Back to Journals » Journal of Inflammation Research » Volume 15
The Effects of Non-Surgical Periodontitis Therapy on the Clinical Features and Serological Parameters of Patients Suffering from Rheumatoid Arthritis as Well as Chronic Periodontitis
Authors Ding N, Luo M, Wen YH, Li RY, Bao QY
Received 28 June 2021
Accepted for publication 9 September 2021
Published 11 January 2022 Volume 2022:15 Pages 177—185
DOI https://doi.org/10.2147/JIR.S326896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Nan Ding,1 Mei Luo,2 Ya-Hui Wen,3 Rong-Yin Li,1 Qi-Yan Bao1
1Department of Stomatology, Beijing LuHe Hospital, Capital Medical University, Beijing, 101149, People’s Republic of China; 2Department of Central Laboratory, Beijing LuHe Hospital, Capital Medical University, Beijing, 101149, People’s Republic of China; 3Department of Internal Medicine, Beijing LuHe Hospital, Capital Medical University, Beijing, 101149, People’s Republic of China
Correspondence: Nan Ding
Department of Stomatology, Beijing LuHe Hospital, Capital Medical University, No. 82 of Xinhua South Road, Tongzhou District, Beijing, 101149, People’s Republic of China
Tel/Fax +86 10 69543901
Email [email protected]
Objective: The present study aims to evaluate the effects of basic periodontal disease therapy on the general condition and serum inflammatory indicators of patients with rheumatoid arthritis (RA) combined with chronic periodontitis (CP).
Methods: Forty patients with RA were enrolled in the study and, based on the results of an oral examination and in line with the 2018 periodontitis diagnostic criteria, they were divided into a group with CP (the RA + CP group) and a group without CP (the RA group). Twenty-nine patients with CP who attended the periodontal department of our hospital were recruited as a group with only CP (the CP group), and 20 volunteers without any systemic or periodontal disease were recruited as a healthy control group (the H group). The periodontal and joint conditions of the subjects in the four groups were recorded; anti-cyclic citrullinated protein antibodies, interleukin 6 (IL-6), C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), and rheumatoid factor levels, which reflect the severity of the RA, were detected, and the differences between the groups were analyzed. The probing depth (PD), clinical attachment loss, and sulcus bleeding index (SBI), which reflect the severity of the periodontitis, were correlated with the factor levels. The RA + CP and CP groups received therapeutic intervention, and the differences in each indicator before and six weeks after the treatment were compared, and their data were compared with those of patients in the RA group and H groups.
Results: Compared with the RA group, the serum expressions of ESR, CRP, and IL-6 were significantly higher in the RA + CP group. There were significant differences in the levels of PD, SBI, IL-6, and CRP in the patients receiving basic periodontal disease therapy before and after the treatment.
Conclusion: A relatively large proportion of patients with RA have chronic periodontitis, and the local inflammatory state of CP might exacerbate the systemic inflammatory response in RA. Basic periodontal disease therapy may improve the oral condition of patients with RA and reduce the serum levels of the inflammatory factors.
Keywords: chronic periodontitis, rheumatoid arthritis, citrullination, C-reactive protein, basic periodontal disease therapy, interleukin 6
Introduction
Periodontitis is the most common destructive inflammatory disease of the oral cavity involving the periodontal support tissues,1 while rheumatoid arthritis (RA) is a chronic systemic autoimmune disease closely correlated with environmental, viral, genetic, sex hormone, and neuropsychiatric factors.1 These diseases share many similar pathogenetic features and mechanisms, and research has found that the two are closely correlated in terms of disease level and severity.1 Concerning the clinical presentations, periodontitis and RA also have certain similarities, as both are chronic inflammatory diseases, and 70–80% of patients with RA also suffer from moderate to severe (chronic) periodontitis (CP).2
In a study concerning the pathogenesis of these two diseases, it was found that protein citrullination occurs in both.3 Anti-cyclic citrullinated peptide antibodies (anti-CCP) are highly specific to RA and are also expressed in the serum of patients with CP. C-reactive protein (CRP) is a sensitive and reliable indicator of the body’s inflammatory state and an important marker of the inflammatory process.4 Interleukin 6 (IL-6) is a multi-effect cytokine involved in tissue destruction due to bone resorption.5 All three indicators can be detected in the peripheral blood in RA and CP patients.2–5 The prevalence and rate of disease progression of the teeth differ in various parts of the mouth. The unique anatomical specificity of the molars, anterior teeth, and adjacent surfaces make them more susceptible to CP of a severe nature.6–10 RA is a systemic autoimmune disease characterized by chronic and destructive arthropathy, mainly manifested as symmetrical polyarthritis of the wrist, hand, and foot joints. It also involves large joints, including the hips and the knees. The incidence of RA in China is about three to five percent, but its pathogenesis is still unclear.11 Periodontitis is a destructive inflammatory disease involving the periodontal supporting tissues, with histopathological manifestations such as the formation of pockets and the resorption of alveolar bone. Periodontitis has become the leading cause of tooth loss among adults in China, and its prevalence is relatively high, with it affecting 40–60% of adults, most of whom suffer from mild to moderate periodontitis.6 In-depth research into the pathogenesis of CP has found that it is closely correlated with many systemic diseases, including immune dysfunction, diabetes mellitus, AIDS, chronic obstructive pneumonia, atherosclerosis, osteoporosis, and RA.7–10 The risk of RA in patients with periodontitis has also increased to a certain extent.12–14 Some pathogens in the mouth can induce hypercitrullination by altering neutrophil levels, and this process leads to high citrullination levels and the release of autoantigens that trigger an autoimmune response in patients with rheumatoid arthritis.15,16
The primary treatment for CP is to control gum inflammation after the thorough removal of plaque and tartar and other irritants using mechanical methods, promote the shallowing of periodontal pockets, and reduce periodontal tissue inflammation.6 It has been shown that basic periodontal disease therapy can significantly improve the local inflammatory status in patients and simultaneously reduce the levels of inflammatory factors in the peripheral blood.7 The purpose of this study was to evaluate the influence of basic periodontal treatment on the local and systemic conditions of patients with periodontitis combined with rheumatoid arthritis and the effect of such treatment on the adjuvant treatment of patients with rheumatoid arthritis by comparing the oral conditions of patients and the inflammatory factors in their peripheral blood serum before and after periodontal treatment. The primary hypothesis was that there would be significant differences in the levels of probing depth (PD), the clinical attachment loss (CAL), and the sulcus bleeding index (SBI) before and after the basic periodontal treatment, and the secondary hypothesis was that compared with the RA only group, the serum expressions of erythrocyte sedimentation rate (ESR), CRP, and IL-6 would be significantly higher in the RA + CP group. Thus, the PD, CAL, and SBI, which reflect the severity of periodontitis, were correlated with the factor levels, namely anti-CCP, ESR, rheumatoid factor (RF), CRP, and IL-6, which reflect the severity of RA.
Materials and Methods
Sample Selection
With the approval of our hospital’s Ethics Committee, patients at the outpatients’ clinic and on the wards of the rheumatology department were enrolled in this observational cross-sectional study between September 2018 and December 2019 based on the results of oral examinations.
With respect to the inclusion criteria, the revised criteria on RA classification issued in 2010 by the American College of Rheumatoid Sciences were used regarding the RA diagnosis, while the American Academy of Periodontology’s classification criteria issued in 2018 were used regarding the CP diagnosis. The study was explained to all the participants in detail before their agreeing to enrollment with signed informed consent, and they were able to withdraw from it at any time. Their ages ranged between 18–75 years old, and there was no gender restriction.
Subjects who had participated in other clinical trials within six weeks of the start of the present study were excluded as were patients with other metabolic diseases that might affect periodontal health, such as diabetes mellitus, severe osteoporosis, Crohn’s disease, nodular disease, and connective tissue diseases other than rheumatoid arthritis. The New York Heart Association classification was developed in 1964, but the standard used by Killip in 1967 is more commonly used in clinic. These criteria were also used in this study: • Level 1: no restrictions on any activity; there are no symptoms during normal activity. • Level 2: mildly restricted activities; patient is comfortable with rest or light exercise. • Level 3: there are noticeable limitations in any activity; the patient is comfortable only at rest. • Level 4: uncomfortable with any activity and symptomatic even when at rest. Patients with grade 3 and 4 cardiac function were excluded from the study. A reference has been added to the paper.
The following is the sample size of RA patients with chronic periodontitis calculated using PASS 11. The proportion of RA patients with periodontitis can reach more than 90%, so the sample size of RA group is relatively small due to the influence of morbidity. This means there is a very small percentage of rheumatoid arthritis patients who do not have periodontitis, in other words, a very small group of patients (Appendix 1).
Study Design
A total of 69 patients, 40 of whom were RA patients, took part in the study. These RA patients underwent an oral examination, in which the latest 2018 diagnostic criteria for periodontitis was used, and they were divided into two groups, those that also had periodontitis (the RA + CP group) and those without periodontitis (the RA group). Twenty-nine patients with periodontitis, being treated in the periodontitis department of the hospital, were also recruited as the CP group, and 20 volunteers without a systemic disease or periodontitis were recruited as the clinically healthy group (the H group).
The following patients were excluded: patients with other genetic disorders that might affect periodontal health eg, Down syndrome, chronic granulomatous disease, hypophosphatasia, and congenital leukocyte granule anomaly syndrome; patients with other hematological disorders that might affect periodontal health, such as acute and chronic myeloid leukemia and granulocyte deficiency; patients who had undergone organ transplantation; patients with severe cardiac disorders (ie, those with class III or higher cardiac function);20 patients with hypertension; patients with significant abnormalities in hepatic and renal function (ie, indicators of more than double the normal reference range); patients with brain disease and abnormal judgment; drug and/or alcohol abusers; pregnant or lactating females.; and smokers with a consumption of ≥5 cigarettes per day.
Clinical Measurements
The general characteristics and joint conditions of the four groups were recorded at the conclusion of the periodontal examinations. Peripheral blood samples were collected so that the levels of the indicators, anti-CCP, IL-6, CRP, ESR, and RF could be detected. The differences between each group were analyzed. Systematic periodontal therapy, including supragingival scaling, subgingival scraping, and root planing, was given to the two groups of patients with CP. Six weeks after treatment, the above indicators were retested by the same rheumatologist and the same trained dentist and peripheral blood was collected for laboratory indicators assays. The differences before and after treatment were analyzed and the effect of periodontal therapy on improving the systemic condition in patients with RA was evaluated.
The general characteristics consisted of name, gender, age, body mass index (BMI), smoking status, educational background, and general systemic condition. The indicators for the periodontal examination were the periodontal PD, CAL, tooth looseness, SBI, and the number of missing teeth. The detection indicators for RA were RF and the 28 joint disease activity score incorporating the erythrocyte sedimentation rate (the DAS28-ESR Score). The morning fasting serum was collected from all subjects, and the serum levels of IL-6, anti-CCP, and CRP were measured using an enzyme-linked immunosorbent assay. Whole blood was collected in the morning from all subjects, and the levels of ESR were determined using the Weiss Method. An RF level greater than 20 IU/mL was considered positive, and an anti-CCP greater than 20 RU/mL was considered positive. The RF and anti-CCP given in the tables are the positive rates.
Statistical Analysis
The sample size was calculated using PASS 11 software. The database was established with EpiData software, and the statistical analysis was performed with SPSS 22.0 software. Quantitative data were statistically analyzed in the form of mean ± standard deviation (X ± S). Analysis of variance was used to compare the age, BMI, PD, IL-6, CRP, and ESR of the four groups. The t-test was used to compare the scores of CAL and DAS-28 between the two RA groups. Qualitative data were statistically described in the form of frequency and rate. A chi-square test was used to compare gender, SBI, RF, CI-S (Plaque index), and anti-CCP between the groups. Correlation analysis was used to analyze the correlations between PD, CAL, SBI, and CI-S and age, BMI, IL-6, anti-CCP, RF, CRP, ESR and DAS28 scores in the RA + CP group. A value of p < 0.05 was considered to be significant.
Results
The General Characteristics of the Four Groups of Patients
There was a statistically significant difference in age between the RA + CP group and the CP and H groups. There existed a statistically significant difference in age between the CP group and the RA and H groups. There existed a statistically significant difference in age between the RA group and the H. There was no statistically significant difference in BMI between the four groups. There was no statistically significant difference between the four groups in the gender composition ratio.
As can be seen in Table 1 the patients with RA were older than those in the other groups, and the difference was statistically significant. The result was consistent with research that has suggested that the prevalence of RA and CP may increase with advancing age. This might be correlated with the fact that both RA and CP are chronic diseases, and therefore their severity will increase with age. However, in the analysis of BMI, no significant differences were found among the four groups, which was inconsistent with the findings of Hotamisligil et al.24,25 This might be correlated with the small sample size, the concentration of the enrolled population in a single region, and the fact that those enrolled were all patients of the same hospital.
 |
Table 1 The General Characteristics of the Four Groups of Patients (mean±SD) |
The Initial Indicators of the Four Groups of Patients
A) There existed a statistically significant difference between the RA + CP group and the CP group. B) There existed a statistically significant difference between the RA + CP group and the RA group. C) There existed a statistically significant difference between the RA + CP group and the H group. D) There existed a statistically significant difference between the CP group and the RA group. F) There existed a statistically significant difference between the CP group and the H group. (See Table 2).
 |
Table 2 The Initial Indicators of the Four Groups of Patients (mean±SD) |
The Results of the First Re-Examination
The age difference was statistically significant between the two groups. The difference in gender composition ratio was not statistically significant between the two groups. The difference in body mass index was not statistically significant between the two groups. (See Table 3).
 |
Table 3 The Results of the First Re-Examination (mean±SD) |
The Comparison Between the RA + CP and CP Groups of the Indicators After the First Re-Examination
The RA + CP and CP groups were re-examined six weeks after having the basic periodontal treatment, and, as shown in Table 4, there were differences in the PD, CAL, RF, IL-6, CRP, ESR, anti-CCP (p < 0.05). Compared with the results shown in Table 2, CRP was significantly decreased in both groups, but it was more obviously so in the CP group. This suggested that the oral hygiene of the patients was improved after treatment, and, thus, the level of inflammatory factors in the blood was reduced.
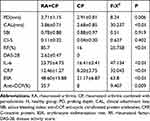 |
Table 4 The Comparison of the Indicators After the First Re-Examination Between the RA+CP and CP Groups (mean±SD) |
The Correlation Between the Indicators for Periodontal Examinations and Serum Inflammatory and Clinical Indicators for RA in the RA + CP Group
In all the RA + CP patients, it was found that the changes in PD were correlated with CRP, RF, IL-6, and anti-CCP, while the changes in CAL were correlated with BMI, CRP, ESR. and anti-CCP. The changes in SBI were correlated with IL-6, CRP, ESR, and anti-CCP, and the changes in CI-S were correlated with BMI and CRP (p < 0.05). (See Table 5).
 |
Table 5 The Correlation Between the Indicators for Periodontal Examinations and Serum Inflammatory and Clinical Indicators for RA |
When compared with the laboratory indicators shown in Table 2, with regard to the results of IL-6, CRP, ESR, and anti-CCP, there were significant differences between the RA + CP group and the CP, RA, and H groups. Laboratory parameters, such as CRP, ESR, IL-6, and anti-CCP, can alter significantly during the active phase of RA. Both ESR and CRP are systemic inflammatory markers, and CRP, ESR, and RF are common indicators for observing the therapeutic effects in the treatment and prognosis of RA. The expression levels of all three indicators correlate with the disease activity of RA. Anti-CCP is a sensitive and specific indicator for RA, manifested when the disease is in its early stages. In the present study, the expressions of ESR, CRP, and IL-6 in patients in the RA+CP group were significantly higher than those in the RA group, which indicates that the local inflammatory state of CP might exacerbate the systemic inflammatory response in RA.
The data in Table 5 shows that there was a certain correlation between the periodontal clinical examination indicators, consisting of PD, CAL, and SBI, and the laboratory indicators for RA, suggesting that the number of patients presenting significant periodontal destruction with RA was relatively high. In particular, CAL, an indicator for the severity of the periodontal disease, correlated with the concentration of anti-CCP, an indicator for the severity of RA, suggesting a possible correlation between the CP and RA in terms of pathogenesis.
The Comparison of the Indicators Before and After Treatment in the RA+CP and CP Groups
As can be seen in Table 6, the indexes of PD, SBI, CI-S, IL-6, and CRP before and after periodontal basic treatment were significantly improved in the RA + CP and CP groups (p < 0.05).
 |
Table 6 The Comparison of the Indicators Before and After Treatment in the RA+CP and CP Groups (mean±SD) |
Discussion
In this study, the index teeth selected from the community periodontal index examination were selected as the examination tooth positions. If an index tooth was found to be missing or to have tooth extraction indications, only the other index tooth was examined. If all the exponential teeth in a segment were missing or had a tooth extraction indication, all the remaining teeth in the segment were scored as the heaviest. The buccal (labial), lingual (palatal) facial gingival crevicular or periodontal pocket of each index tooth was examined and averaged.
IL-6 is a glycoprotein with a molecular weight of 19–28 kDa with the gene locating on the short arm of chromosome 7. IL-6 can be secreted by a variety of cells, such as monocytes and lymphocytes. Bacteria, viruses, parasites, and lipopolysaccharides in the cell wall can increase the gene expression of IL-6 and other inflammatory cytokines (IL-1, IL-2, IL- 3, tumor necrosis factor [TNF-α], etc.). IL-6 is a multi-effect cytokine with a wide range of biological functions. It can induce the differentiation of B lymphocytes and secrete immunoglobulin. It can promote the proliferation of a variety of cells. Il-6 can induce hepatocytes to synthesize and release the acute phase proteins. IL-6 is involved in bone resorption. All these functions are correlated with the destruction of tissues.17,18
Citrullination is one of the hotspots of research in recent years. Citrulline is a non-coding amino acid. With peptidyl deiminase (PAD), the arginine residues of proteins can be converted to citrulline residues, a process called protein citrullination. Citrullination is therefore a form of post-translational modification of the proteins. The phenomenon of protein citrullination can be found in many diseases, such as infections, inflammation, tumors, and autoimmune diseases. Anti-CCP is directed against the target antigens that contain the citrullinated epitopes, which are highly specific to RA, and are strongly correlated with the severity of the disease. One important pathogenic bacterium in periodontitis is Porphyromonas gingivalis (P. gingivalis). P. gingivalis contains PAD, which can modify the peptide chain through citrullination and participate in the autoimmune process of RA, which is the reason for the close relationship between periodontitis and RA.19,20
CRP is a member of the penetrant family in non-specific immune responses, and it is a sensitive and reliable indicator of the body’s inflammatory state and an important marker of the inflammatory process. CRP binds specifically to the CRP receptors on the surface of T lymphocytes, altering or limiting the function of these lymphocytes.21 CRP can cause the monocytes to synthesize tissue factors and inflammatory cytokines, directly promoting the aggregation of leukocytes and apoptosis of the vascular wall cell, leading to disease progression. These biological functions have implications for the onset and development of periodontitis and RA. The synthesis of CRP is regulated by the activated monocytes, fibroblasts, and specific cytokines, such as IL-1, TNF-α, and transforming growth factor.11
ESR was first described by Weiss in 1921 and refers to the rate of erythrocyte sedimentation per time unit and the resting of isolated anticoagulated blood, with a normal value of <20 mm/h.22 The rate of descent of erythrocytes is influenced by the degree of erythrocyte cord formation and the plasma environment in which it is placed, mainly influenced by plasma proteins.23 With the occurrence of acute bacterial inflammation, tissue injury, and necrosis, resulting in an increase in macromolecular proteins such as fibrinogen and immunoglobulins and the formation of red blood cells in the shape of coins, blood sedimentation accelerates after 2–3 days. In chronic inflammatory diseases, such as RA, connective tissue disease, and malignant tumors, the ESR will accelerate during the active phase and slow down after the condition is controlled and improved, returning to normal during the inactive phase.
In the periodontal examination, the PD, CAL, and SBI can be used to describe the degree of periodontitis comprehensively.26,27 Due to the low feasibility of radiological examinations in epidemiological studies, few defined criteria have been proposed based on those indicators. Therefore, the radiological indicators were not compared in the present study. From the data shown in Table 2, it can be seen that before the periodontal therapy, there were significant differences in all the periodontal examinations and serological indicators in the RA + CP group compared with those in the CP, RA, and H groups. In the RA + CP group, due to the combined effect of the RA and CP, the levels of PD and SBI were higher than those in the CP, RA, and H groups, and there was a marked CAL. As widely demonstrated, both RA and PD are characterized by an imbalance between proinflammatory and anti-inflammatory cytokines. This increased expression of proinflammatory cytokines could stimulate signal transducer and activator of transcription 3, thereby playing a key role in the pathophysiology of RA and PD.28 Of the 40 patients with RA initially enrolled in the present study, 32 (80%) were diagnosed with CP, which was consistent with the findings that the prevalence of CP was much higher in patients with RA than in the general population.28,29
Periodontal non-surgical treatment is an essential therapeutic tool in periodontal therapy, and involves plaque control, supragingival scaling, subgingival scraping, and root planing, and improving and maintaining the health of periodontal tissues. There is now increasing evidence that periodontal non-surgical treatment affects the progression of CP and improves the levels of the systemic inflammatory cytokines in patients.30 Periodontal non-surgical treatment can improve oral hygiene and P. gingivalis counts in patients, which may transiently reduce the disease activity of RA. The data in Table 6 shows significant differences in PD, SBI, IL-6, and CRP before and after the basic periodontal disease therapy, indicating that the treatment had a substantial effect on the oral symptoms and serum indicators in patients.
RA is an autoimmune disease. Modern medicine advocates anti-inflammatory and immunotherapy, usually using non-steroidal anti-inflammatory drugs, hormones, immunosuppressants, and other drugs, but the adverse reactions of drugs are considerable, and it is easy to relapse after drug withdrawal. This study intended to observe whether basic periodontal treatment could improve the levels of systemic inflammatory indicators in patients with RA by controlling their oral environment. Although the observation period was relatively short, it was found that the levels of important systemic inflammatory indicators in patients before and after treatment could be significantly reduced. Using western medicine treatment, combined with oral local treatment, can significantly improve the treatment effect and improve patients’ oral health and systemic condition. When patients are found to have RA, a routine oral examination should be conducted at the same time, and active basic periodontal treatment should be given to those patients with CP. On the one hand, it can improve the patient’s periodontal condition, reduce the degree of inflammation, protect the integrity of chewing organs, and maintain oral hygiene. On the other hand, it also plays a positive role in controlling the level of serum inflammatory factors in patients with rheumatoid arthritis. In the future, basic periodontal treatment could become one of the basic treatment measures for the control of rheumatoid arthritis, providing a new treatment approach for the treatment of such patients.
The patients enrolled in the present study, who attended the outpatient clinic and wards of the hospital’s rheumatology department, did so with a wide variation in disease activity, but they were not further sub-grouped. Thus, the DAS28 score in this study failed to improve significantly after the treatment, which was not consistent with the findings of Cosgarea et al.31 In subsequent studies, there was an increase in the number of investigators, the duration of observation was extended, and the periodontal treatment was continued for longer, primarily to observe changes in the DAS28 score and evaluate the systemic improvement in RA.32
RA patients on medication were asked to maintain the type and dose of medications they were taking during the study period without changing or interrupting the medication protocol. However, there is a clear distinction between the degree of disease among RA patients, which can be classified as low, moderate, or severe according to the RA activity, and the different degrees may respond differently to the basic periodontal disease therapy. In the future, our study group intends to observe patients with RA in groups according to their disease severity with the expectation of more definitive results. New data concerning the association between CP and RA has largely confirmed a link between the disorders mediated through P gingivalis.
In the present study, the therapeutic effects of the patients enrolled in the RA + CP and CP groups were observed for just six weeks after the periodontal therapy, and the data obtained only reflected the short-term changes in the periodontal clinical examination indicators, serum cytokines, and anti-CCP. Thus, further observation of the levels of indicators at six months, one year, or even many years after the treatment is needed in the future to understand the long-term effects of basic periodontal disease therapy.28
Conclusion
A relatively large proportion of patients with RA appear to have CP, and it appears that the local inflammatory state of CP might exacerbate the systemic inflammatory response in RA. Basic periodontal disease therapy may improve the oral condition in patients with RA and reduce the serum levels of the inflammatory factors.
Ethics Approval
This study was conducted in accordance with the declaration of Helsinki.This study was conducted with approval from the Ethics Committee of Beijing LuHe Hospital.A written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Funding
2019 Tongzhou District Science and Technology Commission of Beijing Clinical Project: KJ2019CX012-07; 2018 Beijing Tongzhou District Health Development Research Special Project: TFZXPT-20180124.
Disclosure
All authors have contributed significantly to the manuscript and declare that the work is original and has not been submitted or published elsewhere. None of the authors have any financial disclosure or conflicts of interest.
References
1. Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005;76(11 Suppl):2066–2074. doi:10.1902/jop.2005.76.11-S.2066
2. Woodman I. Rheumatoid arthritis: PAD-ing out the ACPA response in RA. Nat Rev Rheumatol. 2013;9(7):381. doi:10.1038/nrrheum.2013.90
3. Trouw LA, Huizinga TW, Toes RE. Autoimmunity in rheumatoid arthritis: different antigens–common principles. Ann Rheum Dis. 2013;72(Suppl 2):ii132–ii136. doi:10.1136/annrheumdis-2012-202349
4. van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7(7):391–398. doi:10.1038/nrrheum.2011.76
5. Ceccarelli F, Saccucci M, Di Carlo G, et al. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediators Inflamm. 2019;2019:6034546. doi:10.1155/2019/6034546
6. Meng HX. Clinical Periodontology.
7. Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 Suppl):2075–2084. doi:10.1902/jop.2005.76.11-S.2075
8. Scannapieco FA, Genco RJ. Association of periodontal infections with atherosclerotic and pulmonary diseases. J Periodontal Res. 1999;34(7):340–345. doi:10.1111/j.1600-0765.1999.tb02263.x
9. Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(Suppl 10S):1123–1137. doi:10.1902/jop.1996.67.10s.1123
10. Kaushal S, Singh AK, Lal N, et al. Effect of periodontal therapy on disease activity in patients of rheumatoid arthritis with chronic periodontitis. J Oral Biol Craniofacial Res. 2019;9:128. doi:10.1016/j.jobcr.2019.02.002
11. Monsarrat P, Fernandez de Grado G, Constantin A, et al. The effect of periodontal treatment on patients with rheumatoid arthritis: the ESPERA randomised controlled trial. Joint Bone Spine. 2019;86(5):600–609. doi:10.1016/j.jbspin.2019.02.006
12. Mikuls TR, Payne JB, Reinhardt RA, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9(1):38–42. doi:10.1016/j.intimp.2008.09.008
13. Dissick A, Redman RS, Jones M, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81(2):223–230. doi:10.1902/jop.2009.090309
14. de Smit M, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14(5):R222. doi:10.1186/ar4061
15. Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014;16(3):408. doi:10.1007/s11926-014-0408-9
16. Miller A, Mahtani KR, Waterfield MA, Timms A, Misbah SA, Luqmani RA. Is rheumatoid factor useful in primary care? A retrospective cross-sectional study. Clin Rheumatol. 2013;32(7):1089–1093. doi:10.1007/s10067-013-2236-0
17. Äyräväinen L, Heikkinen AM, Kuuliala A, et al. Inflammatory biomarkers in saliva and serum of patients with rheumatoid arthritis with respect to periodontal status. Ann Med. 2018;50(4):333–344. doi:10.1080/07853890.2018.1468922
18. Udagawa N, Takahashi N, Katagiri T, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995;182(5):1461–1468. doi:10.1084/jem.182.5.1461
19. Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829(10):1126–1135. doi:10.1016/j.bbagrm.2013.07.003
20. Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi:10.1016/0002-9149(67)90023-9
21. Marnell L, Mold C, Du-Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–111. doi:10.1016/j.clim.2005.08.004
22. Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5(2):e9358. doi:10.1371/journal.pone.0009358
23. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi:10.1056/NEJM199902113400607
24. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–668. doi:10.1126/science.271.5249.665
25. Davison E, Johnston W, Piela K, et al. The subgingival plaque microbiome, systemic antibodies against bacteria and citrullinated proteins following periodontal therapy. Pathogens. 2021;10(2):193. doi:10.3390/pathogens10020193
26. Leroy R, Eaton KA, Savage A. Methodological issues in epidemiological studies of periodontitis –how can it be improved? BMC Oral Health. 2010;10:8. doi:10.1186/1472-6831-10-8
27. Offenbacher S. Commentary: clinical implications of periodontal disease assessments using probing depth and bleeding on probing to measure the status of the periodontal-biofilm interface. J Int Acad Periodontol. 2005;7(4):157–161.
28. Tristiu R, Vesa S, Dumitru RB, et al. Association of oral-health related quality of life and general health assessment in patients with rheumatoid arthritis. Oral Health Prev Dent. 2018;16(3):271–280.
29. Ziebolz D, Rupprecht A, Schmickler J, et al. Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: results from a cross-sectional study. J Periodontol. 2018;89(11):1310–1317. doi:10.1002/JPER.17-0616
30. Hashimoto H, Hashimoto S, Muto A, Dewake N, Shimazaki Y. Influence of plaque control on the relationship between rheumatoid arthritis and periodontal health status among Japanese rheumatoid arthritis patients. J Periodontol. 2018;89(9):1033–1042. doi:10.1002/JPER.17-0575
31. Cosgarea R, Tristiu R, Dumitru RB, et al. Effects of non-surgical periodontal therapy on periodontal laboratory and clinical data as well as on disease activity in patients with rheumatoid arthritis. Clin Oral Investig. 2019;23(1):141–151. doi:10.1007/s00784-018-2420-3
32. Kobayashi T, Yoshie H. Host responses in the link between periodontitis and rheumatoid arthritis. Curr Oral Health Rep. 2015;2(1):1–8. doi:10.1007/s40496-014-0039-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
