Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
The effects of home oxygen therapy on energy metabolism in patients with COPD
Authors Kırıcı Berber N, Yetkin Ö, Kılıç T, Berber İ, Özgel M
Received 18 January 2017
Accepted for publication 9 May 2017
Published 15 May 2018 Volume 2018:13 Pages 1577—1582
DOI https://doi.org/10.2147/COPD.S132718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Nurcan Kırıcı Berber,1 Özkan Yetkin,2 Talat Kılıç,2 İlhami Berber,3 Mehmet Özgel4
1Department of Chest Disease Clinic, Malatya Training and Research Hospital, 2Department of Pulmonary Medicine, Inonu University Faculty of Medicine, Turgut Ozal Medical Center, 3Department of Haemotology Clinic, 4Department of Thoracic Surgery Clinic, Malatya Training and Research Hospital, Malatya, Turkey
Background: COPD is preventable and treatable and is characterized by completely nonreversible airflow obstruction. In this study, we aimed to investigate the effect of long-term oxygen therapy on patients with stage 4 COPD who were followed up and treated at the polyclinic or clinic service. We evaluated the effects of oxygen therapy on energy metabolism and physical activity in patients with COPD.
Methods: Nineteen patients with COPD (16 male/3 female), treated with oxygen therapy for the first time, were included in this study. Analysis of arterial blood gases and pulmonary function test was performed. Metabolic Holter device (SenseWear® Armband) was placed pre- and post-oxygen therapy on the patients’ arm for at least 3 days. This device captures Holter data in a digitized electronic system, and the daily average value was calculated from the data.
Results: Post-oxygen treatment showed a significant increase in energy expenditure by patients with COPD (pretreatment, 1,497±596 joule; posttreatment, 2,977±5,985 joule; P=0.044). Moreover, number of steps during walking (pretreatment, 2,056±256; posttreatment, 2,120±195; P=0.03), resting (pretreatment, 6.36±3.31 hours; posttreatment, 3.47±2.19 hours; P<0.03), and sleeping (pretreatment, 4.23±2.13 hours; posttreatment, 2.33±1.42 hours; P<0.00) showed significant differences. Increased daily energy expenditure in patients with respiratory failure was detected with long-term oxygen therapy. In addition, the immobility of patients decreased and duration of physical activity increased in patients with COPD.
Conclusion: In this study, positive effects of long-term oxygen therapy have been demonstrated with respect to energy metabolism and physical activity of patients with COPD. Thus, we recommend that medication adherence and long-term oxygen therapy should begin early in patients with COPD.
Keywords: sleep time, physical activity, COPD, metabolic holter, long-term oxygen, daily activity
Introduction
In recent years, COPD has shown an increasing trend in morbidity and mortality rate compared to other diseases.1 Its incidence has increased in developing countries and is an important socioeconomic burden for these countries.1 Although smoking is considered to be the leading cause of COPD (which is characterized by completely nonreversible and often progressive airflow obstruction), only 15% of smokers develop COPD,1 suggesting that other factors contribute to the formation of the disease.
In this study, the energy metabolism of COPD patients who were planning to undergo oxygen therapy for the first time at home, was studied. Both baseline and post oxygen therapy measurements were recorded. For the study, a noninvasive metabolic Holter device was placed on the right arm of each patient for at least 3 days; this device detected changes in circulation in subcutaneous capillaries, transferred the calculated energy data and recorded it in an electronic format on the computer. After the follow-up, the data were compared between the treatment days and days without oxygen treatment. The effect of long-acting oxygen therapy on energy metabolism, and whether oxygen therapy is beneficial for patients with COPD, was investigated.
Materials and methods
In this study, we planned to investigate the effects of long-term home oxygen therapy on energy metabolism in patients with COPD. Nineteen patients who were diagnosed with stage 4 COPD and who were followed-up (either remotely or by in-patient appointments) between March 2012 and June 2012 at Department Chest Diseases of Inonu University were selected according to the inclusion and exclusion criteria.
Inclusion criteria
Patients older than 40 years and who were diagnosed with stage 4 COPD and who had not previously undergone home oxygen therapy were included in this study.
Exclusion criteria
Patients who could not use the metabolic Holter device due to any physical or mental disability were excluded from this study.
The energy metabolism of patients before and after oxygen therapy was studied. For the study, a noninvasive metabolic Holter device (SenseWear® Pro3 Armband [SWA]) was placed on the right arm of the patients who were followed-up for at least 3 days. Daily average data were recorded. SWA detected changes in circulation in the subcutaneous capillaries, transferred the calculated energy data to the device, and recorded it in an electronic format on the computer. The pre- and post-oxygen therapy data were compared.
A total of 16 males and 3 females participated in this study (Table 1).
  | Table 1 Patients’ characteristics |
Methods performed
Respiratory function tests, SWA device measurements, and oxygen device measurements.
Respiratory function tests
Respiratory function tests were performed by two technicians in Inonu University, Turgut Özal Medical Center, Respiratory Function Test Laboratory of Chest Diseases Department, by using the Vmax® 22C (Sensor Medics; Yorba Linda, CA, USA).
Simple spirometry
Each patient, while in a seated position, was asked to breathe slowly into the mouth of the spirometer while his/her nose was closed with a soft latch. They were asked to take a deep breath after three normal breaths (resting level) and were asked to discharge all air out with a challenging, deep, and rapid exhalation. At this time, the volumetric time curve was obtained by placing the volume taken by the expiration on the y axis and time on the x axis. The patients were asked to perform three acceptable forced vital capacity (FVC) maneuvers, and the best value was considered. FVC, forced expiratory volume in one second (FEV1), and FEV1/FVC values were obtained from this procedure.
Long-term oxygen therapy was planned for those patients whose PaO2 ≤55 mmHg or saturation ≤88% with hypercapnia or signs of pulmonary hypertension, peripheral edema or congestive heart failure, or polycythemia (hematocrit >55%) or if PaO2 was 55–60 mmHg or saturation <89%.
The pre- and post-oxygen therapy data were compared. The effect of long-acting oxygen therapy on metabolism and whether oxygen therapy was beneficial was investigated.
Metabolic Holter device
SWA is a multi-sensor body monitor capable of collecting lifestyle data for up to 2 weeks. SWA was placed on the triceps of each patient’s right arm. It calculated energy expenditure and metabolic physical activity of the COPD patients in their free-living environment.2,3 Energy expenditure and physical activity, sleep duration, and quality could then be evaluated objectively.3,4 Physiological variables of the body such as total energy expenditure, effective energy expenditure, resting energy expenditure, metabolic equivalency unit (kCal/kg/h), total number of steps, duration of physical activity, and sleep duration were measured. The physiological variables incorporate body signals from four sensors (skin temperature, heat flow, galvanic skin response, and biaxial accelerometers) into “theme recognition” parameters:
- Body surface temperature measurements.
- Galvanic skin response measurements (skin impedance that reflects skin water content and vascular wall construction and dilatation).
- Heat flow sensors measurements (spreading speed of heat from body). If the heat flow is too high and movement is low, it determines resistance and isometric properties (if the heat flow and the motion are both high, it is a sign dense activity; if both the heat flow and the movement are small, quiet activities will be considered).
- Axis Accelerometer measurements (movement).
These parameters have been compared and validated across thousands of experiences in which physiological data were obtained by armband and metabolic devices (gold standard). Confirmation of resting and active energy expenditure is made according to double labeled water5 and metabolic measurement systems6,7 and is highly relevant. SWA is preferred for patients with COPD and obstructive sleep apnea syndrome.
Wilcoxon test was used for test parameters before and after oxygen therapy. All results are expressed as mean ± standard deviation. A Pearson correlation analysis was performed to evaluate the correlation between the variables. A P<0.05 was considered significant.
We obtained ethical approval from Malatya Clinical Research Ethics Committee. Preliminary information was provided to the candidates regarding the study. During the study, the WHO Declaration of Helsinki rules were followed. Each patient provided written informed consent to participate in this study.
Results
Nineteen patients with COPD (male/female; 16/3) were included in this study. The mean age of the patients was 70.2±10 years (range: 40–84).
We found a statistically significant difference in the total daily energy expenditure of the patients (pre- and posttreatment: 1,497±596 and 2,977±5,985 Cal/day, respectively; P=0.044) (Figure 1).
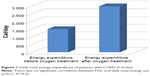  | Figure 1 Daily total energy expenditure of patients with COPD (Cal/day). |
A statistically significant difference was found when daily physical activity (daily walking distance) of patients was compared pre- and posttreatment (2,056±2,569, 2,120±1,958 steps/day, respectively; P=0.03) (Figure 2).
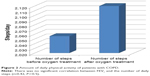  | Figure 2 Amount of daily physical activity of patients with COPD. |
A statistically significant difference was found when the total daily resting period of patients (immobilization, total lengthening time including sleep) was compared pre- and posttreatment (6.36±3.31 vs 3.47±2.19 hours/day, respectively; P<0.00) (Figure 3).
  | Figure 3 Patients’ daily rest time (hours/day). |
Furthermore, a statistically significant difference was found when the patients’ daily total sleeping hours (hours/day) were compared pre- and posttreatment (4.23±2.13 vs 2.33±1.42 hours/day, respectively; P<0.00) (Figure 4).
  | Figure 4 Patients’ daily total sleep time (hours/day). |
Discussion
COPD is one of the leading causes of mortality and morbidity.1 Lack of oxygen caused by COPD is a major cause of insufficient oxygen in all tissues and organs.1 To date, the effects of long-term oxygen therapy on mortality and physiological functions in patients with COPD have been investigated by two research groups. One research group performed the Nocturnal Oxygen Therapy Trial (NOTT) in 1970,8 and the other group (British Medical Research Council [MRC]) performed long-term at-home oxygen therapy trial.9
Oxygen is essential for energy metabolism by the central nervous system.
As cognitive functions are not fully performed under hypoxic conditions, there is a decrease in neurocognitive functions in patients with COPD.10–12 Studies have shown that patients with COPD are more depressed and anxiety-prone.13–16 The causes of depression and anxiety in COPD patients are physical disabilities, dependency on the drugs, withdrawal from physical activity compared to peers in the community, and a tendency to move away from social activities with worry of increase in symptoms. All these limitations arise due to the imbalance between oxygen delivery and consumption.
As we have seen in our study, the posttreatment energy expenditure was significantly increased compared to pretreatment (Figure 1).
In another study, the metabolic Holter device described sleep and wakefulness with moderate sensitivity and clarity.17 A further study18 of patients with COPD wearing SWA showed that there was a positive correlation between maximal voluntary ventilation (MVV), respiratory muscle strength, and physical activity. Among the parameters of the body mass index, obstruction, dyspnea and exercise capacity (BODE) index inspiratory capacity (IC), MVV and FEV1 are variables of spirometric testing reflecting lung function. MVV was found to better predict muscle power and functional energy capacity in COPD than FEV1, body mass index, IC, and the 6 min walk test.18 The correlation between MVV and daily total energy expenditure, the energy spent per day in moderate and vigorous activities, and the numbers of steps per hour were statistically significant. But the correlation between IC and FEV1 and these variables was not statistically significant.18 In another study with SWA, heart rate variability (HRV) assessing cardiac autonomic dysfunction in COPD was not correlated with FEV1 and BODE index. HRV was correlated with physical activity level and body composition.19 Patients with COPD have a higher heart rate during physical activity and a lower heart rate at rest in daily life. In a study using SWA, there was a correlation between total energy expenditure and HRV.20 SWA was compared with indirect calorimetry in young adults and provided accurate estimates of energy expenditure.6 For walking and jogging in heart patients, SWA generally showed the best estimates (Actigraph, Tritrac R3D).21 SWA truly predicted resting energy expenditure in healthy individuals.2,7,22 Resting energy expenditure has been reported in obese patients, in healthy persons during exercise, in patients with COPD, and heart failure and diabetic patients.6,22,23 In a study with SWA, the number of steps in the walking band compared with manual counting, the number of steps calculated by SWA was 3%–4% less than the manual count.24
When the differences in energy expenditure as measured by metabolic Holter device were evaluated, oxygen therapy was the only treatment change and there were no changes made to the pharmacological treatments. This suggests that oxygen therapy resulted in an increased energy metabolism and increased the physical activity of the patients. The patients’ dependence on sleep decreased; therefore, the patient will adapt more easily to social activities. Consequently, clinical signs of depression and anxiety will be less apparent, and the socioeconomic burden due to oxygen insufficiency will decrease. One of the energy-consuming systems in the body is the musculoskeletal system. There is a need for oxygen to convert stored muscle glycogen to energy. Enough energy cannot be produced in patients with COPD because oxygen delivery is inadequate. Patients’ physical activity is, therefore, restricted. It manifests with symptoms such as shortness of breath and muscle cramps. Patients with COPD who use long-term oxygen have increased energy expenditure. Therefore, energy production is considered to be increased.
Cardiac diseases are one of the major causes of mortality in patients with COPD.1 These include ischemia, hypoxemia, secondary arrhythmias, and ischemic cardiomyopathies.1 This suggests that energy is actively required for the optimum functioning of the heart muscle. Therefore, we hypothesized that if oxygen is given to the patient for a long time, then the risk of cardiac mortality and morbidity can be reduced.1
Another finding in our study was a significant increase in the number of daily steps of patients after oxygen therapy. This finding is an indication of the positive contribution of oxygen to energy metabolism. By giving oxygen to the patient, energy production and consumption increases. Consequently, the physical activity of patients increased significantly after oxygen therapy. This increasing number of steps is another finding that shows that energy expenditure after oxygen therapy increases. Oxygen therapy will prevent immobilization and its possible morbidities (pulmonary thromboembolism, constipation, osteoporosis, muscle atrophy, and depression).
In addition, we have shown that patients have significantly reduced resting time outside of sleep. The difference between pre- and post-oxygen therapy periods was significant and the mean pretreatment 6.36 hours of inactivity with oxygen therapy fell to 3.47 hours after oxygen therapy. These results support our hypothesis that oxygen therapy helps to increase physical activity and energy expenditure.
Causes of mortality such as pulmonary thromboembolism, constipation, osteoporosis, muscle atrophy, and depression, which may occur when patients are less active, will be prevented. Another result of our study is that patients’ sleeping times were significantly reduced by oxygen therapy. We believe oxygen therapy decreased resting time and increased sleep quality.
Studies have shown that hypoxic COPD patients have a lower quality of sleep and, thus, need to sleep for a longer time.10,12 Once oxygen levels are increased, it appears that a higher quality of sleep is achieved and, as a result, these COPD patients do not require a prolonged sleep time.
In summary, the findings of our study showed that physical activity and energy expenditure of patients increased significantly, and the periods of inactivity only decreased with the addition of long-term oxygen therapy without any change in pharmacology.
We conclude that oxygen therapy can prevent clinical pathologies such as ischemia, arrhythmia, organ failure, and neurocognitive disorders that come from all-body oxygen deficiency. This suggests that oxygen therapy positively affects energy metabolism in patients with COPD who had respiratory insufficiency when oxygen therapy was initiated.
Because of the increased energy metabolism with oxygen therapy, we observed that the physical activity of the patient increased; the sleep dependency of the patients decreased; the compliance of these patients with social activities was increased; and depression and anxiety were observed to be decreased. Another finding in our study was a marked increase in the number of patients’ steps after oxygen therapy. This is an indication of the positive contribution of oxygen to energy metabolism. Oxygen therapy will result in more mobility in patients with COPD, thereby avoiding the various complications caused by immobilization, and thereby reducing the various causes of morbidity.
Another finding in our study was that patients with COPD experienced significantly fewer periods of rest outside sleep. Consequently, physical activity of patients with COPD significantly increased with prolonged oxygen therapy and the periods of inactivity decreased. In addition, energy production was statistically significant.
Conclusion
Oxygen therapy is essential for hypoxic COPD patients who suffer from lower quality of sleep, prolonged resting time, lower physical activity, and poor quality of life. Oxygen therapy not only improves quality of life but also decreases the complications of COPD. Therefore, all hypoxic COPD patients should be encouraged to adhere to oxygen therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Shapiro SD, Snider GL, Rennard SI. Chronic bronchitis and emphysema. In: Mason RJ, Broaddus VC, Murray JF, Nadel JA, editor. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia: Elsevier-Sunders; 2005:1115–1167. | ||
Malavolti M, Pietrobelli A, Dugoni M, Poli M, De Cristofaro P, Battistini NC. A new device for measuring daily total energy expenditure (TEE) in free living individuals. Int J Body Compos Res. 2005;3:63. | ||
Patel SA, Slivka WA, Sciurba FC. Validation of a wearable body monitoring device in COPD. Am J Respir Crit Care Med. 2004;30:771. | ||
Mignault D, St-Onge M, Karelis AD, Allison DB, Rabasa-Lhoret R. Evaluation of the Portable Health Wear Armband, a device to measure total daily energy expenditure in free living type 2 diabetic individuals. Diabetes Care. 2005;28(1):225–227. | ||
Wiedemann HP, Stoller JK. Lung disease due to alpha 1 – antitrypsin deficiency. Curr Opin Pulm Med. 1996;2(2):155–160. | ||
Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5):897–904. | ||
Malavolti M, Pietrobelli A, Dugoni M, et al. A new device for measuring resting energy expenditure (REE) in healthy subjects. Nutr Metab Cardiovasc Dis. 2007;17(5):338–343. | ||
Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease a clinical trial. Ann Intern Med. 1980;93(3):391–398. | ||
Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic corpulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–686. | ||
Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. | ||
Rafii R, Albertson TE, Louie S, Chan AL. Update on pharmaceutical and minimally invasive management strategies for Chronic Obstructive Pulmonary Disease. Pulm Med. 2011;2011:1–11. | ||
Krop HD, Block AJ, Cohen E. Neuropsychologic effects of continuous oxygen therapy in chronic obstructive pulmonary disease. Chest. 1973;64(3):317–322. | ||
Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65–70. | ||
Heaton RK, Grant I, McSweeny AJ, Adams KM, Petty TL. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1983;143(10):1941–1947. | ||
Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig. 2015;53(6):249–258. | ||
Geenberg GD, Ryan JJ, Bourlier PF. Psychological and neuropsychological aspects of COPD. Psychosomatics. 1985;26(1):29–33. | ||
Germain A, Buysse DJ, Nofzinger E. Sleep-specific Mechanisms Underlying Posttraumatic Stress Disorder: Integrative Review and Neurobiological Hypotheses. Sleep Med Rev. 2008;12(3):185–195. | ||
Pitta F, Takaki MY, de Oliveira NH, et al. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir Med. 2008;102(8):1203–1207. | ||
Camillo CA, Pitta F, Possani HV, et al. Heart rate variability and disease characteristics in patients with COPD. Lung. 2008;186(6):393–401. | ||
Melo RC, Santos MD, Silva E, et al. Effects of age and physical activity on the autonomic control of heart rate in healthy men. Braz J Med Biol Res. 2005;38(9):1331–1338. | ||
Cole PJ, LeMura LM, Klinger TA, Strohecker K, McConnell TR. Measuring energy expenditure in cardiac patients using the Body Media Armband versus indirect calorimetry. A validation study. J Sports Med Phys Fitness. 2004;44(3):262–271. | ||
Papazoglou D, Augello G, Tagliaferri M, et al. Evaluation of a multisensor armband in estimating energy expenditure in obese individuals. Obesity (Silver Spring). 2006;14(12):2217–2223. | ||
Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients. COPD. 2007;4(2):107–112. | ||
Troosters T, Langer D, Vrijsen B, et al. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur Respir J. 2009;33(1):99–106. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
