinki. All patients read and signed an informed consent form, and the Institutional Review Board of Fussa Hospital approved the study in advance. This study was registered in the University Hospital Medical Information Network (UMIN) clinical trials registry (No UMIN000012582).
Participants
Japanese NTG patients diagnosed according to Japanese glaucoma guidelines5 and receiving one to three drugs including PG, without a change of prescription for 3 months, were recruited.
Inclusion criteria for enrolled patients were as follows: 1) aged ≥20 years and ≤80 years who were diagnosed with NTG in both eyes; 2) patients undergoing treatment with PG and/or β-blockers who were judged to require the addition of brimonidine because they were unable to achieve target IOP on their current IOP-lowering therapy; 3) on-treatment IOPs in bilateral eyes of between 13 mmHg and 16 mmHg in at least three consecutive measurements; 4) patients with an IOP of 10–12 mmHg were eligible if the central corneal thickness was ≤470 μm; 5) mean deviation (MD) values <−6 dB using the Humphrey central 30-2/24-2 Swedish Interactive Threshold Algorithm standard program (Carl Zeiss Meditec Inc., Dublin, CA, US), or any patients with central visual field loss (Figure 1); 6) best-corrected visual acuity ≥0.5; and (7) spherical equivalent refraction ≥−10D.
Exclusion criteria were as follows: 1) abnormalities of the anterior segment that disturbed accurate IOP measurement with the Goldmann applanation tonometer; 2) apparent findings of dry eye; 3) any active ocular inflammation or blepharitis; 4) a history of laser therapy or surgical treatment of glaucoma; 5) patients requiring treatment with steroid eye drops during the study; 6) patients who had ocular fundus diseases that may affect visual field; and 7) patients judged ineligible by an investigator.
Procedure
One drop of brimonidine was added to instil into the bilateral conjunctival sac twice a day for 12 weeks at the predefined time (12-hour intervals) for each patient. Measurements of IOP, best-corrected visual acuity, slit-lamp examinations, and interviews for adverse events were conducted in each clinic at 4, 8, and 12 weeks after the addition of brimonidine.
IOP was measured twice consecutively between 9 am and 1 pm by a single examiner using a Goldmann applanation tonometer. The mean of the two IOP measurements at each time point was calculated and used for statistical analyses.
The degrees of conjunctival hyperemia and superficial punctate keratitis (SPK) were scored from 0 to 3 and 0 to 4 based on the standard photogram, respectively.11–13 For evaluation of adverse events, patients were examined with a slit lamp for the presence or absence of red eyelid, eyelid edema, conjunctival edema, and conjunctival follicles.
Each adverse event, such as ocular irritation, itching and foreign body sensation, and/or dry eye sensation, was classified into four grades: “nothing”, “almost nothing”, “sometimes” and “always”. Patients were interviewed at each visit to confirm their experiences of dizziness or sleepiness.
Blood pressure and pulse rate were measured for each patient at the start and end of the study. MD values were compared before and after the study.
Statistical analysis
All data collected from patients who fulfilled all of the inclusion criteria and did not meet any of the exclusion criteria were sent to Kinki University, Nara, Japan, and were analyzed by a statistician who did not directly collect data in the clinical study.
In each patient, one eye with a higher on-treatment IOP before the addition of brimonidine (the study eye) was used for statistical analysis. If both eyes had the same IOP values, the eye with the lower MD value served as the study eye.14 In patients with the same IOP values and MD values in both eyes, the right eye was selected. IOPs obtained before and after add-on with brimonidine in the patients who were able to be followed up for at least 4 weeks were used in the analysis using a paired t-test with Bonferroni correction after repeated analysis of variance.
The relationship between the magnitude of IOP reduction in those eyes that were followed for up to 12 weeks, baseline characteristics, and the number of pretreated drugs was analyzed by multivariable logistic regression analysis.
Scores of conjunctival hyperemia and SPK in the study eyes were examined using a Wilcoxon signed rank test. Cumulative incidences of adverse events such as conjunctival and eyelid findings were calculated and statistically tested by a chi-square test. Blood pressures, pulse rates, and MD values before and after treatment with brimonidine were compared using a paired t-test. For statistical analysis, JMP® software (version 9.0.2; SAS Institute Inc., Cary, NC, USA) was used. P-values <0.05 were considered to be significant.
Results
A total of 75 out of 83 enrolled patients (31 males and 52 females; mean age: 63.4±11.6 years) completed the study (Table 1). Eight patients did not complete the study due to dropping out (two patients) and adverse events (six patients; dizziness: three, sleepiness: one, eye pain: one, eye itching: one).
Compared with the IOPs before the addition of brimonidine, the IOPs in the study eyes showed a significant reduction at each time point (P<0.001) (Figure 2). At 12 weeks, the IOP was lowered by 0–1 mmHg in 33 patients (44.0 %), 2–3 mmHg in 25 patients (33.3%), and ≥4 mmHg in seven patients (9.3%). A 2 mmHg or greater reduction was observed in 32 of 75 eyes (42.7%). Brimonidine significantly lowered IOP compared with preaddition, regardless of the number of previous antiglaucoma drugs (Figure 3).
The correlation between various factors, including demographic characteristics, and the change from on-treatment IOP before the addition of brimonidine were analyzed using logistic regression analysis, and the magnitude of IOP reduction at 12 weeks significantly correlated with MD value and degree of refraction (Table 2).
Scores of conjunctival hyperemia in the study eyes were not significantly increased at each time point (Table 3). No significant differences in the SPK scores were observed at any time point in the study eyes (Table 3). Conjunctival edema, conjunctival follicles, reddish eyelid, and eyelid edema were observed in 1.2%, 4.8%, 2.4%, and 1.2% of patients, respectively, but no significant increase in frequency of these side effects was observed during the follow-up period (Figure 4). There were no significant changes in the cumulative incidence of adverse events over time (irritation, itching, foreign body sensation, and dry eye sensation) (P=0.6561, P=0.8270, P=0.7932, P=0.8803; Figure 5).
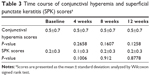  | Table 3 Time course of conjunctival hyperemia and superficial punctate keratitis (SPK) scoresa |
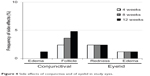  | Figure 4 Side effects of conjunctiva and of eyelid in study eyes. |
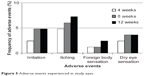  | Figure 5 Adverse events experienced in study eyes. |
No apparent changes were observed in systolic and diastolic blood pressure (130.4±18.2 mmHg, 80.5±12.7 mmHg, 130.1±19.2 mmHg, 79.9±14.5 mmHg, P=0.6849, P=0.4692) or pulse rate (69.2±9.2 bpm and 70.3±9.2 bpm, P=0.1685) before or after add-on treatment with brimonidine.
Adverse events such as dizziness (five cases, 6.0%), sleepiness (four cases, 4.8%), eye pain (three cases, 3.6%), and itching (mild to moderate) (three cases, 3.6%) were observed in 15 of 83 cases (18.1%) during the study. There was no significant difference in MD values between before and after brimonidine instillation (10.72±4.63 dB and −12.01±4.94 dB, P=0.9461).
Discussion
Additive treatment with newly formulated brimonidine provided further IOP-lowering effects in Japanese NTG patients with on-treatment IOP between 13 mmHg and 16 mmHg who had been treated with one to three glaucoma eye drops, without serious local and systemic side effects or adverse events leading to discontinuation of administration.
As the main purpose of the present study was to examine the additive IOP-lowering efficacy of brimonidine in on-treatment Japanese NTG patients under a multicenter study design, inclusion criteria were strictly defined in respect of IOP levels and the visual field condition of the study candidates. Therefore, only those patients with on-treatment IOP between 13 mmHg and 16 mmHg were included, as IOP levels of ≤12 mmHg are generally considered not to need further IOP lowering,15 whereas those patients with on-treatment IOP of ≥17 mmHg might need to undergo surgery or laser therapy.5 The thickness of the cornea may affect the IOP measurement;5,16 however, patients with on-treatment IOP of 10–12 mmHg were eligible if the central corneal thickness was ≤470 μm. Regarding the grade of visual field defects, the necessity of add-on therapy for patients with mild visual field loss is thought to be low. Therefore, patients with an MD value <-6D who were thought to have moderate visual field loss were recruited as study subjects. On the other hand, patients with central visual field defects, as shown by the Swedish Interactive Threshold Algorithm standard program (C10-2) visual field test, who met the new criteria provided in Figure 1 were enrolled because such defects may cause a threat to vision and such patients are thought to need further IOP lowering.17 Patients with severe myopia (<-10D) were excluded from the study because myopic optic neuropathy may be confused with glaucoma.
Although the speed of progression of visual field loss in NTG18 is recognized to be slower than that in POAG, it inevitably progresses. Therefore, IOP-lowering therapy is required for NTG, as it is with POAG. In the treatment of NTG, a decrease in IOP of around 20% from baseline was generally achieved by applying PG analogs as monotherapy.19 However, in those cases with visual field loss progression, additive treatment is required,20–22 and a proportion of NTG patients receive multiple antiglaucoma drugs. The magnitude of IOP reduction observed in the present study was 1.3 mmHg, a 9.4% reduction from preaddition of brimonidine in on-treatment NTG with IOP of 13–16 mmHg. The IOP reduction of 1 mmHg, despite being small in amount, is reported to be valuable for slowing down visual field loss, as shown in POAG in the Early Manifest Glaucoma Trial (EMGT) study.23 It is considered that IOP lowering of 1.3 mmHg is clinically relevant in NTG.
The IOP-lowering effects of brimonidine are suspected to have individual differences, as the magnitude of IOP reduction at 12 weeks was widely distributed, with 44.0% of patients having IOP lowered by 0–1 mmHg, and 42.7% lowered by ≥2 mmHg, including 9.3% of patients by ≥4 mmHg. Therefore, we tried to identify potential factors contributing to IOP reduction. The baseline IOP in patients was relatively low and the additive mean IOP-lowering efficacy was 1.3 mmHg. We selected a logistic regression for this analysis. A cutoff value of 2 mmHg in IOP reduction should be used considering IOP measurement fluctuation of ±1 mmHg. The results of the present analysis suggest that IOP in patients with nonmyopia and better MD values would be further lowered by brimonidine.
Brimonidine is known to lower IOP by reducing aqueous humor formation and increasing uveoscleral outflow.6 It is known that myopic eyes are structurally elongated while having mechanically weakened ciliary muscles.24 These changes in myopic eyes might alter the IOP-lowering effect of brimonidine.
Addition of brimonidine was statistically prominent in patients with worse MD value. The actual time period in which patients had received glaucoma treatment could not be confirmed. However, patients with severe visual field defect, ie, worse MD value, in general, used eye drops for a longer period of time. Although no significant difference in IOP-lowering efficacy was observed based on the number of pretreated drugs, it can be postulated that patients with severe NTG might have already reached a sufficient level of pretreatment efficacy.
Significant local side effects were not observed during the 3 months of brimonidine add-on therapy. Moreover, no exacerbation of SPK or hyperemia was noted, which might be attributable to the exclusion of patients with severe dry eye from the study. In the present study we evaluated the efficacy and safety of newly formulated brimonidine, which had a decreased concentration of brimonidine and a changed preservative with less irritation. Therefore, another possible factor contributing to this favorable safety profile was the presence of SC in the brimonidine formulation as a non-BAK preservative, which may have the potential to reduce corneal disorders.25–27
The literature indicates that brimonidine therapy is associated with an increased risk of allergic reactions.28 Patients in this study were therefore carefully monitored in combination with subjective symptoms and slit-lamp evaluation. However, a marked increase in allergic reactions was not evident in our 3-month study. A longer follow-up study may be warranted for evaluation of these findings.
During this study, blood pressure and pulse rate did not change after brimonidine add-on, as reported in the US.28 However, some patients experienced dizziness and sleepiness. Brimonidine may lead to dizziness or sleepiness like other α2-adrenergic receptor agonists such as clonidine and apraclonidine, which can penetrate the blood–brain barrier.29,30 Therefore, before starting brimonidine therapy, patients should be informed about the risks of dizziness and sleepiness.31
Conclusion
The results of this study suggest that the addition of newly formulated brimonidine effectively lowered IOP in Japanese NTG patients with IOP of 13–16 mmHg, with minimal clinically significant side effects or adverse events. Therefore, brimonidine is thought to be useful as an add-on therapy for on-treatment NTG.
Acknowledgment
The authors thank David P Figgitt of Content Ed Net for reviewing the manuscript.
Disclosure
This study was performed without support from any associations or companies, and none of the authors has any conflicts of interest associated with this study.
References
Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004; 111(9):1641–1648. | ||
Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112(10):1661–1669. | ||
Collaborative Normal Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. | ||
Koseki N, Araie M, Tomidokoro A, et al. A placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low-normal pressure. Ophthalmology. 2008;115(11): 2049–2057. | ||
[The Japan glaucoma society guidelines for glaucoma (third edition)]. Nippon Ganka Gakkai Zasshi. 2012;116(1):3–46. Japanese. | ||
Arthur S, Cantor LB. Update on the role of alpha-agonists in glaucoma management. Exp Eye Res. 2011;93(3):271–283. | ||
Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006;2(4):337–346. | ||
Liu CL. Changes in intraocular pressure and ocular perfusion pressure after latanoprost 0.005% or brimonidine tartrate 0.2% in normal-tension glaucoma patients. Ophthalmology. 2002;109(12):2241–2247. | ||
Cheng JW, Cai JP, Wei RL. Meta-analysis of medical intervention for normal tension glaucoma. Ophthalmology. 2009;116(7):1243–1249. | ||
Day DG, Hollander DA. Brimonidine purite 0.1% versus brinzolamide 1% as adjunctive therapy to latanoprost in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2008;24(5):1435–1442. | ||
Nakano T, Yoshikawa K, Kimura T, Suzumura H, Nanno M, Noro T. Efficacy and safety of tafluprost in normal-tension glaucoma with intraocular pressure of 16 mmHg or less. Jpn J Ophthalmol. 2011;55(6): 605–613. | ||
Lemp MA. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221–232. | ||
Yamazaki S, Nanno M, Kimura T, Suzumura H, Yoshikawa K. Effects of switching to SofZia-preserved travoprost in patients who presented with superficial punctate keratopathy while under treatment with latanoprost. Jpn J Ophthalmol. 2010;54(1):7–14. | ||
Tomita G, Araie M, Kitazawa Y, Tsukahara S. A three-year prospective, randomized and open comparison between latanoprost and timolol in Japanese normal-tension glaucoma patients. Eye (Lond). 2004;18(10): 984–989. | ||
Iwata K. [Primary open angle glaucoma and low tension glaucoma: pathogenesis and mechanism of optic nerve damage]. Nihon Ganka Gakkai Zasshi. 1992;96(12):1501–1531. Japanese. | ||
Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh). 1975;53(1):34–43. | ||
Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10° of the visual field. JAMA Ophthalmol. 2014;132(3):291–297. | ||
Kim M, Kim DM, Park KH, Kim TW, Jeoung JW, Kim SH. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol. 2013;91(4):e270–e275. | ||
Tsumura T, Yoshikawa K, Suzumura H, et al. Bimatoprost ophthalmic solution 0.03% lowered intraocular pressure of normal-tension glaucoma with minimal adverse events. Clin Ophthalmol. 2012;6:1547–1552. | ||
Kokuzawa A, Kondo Y, Yamamoto T. [Current status of medication for glaucoma in a university hospital]. Rinsho Ganka (Jpn J Clin Ophthalmol). 2006;60(9):1679–1684. Japanese. | ||
Nakai Y, Inoue K, Moriyama R, Wakakura M, Inoue J, Tomita G. [Current status of glaucoma therapy at private practices and a private ophthalmology hospital]. Atarashii Ganka (Journal of the Eye). 2008; 25(11):1581–1585. Japanese. | ||
Inoue K, Shiokawa M, Okayama R, Soeda S, Hori S, Tomita G. Current status of the pattern of medication in 2012: difference between elder glaucoma patients and younger or middle aged glaucoma patients. Ganka Rinsho Kiyou. 2013;6(11):869–874. Japanese. | ||
Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. | ||
Tokoro T. [Mechanism of axial elongation and chorioretinal atrophy in high myopia]. Nihon Ganka Gakkai Zasshi. 1994;98(12):1213–1237. Japanese. | ||
Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma. 2002;11(2):119–126. | ||
Wada T, Burke JA, Wheeler LA. [Characteristic of brimonidine tartrate ophthalmic solution (AIPHAGAN 0.1%)]. Igaku to Yakugaku. 2012; 67(4):547–555. Japanese. | ||
Kaneko E, Wada T, Minagawa Y, Inoue Y. [Pharmacological profile and clinical efficacy of brimonidine tartrate (AIPHAGAN® ophthalmic solution 0.1%)]. Nihon Yakurigaku Zasshi. 2012;140(4):177–182. Japanese. | ||
Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9(3):483–491. | ||
Derck RJ. Adrenergic agonist medications: basic mechanisms. J Glaucoma. 1995;4 Suppl 1:S1–S7. | ||
Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996;41 Suppl 1:S9–S18. | ||
Araie M, Yamazaki Y, Sugiyama K, Kuwayama Y, Tanihara H. Long-term safety and efficacy of brimonidine ophthalmic solution in patents with primary open angle glaucoma or ocular hypertension. Atarashii Ganka (Journal of the Eye). 2012;29(5):679–686. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





