Back to Journals » Drug Design, Development and Therapy » Volume 14
The Effects of Short-Chain Fatty Acids on Rat Colonic Hypermotility Induced by Water Avoidance Stress
Authors Yuan F, Tan W, Ren H, Yan L, Wang Y, Luo H
Received 19 January 2020
Accepted for publication 24 September 2020
Published 2 November 2020 Volume 2020:14 Pages 4671—4684
DOI https://doi.org/10.2147/DDDT.S246619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
FangTing Yuan,1,* Wei Tan,1,* HaiXia Ren,1 Lin Yan,1 Ying Wang,1,2 HeSheng Luo1
1Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China; 2Key Laboratory of Hubei Province for Digestive System Diseases, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: HeSheng Luo Email [email protected]
Purpose: Short-chain fatty acids (SCFAs) have been reported to play an important role in regulating gastrointestinal motility. The aim of this study is to investigate the possible role of SCFAs in water avoidance stress-induced colonic hypermotility.
Methods: A rat IBS model was established by water avoidance stress (WAS). Intestinal motility was assessed by fecal pellets expulsion. The fecal SCFA level was detected using gas chromatography-mass spectrometry (GC-MS). Western blotting was performed to assess the expression of SCFAs receptors. To determine the role of SCFAs in gut dysmotility, the rats of the WAS+SCFAS and SCFAs group were administrated with oral SCFAs. The colonic contractile activity was recorded with a RM6240 multichannel physiological signal system.
Key Results: WAS induced gastrointestinal hypermotility and increased defecation in rats. After repeated stress, the fecal SCFAs decreased significantly and the proportion of acetic acid, propionic acid, and butyric acid had changed from Control 2.6:1:1.5 to WAS 2:1:2.3. Protein levels of SCFAs receptors in the colon were promoted by WAS. In addition, oral SCFAs partly inhibited the colonic spontaneous motility both for SCFAs and WAS+SCFAs group in vivo. Meanwhile, we observed acetate had no effect on the contractile amplitudes of muscle strips, but it could slow down contractile frequency in a dose-dependent manner (1– 100 mM). Propionate significantly inhibited the motor activity of colonic strips (1– 30 mM). Butyrate inhibited the contractile amplitude of CM strips in a dose-dependent manner (1– 30 mM), but for LM, it exhibited a stimulating effect at low concentrations of butyrate 1 mM– 10 mM and was suppressed at high concentrations of 30 mM butyrate. Total SCFAs increased the contractile amplitude at low concentration (5– 50 mM) and inhibited it at high concentration (50– 150 mM). All SCFAs slowed down the frequency of colonic activity.
Conclusion: The stress-induced colonic hypermotility by WAS could be ameliorated through oral SCFA supplementation. SCFAs may have potential clinical therapeutic use in modulating gut motility.
Keywords: gastrointestinal motility, SCFAs, chronic stress, IBS
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder, whose pathological mechanism is poorly understood.1 It is proved that stress has a close relationship with IBS.2–4 Several lines of evidence show that chronic stress can increase gastrointestinal motility. For instance, corticotropin-releasing hormone (CRH) mediates stress-induced motility and fecal pellet output.5 The gastric emptying and the intestinal transit are significantly increased in heat exposed young rats.6 Recent years have seen a growing interest in the mechanism of stress on gut motility. Accumulating evidence suggested the possible mechanisms underlying the stress-induced hypermotility in the colon, such as the upregulation of ion channel TMEM16A,7 the activation cholinergic myenteric neurons,8 and the increased 5-HT expression in the gut.9
SCFAs, mainly including acetate, propionate, and butyrate in a ratio of approximately 60:20:20, are major metabolic products fermented by colonic intestinal bacteria through dietary carbohydrates and some amino acids mainly.10 Subsequently they are secreted into the blood to circulate systematically, where they can exert functions in all parts of the body cells by providing energy through β-oxidation in mitochondria.11 These SCFAs partly exert their actions via G-coupled protein receptors 41 and 43 (GPR41 and GPR43).12 In parallel, GPR activation by SCFAs plays a key role in maintaining a healthy intestine, such as providing energy for the colonic epithelium, modulating colonic and intracellular pH, inhibiting tumor growth, and regulating ion transport.13 There is increasing evidence that SCFAs are associated with dysmotility of the gut. It was reported that alterations of the SCFAs might slow intestinal transit time and weaken spontaneous contractions of colonic smooth muscle in mice.14 On the contrary, propionate and butyrate have been shown to increase the longitudinal smooth muscle contractions of both the proximal and distal colon of cats in vitro with a dose of 10–100 mM.15 Furthermore, intracolonic infusion of solutions containing SCFAs mixture (100 mM) did not change the phasic and tonic motor activity of the human colon.16 In a study, luminal SCFAs had differing effects on proximal and distal colonic motility of the guinea pig analyzed with spaciotemporal maps.17 These data suggested that the effects of SCFAs on the intestinal motility are incompletely elucidated. Also, reports implicated SCFAs in the regulation of chronic stress-induced colonic motor activity have not previously been published.
Due to the discussion of SCFAs in gut motility, we investigated whether SCFAs could ameliorate the hypermotility induced by stress. As such, we aimed to assess the alterations in fecal SCFAs and in expression of SCFAs receptors in the colon and to determine the role of SCFAs in a rat model induced by chronic stress.
Materials and Methods
Animals
All adult male Wistar rats (180–200 g) were purchased from Vital River (Beijing, China). Animals were maintained in a constant temperature (22±1°C) under a 12 hour light–dark cycle and provided with free food and water. All protocols were approved by the Institutional Animal Care and Use Committee of Renmin Hospital of Wuhan University (Approval ID: SYXK 2015–0027, Hubei, China) and adhered to the ethical guidelines of the International Association for the Study of Pain.
Chemicals
The following agents were used in this study: sodium acetate, sodium propionate, and sodium butyrate were purchased from Sigma-Aldrich. SCFAs were a mixture of sodium acetate, sodium propionate, and sodium butyrate, which was dissolved in Tyrode’s buffer at a ratio of 3 mM Sodium acetate, 1 mM Sodium propionate, and 1 mM Sodium butyrate. Both the rabbit anti-GPR41 and anti-GPR43 antibodies were purchased from Santa. The rabbit anti-GAPDH antibody was purchased from CST. The goat Anti-Rabbit IgG and the goat Anti-mouse IgG were purchased from Bio swamp. RIPA lysis buffer and the BCA protein assay kit were obtained from Bio swamp.
Experimental Protocol
The rats were randomly divided into four groups: SCFAs group, Control group, Water Avoidance Stress (WAS) group, and WAS+SCFAs group.
The WAS procedure was performed to induce IBS as described previously.18 Briefly, rats were placed on a platform (10×8×8 cm) in the center of a water‐filled (25°C) tank (45×25×35 cm) for 1 hour daily for 10 consecutive days. The water level in the tank was kept at 1 cm below the platform. The Control rats and SCFAs rats were submitted to the same block without water. The procedures were performed between 8:00 and 10:00 AM to minimize the effects of circadian rhythm. Fecal pellets found in the tank were counted at the end of each 1-hour session for the four groups.
The experimental protocol was as follows. After 3 days acclimation, the animals in WAS+SCFAs and SCFAs group were administered drinking water which contained SCFAs described as above before the WAS session for 7 consecutive days. On day 8, the protocol WAS began for all four groups, during the consecutive 10 days, the rats of WAS+SCFAs and SCFAs group were continued to be administered SCFAs water until the end of the session.
Fecal Sample Collection and SCFAs Determination by GC-MS
Fecal pellets from control and WAS rats during each 1 hour session were collected in a sterile plastic tube no later than 24 hours. SCFAs were extracted under 4°C to protect the volatile SCFAs and then quantified by GC-MS. GC-MS analysis was performed using an Agilent 7890 gas chromatograph system coupled with a Agilent 7000D mass spectrometer. The system utilized a HP-FFAP capillary column. A 1 μL aliquot of the fecal extraction was injected in split mode (5:1). Helium was used as the carrier gas, the front inlet purge flow was 3 mL/min, and the gas flow rate through the column was 1 mL/min. The initial temperature was kept at 100°C for 1 minute, then raised to 190°C at a rate of 5°C/min, then kept for 7.5 minutes at 240°C at a rate of 40°C/min. The injection, transfer line, quad, and ion source temperatures were 260°C, 260°C, 150°C, and 230°C, respectively. The energy was −70eV in electron impact mode. The mass spectrometry data were acquired in MRM mode with the solvent delay of 3 minutes.
Western Blot
The proximal colons both for control and WAS rats were extracted under dry ice and stored at −80°C until they were processed. Total proteins were collected using RIPA lysis buffer and centrifuged at 12,000 rpm, 4°C for 15 minutes. We collected supernatants and then detected protein concentration by BCA protein assay kit. The protein samples were mixed with loading buffer and boiled for 5 minutes at 100°C to denature the proteins. The proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane for immunoblotting. The membranes were blocked with 5% skimmed milk for 1 hour at room temperature and then incubated overnight at 4°C with rabbit anti-GPR41 and anti-GPR43 antibodies, respectively. After several washes, they were then incubated for 2 hours at room temperature with the goat Anti-Rabbit IgG and the goat Anti-mouse IgG. Finally, a specific band was detected by ECL chemiluminescent substrate.
Tissue Preparation and Contraction Recordings
The rats were killed by cervical dislocation, the proximal colon was removed and cleaned in Ca2+‐free physiological saline solution (Ca2+‐free PSS) which contained 135.0 mM NaCl, 5.0 mM KCl, 10.0 mM glucose, 1.2 mM MgCl2, and 10.0 mM Hepes (the pH was adjusted to 7.35–7.45 with NaOH) and perfused with carbogen (95% O2 and 5% CO2). The colonic tissue was carefully cut into Circular muscle (CM) strips and Longitudinal muscle (LM) strips (3×10 mm, width x length), respectively, along the edge of the mesentery.
Each smooth muscle strip was fixed in a four‐channel tissue chamber and immersed in 7 mL Tyrode’s buffer containing 147.0 mM NaCl, 4.0 mM KCl, 2.0 mM CaCl2, 0.42 mM NaH2PO4, 2.0 mM Na2HPO4, 1.05 mM MgCl2, and 5.5 mM glucose (adjusted pH to 7.35‐7.45 with NaOH). The solution was maintained at 37°C using a circulating water jacket and bubbled with carbogen constantly.
The strips were placed under an initial resting tension equivalent to a 1.0 g load and washed with Tyrode’s buffer every 30 minutes during the equilibration period. One end of the strip was fixed to the bottom of the chamber, and the other end was connected to an isometric force transducer (JZJOIH, Chengdu, China). The colonic motor activity was recorded with a RM6240 multichannel physiological signal system (Cheng Du, China).
Statistical Analysis
The data were analyzed using SPSS 24.0. All data in the figures were expressed as Mean±SEM. Significant differences between all groups were evaluated using the independent sample t-test and one-way ANOVA. P<0.05 was considered significant.
Results
The Comparison of SCFAs in Fecal Samples of Control and WAS Rats
None of rats died in the course of the experiment. As shown in Table 1, WAS induced a significant change of principal fecal SCFAs acetic acid, propionic acid, butyric acid, and SCFAs. The contents of acetic acid, propionic acid, butyric acid, and SCFAs in fecal samples of Control rats were 622.06±33.42 mg/kg, 306.48±15.49 mg/kg, 703.85±125.63 mg/kg, and 1632.40±150.92 mg/kg, after stress they turned to 87.46±36.13 mg/kg, 72.05±30.07 mg/kg, 109.71±19.16 mg/kg, and 360.67±81.98 mg/kg, respectively. Moreover, the proportion of three acids (acetic acid, propionic acid, and butyric acid) had changed from Control 2.6:1:1.5 to WAS 2:1:2.3.
 |
Table 1 The Fecal SCFAs Content of Control and WAS Rats |
The Fecal Pellets Expulsion of Four Groups
Stress induced a significant increase in colon motor function, and defecation could be used to evaluate colonic activity. Figure 1A shows fecal pellet expulsion during the session for rats of WAS groups, WAS+SCFAs group, Control group, and SCFAs group. Figure 1B showed the mean numbers of fecal expulsion of four groups. WAS induced a greater rate of fecal pellets expulsion. During the experiment, the fecal pellets expulsion per hour of WAS group were higher than that of the Control group (WAS 8.56±0.43 vs Control 2.99±0.13, P<0.01), After being treated with SCFAs, defecation of the rats from the WAS+SCFAs group was lower than that of the WAS group (WAS 8.56±0.43 vs WAS+SCFAs 6.35±0.17, P<0.01), but it was still higher than that of the Control group (WAS+SCFAs 6.35±0.17 vs Control 2.99±0.13, P<0.01). In the SCFAs group, defecation of the rats was lower than that of the Control group (SCFAs 1.57±0.12 vs Control 2.99±0.13, P<0.01).
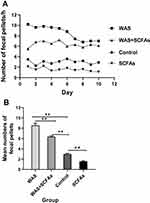 |
Figure 1 The fecal pellets expulsion of four groups. (A) Effect of repeated WAS and SCFAs on rat Defecation. (B) The difference among SCFAs group, Control group, WAS group, and WAS+SCFAs group. |
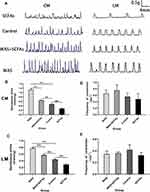
The Influence of Repeated Stress on Contractile Activity of Proximal Colonic Strips
In Figure 2A, WAS induced a significant increase in colonic spontaneous contraction of the proximal colon compared with that of the Control rats. In Figure 2B, the average contraction amplitude of the CM from the WAS rats was significantly higher than that from the Control rats (1.13±0.05g vs 0.48±0.03g, P<0.01). Similarly, In Figure 2C, the average contraction amplitude of the LM from the WAS rats was significantly higher than that from the Control rats (0.77±0.01g vs 0.44±0.02g, P<0.01). However, there was no significance between the contractile frequency of CM and LM between the WAS and the Control group (Figure 2D and E).
Increased Expression of the Short-Chain Fatty Acids Receptors in the Colon of WAS Rats
To determine whether SCFAs receptors can be regulated by stress in the rat proximal colon, the expression of GPR41 and GPR43 was detected using Western blot (Figure 3A). We observed the WAS rats showed a significant upregulation of SCFAs receptors GPR41 and GPR43 in the colonic tissue compared with the Control rats. As shown in Figure 3B, the level of GPR41 in WAS rats was significantly higher than that in Control rats (WAS: 0.43±0.12 vs Control: 0.32±0.11, P<0.05). Similarly, the level of GPR43 in WAS rats was significantly higher than that in Control rats (WAS: 0.78±0.07 vs Control: 0.35±0.04, P<0.05).
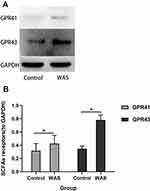 |
Figure 3 (A) Expression levels of SCFAs receptors were detected by Western blot. (B) Repeated WAS increased the expression of SCFAs receptors in the proximal colon. |
SCFAs Alleviate Colonic Hypermotility in vivo
In this experiment, SCFAs were made with 3 mM Sodium acetate, 1 mM Sodium propionate, and 1 mM Sodium butyrate. SCFAs were used at a concentration of 150 mM and then PH of solution was adjusted to 7.6 using NaOH. SCFAs solutions were administered to explore the role of SCFAs in intestinal hypermotility of stress‐exposed rats. In Figure 2B and C, the data indicated that SCFAs partly inhibited the colonic spontaneous high-amplitude contractions of WAS-exposed rats (CM: 0.65±0.02 g vs 1.13±0.05 g, P<0.01; LM: 0.57±0.01 g vs 0.77±0.01 g, P<0.01), Although SCFAs alleviate colonic hypermotility, the spontaneous contractions from WAS-exposed rats treated with SCFAs is still higher than that of Control rats (CM: 0.65±0.02 g vs 0.48±0.02 g, P<0.01; LM: 0.57±0.01 g vs 0.44±0.02 g, P<0.01). In the SCFAs group, the oral SCFAs alone alleviates colonic motility compared with the Control group (CM: 0.27±0.03 g vs 0.48±0.03 g, P<0.01; LM: 0.29±0.03 g vs 0.44±0.018 g, P<0.01). There was no significance between the contractile frequency of CM and LM among them (Figure 2D and E).
Effects of SCFAs on the Spontaneous Contractile Activities of Colonic Muscle Strips in vitro
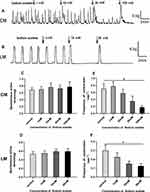
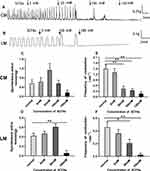
As shown in Figure 4A and B, Sodium acetate had no effect on the amplitudes of spontaneous contraction of colonic strips. The average amplitudes of CM and LM had no changes under different concentrations of sodium acetate in Figure 4C and D. Interestingly, we observed that contractile frequency was inhibited by sodium acetate in a concentration-dependent manner. The mean frequency of CM before adding sodium acetate was 0.7±0.15/min, after the addition of sodium acetate (1–100 mM), it was reduced to 0.58±0.11/min, 0.35±0.14/min, and 0.18±0.05/min, respectively (Figure 4E). However, at the concentration of 1 mM, the frequency of CM 0.78±0.13/min increased slightly compared with basal contractile frequency. Meanwhile, the mean frequency of LM before adding sodium acetate was 0.3±0.06/min, after applying sodium acetate (1–30 mM), it was reduced to 0.23±0.05/min, 0.15±0.03/min, and 0.13±0.03/min, respectively (Figure 4F).
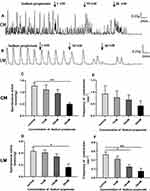
As shown in Figure 5A and B, sodium propionate significantly inhibited spontaneous contraction of the colonic strips in a concentration‐dependent manner. For the CM strips, the average contractile amplitude before adding sodium propionate was 1.26±0.13 g, whereas in the presence of sodium propionate (1, 10, and 30 mM), it was dropped to 1.11±0.20 g, 0.95±0.17 g, and 0.50±0.05 g, respectively (Figure 5C). For the LM strips, the mean amplitude was 0.43±0.06 g before the addition of sodium propionate and decreased to 0.41±0.04 g, 0.34±0.05 g, and 0.16±0.06 g, respectively (Figure 5D) after adding sodium propionate 1.10 and 30 mM.
Similarly, sodium propionate significantly inhibited the frequency of LM strips in a concentration-dependent manner. The average contractile frequency before adding sodium propionate was 0.53±0.06/min, and it changed to 0.43±0.06/min, 0.25±0.05/min, and 0.15±0.07/min, respectively (Figure 5F) with different concentrations of sodium propionate at 1.10 and 30 mM. Although there was no significant difference in contractile frequency of CM strips (Figure 5E), the frequency of contraction still presented a downward trend with the increase of concentration (1–30 mM) compared with basal frequency 0.93±0.33/min (0.78±0.28/min, 0.68±0.21/min, and 0.43±0.20/min).
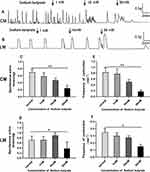
As shown in Figure 6A and B, Sodium butyrate influenced the contraction of colonic strips. When adding 1, 10, and 30 mM sodium butyrate, the average amplitudes of CM strips were 0.58±0.20 g, 0.47±0.13 g, and 0.24±0.16 g, respectively (Figure 6C), compared with the basal amplitudes (0.70±0.18 g) before adding Sodium butyrate. For the LM strips, the basal amplitude was 0.71±0.05 g, but the mean amplitude was slightly increased at concentrations of 1 mM and 10 mM, it was, respectively, 0.72±0.04 g and 0.90±0.11 g; in the presence of the highest sodium butyrate concentration 30 mM, it was reduced to 0.38±0.08 g (Figure 6D).
Sodium butyrate produced a decrease in the contractile frequency in a dose‐dependent manner both for CM strips and LM strips. In CM strips, the frequency changed from 0.83±0.30/min to 0.78±0.35/min, 0.5±0.16/min, and 0.18±0.1/min (Figure 6E) in response to sodium butyrate treatment at different concentrations of 1, 10, and 30 mM. Similarly, the frequency in LM strips changed from 0.35±0.05/min to 0.30±0.04/min, 0.28±0.03/min, and 0.15±0.03/min (Figure 6F) in response to sodium butyrate at concentrations of 1, 10, and 30 mM.
SCFAs application (3 mM acetate, 1 mM butyrate, 1 mM propionate) with an increasing concentration (5, 50, 100, 150 mM) to the organ bath changed the motility of the colon. As shown in Figure 7A and B, the SCFAs at concentration of 5 and 50 mM slightly increased the amplitudes of contraction both for CM and LM strips, although there was no significant difference. For CM strips, prior to the addition of SCFAs, the average amplitude was 0.56±0.16 g, after the addition of SCFAs at concentrations of 5.50, 100, and 150 mM, the amplitude changed to 0.63±0.17 g, 1.13±0.28 g, 0.55±0.14 g, and 0.17±0.10 g, respectively (Figure 7C). For LM strips, prior to the application of SCFAs, the average amplitude was 0.42±0.06 g, after the application of SCFAs at concentrations of 5, 50, and 100 mM, the amplitude changed to 0.47±0.05 g, 0.61±0.07 g, and 0.06±0.06 g, respectively (Figure 7D). Moreover, the inhibitory effect of different dose of SCFAs are shown on the contractile frequency of the colon. The frequency was reduced to 1.3±0.21/min, 0.43±0.14/min, 0.35±0.0/min, and 0.18±0.11/min, respectively (Figure 7E) compared with the basal contractile frequency 1.5±0.20/min in response to SCFAs (5–150 mM) in CM strips. Similarly, the frequency was reduced to 0.36±0.61/min, 0.21±0.06/min, and 0.03±0.02/min, respectively (Figure 7F), compared with the basal contractile frequency (0.46±0.08/min) in response to SCFAs (5–100 mM) in LM strips.
Discussion
Chronic stress affects people’s daily life seriously, for its association with various diseases including psychological illness such as depression and anxiety19 as well as gastrointestinal disorders like Irritable bowel syndrome4 and Inflammatory bowel disease.20 It is reported that repeated exposure to WAS had frequently been used to establish animal models of stress-induced IBS with increased colonic motor function.18 Repeated WAS could induce colonic hyperactivity, which is manifested in an increased defecation of animals and increased spontaneous contraction of smooth muscle. We observed that WAS increased the number of fecal pellets per hour and the spontaneous contractile activities of colonic muscle strips, indicating stress-induced dysmotility was successfully performed in our study, which was consistent with previous studies.7,21
Reports have shown that SCFAs were associated with reduced risk of some disease, such as intestinal diseases,22 cardiovascular diseases,23 neurological diseases,24 and cancer.25 Recently, the relationship between SCFAs and stress-induced IBS has received wide attention, but the current mechanism has not been fully clarified. In our study, WAS decreased the total fecal SCFAs, with a significant reduction in acetate, propionate, butyrate, and total SCFAs compared to the Control group. Yet, this observation seems to contradict findings from a previous study, showing that stress increased fecal acetate and total SCFA levels.26 We have also seen similar results showing mice were exposed to a social disruption stress, thus leading to a decrease in fecal SCFAs in the absence of infection.27 The discrepancy among studies may be due to the different experimental protocol. Based on these data, we may infer that chronic psychological stress influenced the production and excretion of SCFAs in the gut. Microbiota-derived metabolites play a key role in the communication between microbes and their host, with SCFAs being perhaps the most studied. The production of SCFAs depends on species and amounts of microbiota in the colon, substrate source, and gut transit time;13 a report that the amount of fecal SCFAs was significantly lower in Germ-Free rats than in fecal microbiota transplantation rats28 could support this argument. Besides, a study showed that WAS led to reduction in the abundance of butyrate-producing bacteria, which could restore the IBS symptoms.29 Further, it has been reported that SCFAs-producing bacteria were positively correlated with colonic stress, which could decrease the colonic bacteria relevant to SCFAs.27,29 Therefore, the long-term stress-induced reduction in gut microbiota might explain the decrease of fecal SCFAs in this study, our present research needs further study.
The previous study showed ingestion of butylated starch increased large bowel butyrate levels and decreased colonic contractility.30 Rats given a butyrate starch diet had showed increased colonic transit.31 In our work, we proved that application of SCFAs via drinking water to rats undergoing stress alleviated the colonic hypermotility in vitro and reduced defecation in vivo. Moreover, given oral administration of SCFAs to normal rats also alleviated the colonic motility and reduced defecation. As mentioned above, the relationship among stress, gut microbiota, and SCFAs is complex. A similar study showed the cocktail of SCFAs rectified abnormal motility in GF mice.32 The loss of SCFAs caused by stress in the colon could be supplemented by the treatment of SCFAs, which partially restored the gut motility accompanied by the decrease in fecal pellet expulsion.
In addition to being an energy source for both the host and microbiota,11,13 SCFAs are also signaling molecules through binding to G-protein coupled receptors GPR41 and GPR43 which is known to be expressed in adipose tissue, intestines, endocrine, and immune cells.12 Several lines of studies indicated that altered expression of SCFAs receptors could be related to psoriasis,33 colon cancer,34 and diabetes.35 However, only a few studies reported stress modulated the expression of SCFAs receptor in the gastrointestinal tract.26,27 In our colonic hypermotility model, we detected SCFAs receptor GPR41 and GPR43 were significantly increased expression in colonic tissues of the WAS rats, which has not been explored before, indicating that SCFAs receptors played a role in stress-induced colonic motility. Stress is defined as the psychophysiological reaction in which the steady state is disturbed or threatened.36 It was reported that chronic psychosocial stress could lead to an imbalanced immune response in body, such as the dysregulation of macrophages37 and lymphocyte,38 accompanied by energy metabolism disorder.39 Besides, SCFAs are an energy source, which provides 60–70% energy needs to the colon.40 A recent study demonstrated psychosocial stress increased stress-responsivity and intestinal permeability, in the presence of an upregulation of intestinal GPR43, both of which could be ameliorated by oral SCFAs administration.26 Maybe the upregulation of GPR41 and GPR43 in the colon is an adaptive response to environmental irritation for the maintenance of intestinal homeostasis. Based on these findings, we inferred that G-protein-coupled receptor agonist SCFAs is related to the pathological course of motility disorder in gastrointestinal tract.
Further studies regarding the action of SCFAs were performed in colonic strips with a multichannel physiological signal system. The number of animal experiments confirmed that SCFAs is involved in regulating intestinal motility, but there are conflicting results.14–16 The contradictory data could be explained by differences in the signaling pathway, different drug concentrations, the species used, and the type of preparation. Here, in the present work, we tested the effect of acetate, propionate, butyrate, and total SCFAs on the colonic muscle strips.
We observed acetate had no effect on the muscle strips, but it could slow down contractile frequency. Propionate significantly inhibited contractile amplitudes of the colonic strips. Colonic infusion of propionate induced secretion of gut hormone peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) through the activation of GPR43 located on colonic enteroendocrine L cells.41 As we know, PYY and GLP-1 are inhibitory hormones which have also been involved in inhibiting motility,42,43 it seems possible that the inhibitory effect from propionate is a result of the PYY and GLP-1, so far, although no study had shown propionate had a greater effect on the colonic enteroendocrine cells than any other SCFA.
Butyrate inhibited the CM strips in a dose-dependent manner, but for LM, it exhibited a contractile effect at low concentration of butyrate 1 mM–10 mM and were suppressed at high concentrations of 30 mM butyrate, which was perhaps due to different contraction mechanisms between the CM and LM strips. Butyrate enemas 2.5 mM and 5 mM but not 10 mM increased distal colonic transit time compared with control via the myenteric neurons.44 Further, butyrate 1 mM neuronally mediated contractile response in the colon, neither acetate nor propionate did modify the proportion of myenteric neurons.31 Therefore, the effect of butyrate on motility might tend to be mediated by enteric neurons.
It is demonstrated that administration of SCFAs (100 mM) into the proximal colon significantly increased the colonic motility in conscious rats, via the release of 5-HT from enterochromaffin cells which expressed 5-HT3 receptors located on the vagal sensory fibers.45 However, SCFAs had no effect on the phasic and tonic motor activity of the human colon.16 In this work, for CM and LM, we showed total SCFAs increased contraction at low concentration (5–50 mM) and inhibited contraction at high concentration (50–150 mM).
The response to SCFAs is likely to be co-mediated by two SCFA receptors, GPR43 and GPR41, on the gut wall. GPR41 is alone expressed in enteric neurons of submucosal and myenteric ganglia, while GPR43 is strongly expressed in enteroendocrine cells.46 Hence, the effects of SCFAs on the colon may be due to the enteric neurons, such as cholinergic neurons and nitrergic neurons, as well as gut hormones, including 5-HT, PYY, GLP-1, and so on. And their mechanism involved in the differential effect on motility is unknown in our study. Each SCFA produced in the colon may have its own specific characteristic, and each SCFA needs to be explored separately to understand its specific physiological effect.
Conclusion
Based on these data, our research demonstrated for the first time that the administration of oral SCFAs could restore chronic stress-induced colonic motility dysfunction. We know that more research is warranted to elucidate the exact relationship of stress-induced dysmotility and SCFAs, for example, the alteration of intestinal microbiota, gut hormones, and exploration of the mechanism from the perspective of cell level using the whole-cell patch-clamp technique.
Acknowledgments
Thanks to all who contributed to this research. This study was supported by the National Natural Science Foundation of China [NSFC: 81600423]. FangTing Yuan andWei Tan share first authorship.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi:10.1053/j.gastro.2005.11.061
2. Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008;64(2):119–128. doi:10.1016/j.jpsychores.2007.10.010
3. Vasquez-Rios G, Machicado JD, Ticse R, et al. Stress and a sedentary lifestyle are associated with irritable bowel syndrome in medical students from Peru: a cross-sectional study. Eur J Gastroenterol Hepatol. 2019;1. doi:10.1097/MEG.0000000000001479
4. Gwee KA, Ghoshal UC, Chen M. Irritable bowel syndrome in Asia: pathogenesis, natural history, epidemiology, and management. J Gastroenterol Hepatol. 2018;33(1):99–110. doi:10.1111/jgh.13987
5. Murakami T, Kamada K, Mizushima K, et al. Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion. 2017;95(1):55–60. doi:10.1159/000452364
6. Datta UK. Effect of heat stress on gastro-intestinal motility in young albino rats. Indian J Physiol Pharmacol. 2001;45(2):222–226.
7. Lin MJ, Yu BP. Colonic hypermotility in a rat model of irritable bowel syndrome is associated with upregulation of TMEM16A in myenteric plexus. Dig Dis Sci. 2018;63(12):3329–3338. doi:10.1007/s10620-018-5261-7
8. Miampamba M, Million M, Yuan PQ, et al. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport. 2007;18(7):679–682. doi:10.1097/WNR.0b013e3280bef7f8
9. Yu YC, Li J, Zhang M, et al. Resveratrol improves brain-gut axis by regulation of 5-HT-dependent signaling in the rat model of irritable bowel syndrome. Front Cell Neurosci. 2019;13:30. doi:10.3389/fncel.2019.00030
10. Roy CC, Kien CL, Bouthillier L, et al. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21(4):351–366. doi:10.1177/0115426506021004351
11. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi:10.1016/j.cmet.2011.02.018
12. Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119.
13. Wong JM, de Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–243. doi:10.1097/00004836-200603000-00015
14. Ge X, Zhao W, Ding C, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. 2017;7(1):441. doi:10.1038/s41598-017-00612-y
15. Rondeau MP, Meltzer K, Michel KE, et al. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg. 2003;5(3):167–173. doi:10.1016/S1098-612X(03)00002-0
16. Jouet P, Moussata D, Duboc H, et al. Effect of short-chain fatty acids and acidification on the phasic and tonic motor activity of the human colon. Neurogastroenterol Motil. 2013;25(12):943–949. doi:10.1111/nmo.12212
17. Hurst NR, Kendig DM, Murthy KS, et al. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26(11):1586–1596. doi:10.1111/nmo.12425
18. Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G42–G53. doi:10.1152/ajpgi.00500.2004
19. Jiang N, Lv JW, Wang HX, et al. Antidepressant-like effects of 20(S)-protopanaxadiol in a mouse model of chronic social defeat stress and the related mechanisms. Phytother Res. 2019;33(10):2726–2736. doi:10.1002/ptr.6446
20. Oligschlaeger Y, Yadati T, Houben T, et al. Inflammatory bowel disease: a stressed “gut/feeling”. Cells. 2019;8(7):659. doi:10.3390/cells8070659
21. Liu Y, Luo H, Liang C, et al. Actions of hydrogen sulfide and ATP-sensitive potassium channels on colonic hypermotility in a rat model of chronic stress. PLoS One. 2013;8(2):e55853. doi:10.1371/journal.pone.0055853
22. Manrique VD, Gonzalez SM. Short chain fatty acids (butyric acid) and intestinal diseases. Nutr Hosp. 2017;34(Suppl 4):58–61.
23. Ahmadmehrabi S, Tang W. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol. 2017;32(6):761–766. doi:10.1097/HCO.0000000000000445
24. Castillo-Alvarez F, Marzo-Sola ME. Role of the gut microbiota in the development of various neurological diseases. Neurologia. 2019.
25. Wang G, Yu Y, Wang YZ, et al. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J Cell Physiol. 2019;234(10):17023–17049. doi:10.1002/jcp.28436
26. van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–4944. doi:10.1113/JP276431
27. Maltz RM, Keirsey J, Kim SC, et al. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J Pediatr Gastroenterol Nutr. 2019;68(4):533–540. doi:10.1097/MPG.0000000000002226
28. Wang B, Zhang L, Zhu SW, et al. Short chain fatty acids contribute to gut microbiota-induced promotion of colonic melatonin receptor expression. J Biol Regul Homeost Agents. 2019;33(3):763–771.
29. Zhang J, Song L, Wang Y, et al. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2018. doi:10.1111/jgh.14536
30. Bajka BH, Clarke JM, Topping DL, et al. Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats. Nutr Res. 2010;30(6):427–434. doi:10.1016/j.nutres.2010.06.003
31. Soret R, Chevalier J, De Coppet P, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772–1782. doi:10.1053/j.gastro.2010.01.053
32. Vincent AD, Wang XY, Parsons SP, et al. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am J Physiol Gastrointest Liver Physiol. 2018;315(5):G896–G907. doi:10.1152/ajpgi.00237.2017
33. Krejner A, Bruhs A, Mrowietz U, et al. Decreased expression of G-protein-coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch Dermatol Res. 2018;310(9):751–758. doi:10.1007/s00403-018-1865-1
34. Tang Y, Chen Y, Jiang H, et al. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128(4):847–856. doi:10.1002/ijc.25638
35. Shi G, Sun C, Gu W, et al. Free fatty acid receptor 2, a candidate target for type 1 diabetes, induces cell apoptosis through ERK signaling. J Mol Endocrinol. 2014;53(3):367–380. doi:10.1530/JME-14-0065
36. Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi:10.1146/annurev.clinpsy.1.102803.144141
37. Hanke ML, Powell ND, Stiner LM, et al. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26(7):1150–1159. doi:10.1016/j.bbi.2012.07.011
38. Gurfein BT, Hasdemir B, Milush JM, et al. Enriched environment and stress exposure influence splenic B lymphocyte composition. PLoS One. 2017;12(7):e180771. doi:10.1371/journal.pone.0180771
39. Williams ED, Magliano DJ, Tapp RJ, et al. Psychosocial stress predicts abnormal glucose metabolism: the Australian diabetes, obesity and lifestyle (AusDiab) study. Ann Behav Med. 2013;46(1):62–72. doi:10.1007/s12160-013-9473-y
40. Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21(9):793–798. doi:10.1136/gut.21.9.793
41. Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–429. doi:10.1038/ijo.2014.153
42. Hellstrom PM, Naslund E, Edholm T, et al. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. 2008;20(6):649–659. doi:10.1111/j.1365-2982.2007.01079.x
43. Cherbut C, Ferrier L, Roze C, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275(6):G1415–G1422.
44. Suply E, de Vries P, Soret R, et al. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;302(12):G1373–G1380. doi:10.1152/ajpgi.00338.2011
45. Fukumoto S, Tatewaki M, Yamada T, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1269–R1276. doi:10.1152/ajpregu.00442.2002
46. Nohr MK, Pedersen MH, Gille A, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi:10.1210/en.2013-1142
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.