Back to Journals » Drug Design, Development and Therapy » Volume 14
The Effects of Dexmedetomidine and Ketamine on Oxidative Injuries and Histological Changes Following Blunt Chest Trauma
Authors Kartal S , Kip G, Küçük A , Aşçı SS , Erdem Ö, Arslan M , Kavutçu M
Received 28 April 2020
Accepted for publication 25 June 2020
Published 23 July 2020 Volume 2020:14 Pages 2937—2943
DOI https://doi.org/10.2147/DDDT.S258921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Seyfi Kartal,1 Gülay Kip,2 Ayşegül Küçük,3 Seyhan Sümeyra Aşçı,1 Özlem Erdem,4 Mustafa Arslan,2 Mustafa Kavutçu5
1Health Science University, Kanuni Training and Research Hospital, Department of Anaesthesiology and Reanimation, Trabzon, Turkey; 2Gazi University, School of Medicine, Department of Anaesthesiology and Reanimation, Ankara, Turkey; 3Kütahya Health Science University, School of Medicine, Department of Physiology, Kütahya, Turkey; 4Gazi University, School of Medicine, Department of Medical Pathology, Ankara, Turkey; 5Gazi University, School of Medicine, Department of Medical Biochemistry, Ankara, Turkey
Correspondence: Mustafa Arslan
Gazi University, School of Medicine, Department of Anaesthesiology and Reanimation, Ankara 06510, Turkey
Tel +90 312 202 53 13
Fax +90 312 202 4166
Email [email protected]
Background: The objective of this research was to evaluate the oxidative and histopathological effects of dexmedetomidine and ketamine on the pulmonary contusion model resulting from blunt chest trauma.
Methods: Rats were randomly assigned to 5 equal groups (n=6): control group (Group C), pulmonary contusion group (Group PC), PC-dexmedetomidine group (Group PC-D), PC-ketamine group (Group PC-K), and PC-dexmedetomidine + ketamine (Group PC-D+K). The PC was performed by dropping a weight of 500 g (2.45 Joules) from a height of 50 cm. In Group PC-D, after chest trauma, dexmedetomidine (100 μg/kg) was administered intraperitoneally. In Group PC-K, after chest trauma, ketamine (100 mg/kg) was administered intraperitoneally. In Group PC-D+K, dexmedetomidine and ketamine were administered together. At the end of the 6th hour, rats were sacrificed. Malondialdehyde (MDA) level, superoxide dismutase (SOD) enzyme activities, neutrophil infiltration/aggregation, and thickness of the alveolar wall were evaluated.
Results: MDA levels were significantly higher in Group PC than Groups C, PC-D, and PC-D+K. SOD enzyme activity was significantly higher in Group PC than Groups C, PC-D, and PC-D+K. In addition, neutrophil infiltration/aggregation and total pulmonary injury scores were significantly higher in Group PC than in other groups, and the thickness of the alveolar wall was significantly higher in Group PC compared to Groups C, PC-D, and PC-D+K. MDA level, SOD enzyme activities, neutrophil infiltration/aggregation, and thickness of alveolar wall were similar in PC-D and PC-D+K groups.
Conclusion: Dexmedetomidine and dexmedetomidine+ketamine have protective effects on blunt chest trauma but no protective effect was observed when ketamine was administered alone. We concluded that the administration of dexmedetomidine and ketamine after contusion is beneficial against pulmonary injury in rats.
Keywords: dexmedetomidine, ketamine, pulmonary contusion, oxidative injury, neutrophil infiltration/aggregation
Introduction
Blunt chest trauma (BCT) is a condition that may occur in various work and motor vehicle accidents in daily life.1 It accounts for as much as 20% of adult cases admitted to the emergency department.2 Pulmonary contusion (PC), which occurs after BCT, may cause hypoxemia, lactic acidosis, pulmonary and systemic inflammatory response, and acute lung injury (ALI), since lung tissue is highly sensitive to trauma. Studies have shown that proinflammatory cytokine increase after BCT can cause apoptosis.3,4 Morphologically, pulmonary edema, neutrophil infiltration/aggregation, accumulation of inflammatory cells, disruption of barrier function in the alveolar epithelium and capillary endothelium are observed in ALI.5 ALI is an important factor that increases the mortality and morbidity risk of the patient.6
There are many pathophysiological changes in BCT-induced PC, but the actual mechanism has not been fully elucidated. Therefore, there is no standard pharmacological approach in the treatment.2 The search for effective treatment is still ongoing in addition to the various substances with positive effects.2,7
Dexmedetomidine is an analgesic, sedative, and selective α-2 adrenergic receptor agonist. It is widely used as a sedative and analgesic in postoperative and especially intensive care units due to its ability to provide short-acting sedation without causing respiratory depression.1 In studies, dexmedetomidine has been shown to suppress stress response without disturbing hemodynamic stability in surgery, to suppress the sepsis-induced inflammatory response, and to increase survival rate and have anti-apoptotic and anti-inflammatory effects.5 Again, it has been found to have a protective effect against ischemia-reperfusion (I/R) injury in the lung, liver, heart, and kidneys.8 Dexmedetomidine is known to prevent pulmonary injury in hemorrhagic shock, ventilator-associated pneumonia, and pneumoperitoneum.9 It has been shown that dexmedetomidine decreases hemorrhage and edema formation in BCT-induced PC and suppresses pro-inflammatory cytokine response and reduces the amount of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β).1,8
Ketamine is an N-methyl-d-aspartate receptor (NMDAR) antagonist. Besides anesthetic, analgesic, and sedative effects, it has a protective effect on hemodynamic stability. Due to these effects, it is often preferred in emergency and trauma patients.10 Some undesirable dose-dependent effects of ketamine have also been reported, such as agitation, increased intracranial pressure, and hallucinations.11 To avoid these side effects, it is recommended to use ketamine in combination with other low-dose anesthetic drugs, such as dexmedetomidine.10,12
Ketamine and dexmedetomidine have a strong anti-inflammatory effect.10 In the cardiac I/R model, dexmedetomidine and ketamine have been shown to have similarly protective effects by reducing MDA levels and SOD enzyme activities.13 In another study, it has been reported that clinical doses of dexmedetomidine and subanaesthetic doses of ketamine alone did not have a significant effect in avoiding ALI in rats with hemorrhagic shock. However, the combination of ketamine and dexmedetomidine has been reported to improve ALI in these rats.11
Following these studies we aim to evaluate the oxidative and histopathological effects of dexmedetomidine and ketamine on the BCT-induced PC model.
Materials and Methods
Animals and Experimental Protocol
This study was carried out in Gazi University Animal Experiments Laboratory after obtaining the approval of the Gazi University Local Ethics Committee for Animal Experiments (2017/36). The animal studies were carried out in line with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1986). Thirty 10–12-week-old male Wistar albino rats weighing 250–300 g were used. The animals were allowed to reach water and food freely up to 2 hours before the procedure with a 12-hour dark-light cycle in standard temperatures (22–24°C) in metal cages.
Rats were randomly assigned to 5 equal groups (n=6): control group (Group C), pulmonary contusion group (Group PC), PC-dexmedetomidine group (Group PC-D), PC-ketamine group (Group PC-K), PC-dexmedetomidine + ketamine (Group PC-D+K).
All procedures were performed using the specific platform model described previously for PC14 in supine position. No drugs or PCs were applied to Group C. After waiting for 6 hours, rats were sacrificed. The PC was performed by dropping a weight of 500 g (2.45 Joules) from a height of 50 cm.
In Group PC-D, dexmedetomidine (Precedex ®, Abbott Laboratories Ltd, North Chicago USA) was administered intraperitoneally (i.p) at a dose of 100 µg/kg after chest trauma. After chest trauma in Group PC-K, ketamine (Ketalar®, Eczacıbaşı Parke-Davis, Istanbul, Turkey) was administered at a dose of 100 mg/kg (i.p). In Group PC-D+K, both 100 µg/kg dexmedetomidine and 100 mg/kg ketamine were administered after chest trauma. Post-PC sedation was applied to all groups, and half of the first dose of dexmedetomidine/ketamine was re-administered, while the rats were immobilized for 6 hours. At the 6th hour of the PC, under anesthesia, the thorax was opened, lung tissues were excised and removed, and then rats were sacrificed. Oxidative and histopathological parameters were evaluated.
Oxidative Parameters
The lung tissue was first washed with cold deionized water to discard blood contamination and then homogenized in a homogenizer. Measurements of cell contents require an initial preparation of the tissues. The preparation procedure may involve grinding of the tissue in a ground glass tissue blender using a rotor driven by a simple electric motor. The homogenizer, a tissue blender similar to the typical kitchen blender, is used to emulsify and pulverize the tissue (Heidolph Instruments GMBH & CO KGDiax 900 Germany) at 1000 U for about 3 min. After centrifugation at 10,000 g for about 60 min, the upper clear layer was taken.
SOD activity was measured by Durak et al’s method.15 The SOD activity method is based on the measurement of absorbance increase at 560 nm due to the reduction of NBT to NBTH2. One unit of SOD activity was defined as the enzyme protein amount causing 50% inhibition in the NBTH2 reduction rate and results were expressed in U/mg protein.
For measurement of MDA levels, the thiobarbituric acid (TBA) reactive substance assay was performed by Van Ye et al’s method.16 The reaction with TBA at 90–100°C was used to determine the MDA level, as MDA or similar substances react with TBA and produce a pink pigment that has an absorption maximum of 532 nm. To ensure protein precipitation, the sample at room temperature is mixed with cold 20% (wt/vol) trichloroacetic acid and the precipitate is then centrifuged for 10 min at 3000 rpm and room temperature to form a pellet. An aliquot of the supernatant is then placed into an equal volume of 0.6% (wt/vol) TBA in a boiling water bath for 30 min. Following cooling, sample and blank absorbance were read at 532 nm and the results expressed as nmol/mg protein, based on a graph where 1,1,3,3-tetramethoxypropane has been used as the MDA standard. Sample protein amount was determined by the Lowry O method, and BSA was used as the standard protein.17
Histopathological Parameters
Histopathological analysis in lung tissue sections was performed blindly by a pathologist in twenty-five different areas randomly determined without overlap in each preparation. Histological tissue damage was evaluated by light microscopy by hemotoxylin-eosin staining. The severity of lung injury was evaluated with a 4-point scale (0: no damage, 1: mild injury, 2: moderate injury, 3: severe injury).
Statistical Analysis
All data obtained from the semi-quantitative analysis were calculated using the SPSS 22.0 (IBM, Armonk, NJ, USA) statistics program. The Kolmogorov–Smirnov test was used to compare the distribution of all variable groups. In the evaluation of more than two independent groups with normal distribution, analyses were performed by using the Kruskal–Wallis test. To determine which group differs from the others, we used the Bonferroni-adjusted Mann–Whitney U-test after the Kruskal–Wallis test. Results are expressed as mean±standard error (SE). Statistical significance was set at p<0.05.
Results
Biochemical Analysis
MDA levels were significantly higher in Group PC than in Groups C, PC-D and PC-D+K (p<0.0001, p=0.029, p=0.009, respectively). SOD enzyme activity was significantly higher in Group PC than in groups C, PC-D, and PC-D+K (p<0.0001, p=0.003, p=0.002, respectively) (Table 1). MDA level, SOD enzyme activities were similar in PC-D and PC-D+K groups (p=0.273, p=0.230, respectively).
 |
Table 1 Oxidant Status Parameters of Rat Lung Tissue [Mean ± SE] |
Histopathological Analysis
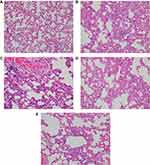
Neutrophil infiltration/aggregation and total lung injury scores were significantly higher in the PC group compared to all groups. In addition, the thickness of the alveolar wall was significantly higher in Group PC than in Groups C, PC-D, and PC-D+K (p<0.0001, p=0.031, p=0.020, respectively) (Table 2, Figure 1A–E). Neutrophil infiltration/aggregation and thickness of the alveolar wall were similar in PC-D and PC-D+K groups (p=0.588, p=0.529, respectively).
 |
Table 2 Histopathological Findings of Rat Lung Tissue [Mean ± SE] |
Discussion
In this study, it was found that PC occurred due to BCT, and that the combined administration of dexmedetomidine and dexmedetomidine + ketamine has protective effects against PC. With PC, an inflammatory response accompanied by progressive local and systemic changes begins.18–20 Macrophages and neutrophils, which are inflammatory mediators, are activated after BCT. After activation, reactive oxygen radicals and proteolytic enzymes are released, causing disruption of the oxidant-antioxidant defense system by alveolar edema and lipid peroxidation.21 Since these enzymes (such as MDA, SOD, and catalase) are a good indicator of lipid peroxidation and formation of oxygen-free radicals, they are often used in the laboratory evaluation of experimental studies.22,23 Türüt et al showed that the inflammatory process begins due to impaired oxidant-antioxidant balance in BCT-induced PC.2 Therefore, we planned our study in a similar way.
In this study, we demonstrated that dexmedetomidine was protective against PC in BCT. Previous studies have shown plasma TNF- α, IL-1β and NF-KB activation in BCT induced PC, and that these cytokines with dexmedetomidine inhibit lung inflammation and injury by suppressing activation.1 In another study,24 dexmedetomidine was shown to inhibit the formation of proinflammatory cytokines (TNF- α, IL-6) 1 hour after ischemia, in a 2-hour reperfusion period. On the other hand, it was shown that the inflammatory response was suppressed (MDA, catalase, TNF-α) by dexmedetomidine after renal,5 myocardial,9 and intestinal I/R.25
In addition to neuroprotective, anti-inflammatory, and organoprotective effects of dexmedetomidine in clinical use,5 it has a protective effect against the formation of ventilator-associated lung injury,26 hyperoxia-induced lung injury,8 endotoxin-induced ALI,27 lung injury after pneumoperitoneum in ventilated rats,28 and traumatic brain injury-induced ALI.29
In BCT, dexmedetomidine has been shown to reduce the degree of lung injury, maintain its normal structure, inhibit the formation of pulmonary edema, and prevent active infiltration of PMNL into the lung tissue, and prevent apoptosis in type 2 epithelial cells.1 In addition, significant lung injury has been observed in the I/R model,24 lower extremity I/R model,30 and renal I/R model,5 after I/R.
Intraalveolar and perivascular edema and thickness of the alveolar wall formed after PMNL deposition are considered to be good markers of inflammation. In the myocardial I/R model,9 dexmedetomidine has been shown to inhibit the thickening of the lung alveolar wall. In this study, dexmedetomidine was shown to have significant lung-protective effects, and this result supports the literature.
While ketamine has been shown to have an antiinflammatory protective effect against liver damage,31 lipopolysaccharide-induced gastric injury,32 and cardiac damage in a cardiac I/R model13 in rats with sepsis-induced endotoxemia, in some other studies11 it has been reported to have no effect alone, especially in subanesthetic doses. In our study, when ketamine was used alone in BCT-induced PC, although it had an anti-inflammatory effect histopathologically, this effect was not biochemically significant.
The anti-inflammatory effect of dexmedetomidine and ketamine is reported to be dose-dependent and not effective at low doses.33 In contrast, there are studies reporting that the dose-dependent effect variability of dexmedetomidine and ketamine is minimal.11 On the other hand, the combination of dexmedetomidine and ketamine has been reported to have a protective effect against ventilator-associated lung damage in septic rats,10 and to have a protective effect against lung injury in rats with hemorrhagic shock.11 Dexmedetomidine and ketamine (in clinical doses) have been shown to reduce pulmonary injury in BCT-induced PC. Our results support these studies.
There are some limitations to our study. The first of them is that the hemodynamic parameters (such as invasive pressure monitoring, pre-and post-PC blood gas analysis) of rats in PC after BCT were not evaluated in detail. However, since histopathological changes (neutrophil infiltration/aggregation) in BCT were deemed sufficient for PC in previous similar trauma model studies,1 detailed monitoring and blood gas analysis were not used in our study because of the increased risk of mortality. Another limiting factor was that dexmedetomidine and ketamine were administered as an effective dose intraperitoneally at once. Here, the efficacy of the drugs may vary according to their pharmacokinetic and pharmacodynamic properties.
Conclusion
We found that 100 µg/kg dexmedetomidine or dexmedetomidine+ketamine combination administered intraperitoneally immediately after contusion had a protective effect on BCT-induced PC. No protective effect was observed when ketamine was administered alone. We believe that dexmedetomidine or dexmedetomidine+ketamine combination administered after contusion is beneficial against pulmonary injury in rats.
In light of this research demonstrating the protective effect of dexmedetomidine/ketamine in BCT-induced PC in rats, detailed clinical research is needed for its effects in clinical practice.
Acknowledgment
This research was presented as an oral presentation (S-086) at the 52nd National Congress of the Turkish Anaesthesiology and Reanimation Association (TARK 2018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu X, Song X, Li N, Zhan L, Meng Q, Xia Z. Protective effects of dexmedetomidine on blunt chest trauma-induced pulmonary contusion in rats. J Trauma Acute Care Surg. 2013;74:524–530. doi:10.1097/TA.0b013e31827d5de3
2. Türüt H, Ciralik H, Kilinc M, Ozbag D, Imrek SS. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant-antioxidant status after lung contusion in rats. Injury. 2009;40:521–527. doi:10.1016/j.injury.2008.05.001
3. Seitz DH, Perl M, Mangold S, et al. Pulmonary contusion induces alveolar type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock. 2008;30(5):537–544. doi:10.1097/SHK.0b013e31816a394b
4. Hoth JJ, Martin RS, Yoza BK, Wells JD, Meredith JW, McCall CE. Pulmonary contusion primes systemic innate immunity responses. J Trauma. 2009;67(1):14–21. doi:10.1097/TA.0b013e31819ea600
5. Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55(10):1272–1278. doi:10.1111/j.1399-6576.2011.02526.x
6. Cohn SM, Dubose JJ. Pulmonary contusion: an update on recent advances in clinical management. World J Surg. 2010;34(8):1959–1970. doi:10.1007/s00268-010-0599-9
7. Gokce M, Saydam O, Hanci V, Can M, Bahadir B. Antioxidant vitamins C, E and coenzyme Q10 vs dexamethasone: comparisons of their effects in pulmonary contusion model. J Cardiothorac Surg. 2012;7(1):92. doi:10.1186/1749-8090-7-92
8. Zhang Q, Wu D, Yang Y, Liu T, Liu H. Effects of dexmedetomidine on the protection of hyperoxia-induced lung injury in newborn rats. Int J Clin Exp Pathol. 2015;8(6):6466–6473.
9. Kip G, Çelik A, Bilge M, et al. Dexmedetomidine protects from post-myocardial ischaemia reperfusion lung damage in diabetic rats. Libyan J Med. 2015;10(1):27828. doi:10.3402/ljm.v10.27828
10. Yang CL, Chen CH, Tsai PS, Wang TY, Huang CJ. Protective effects of dexmedetomidine-ketamine combination against ventilator-induced lung injury in endotoxemia rats. J Surg Res. 2011;167:e273–e281. doi:10.1016/j.jss.2010.02.020
11. Yang CH, Tsai PS, Wang TY, Huang CJ. Dexmedetomidine-ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation. 2009;80:1204–1210. doi:10.1016/j.resuscitation.2009.06.017
12. Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20(4):515–519. doi:10.1053/j.jvca.2005.07.018
13. Guler L, Bozkirli F, Bedirli N, et al. Comparison of the effects of dexmedetomidine vs. ketamine in cardiac ischemia/reperfusion injury in rats – preliminary study. Adv Clin Exp Med. 2014;23(5):683–689. doi:10.17219/acem/37214
14. Raghavendran K, Davidson BA, Helinski JD, et al. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesth Analg. 2005;101(5):1482–1489. doi:10.1213/01.ANE.0000180201.25746.1F
15. Durak I, Canbolat O, Kavutcu M, Ozturk HS, Yurtarslani Z. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10(1):17–20. doi:10.1002/(SICI)1098-2825(1996)10:1<17::AID-JCLA4>3.0.CO;2-I
16. Van Ye TM, Roza AM, Pieper GM, Henderson J
17. Lowry O, Rosenbraugh N, Farr L, Randall R. Protein measurement with folin phenol reagent. J Biol Chem. 1951;182:265–275.
18. Hoth JJ, Stitzel JD, Gayzik FS, et al. The pathogenesis of pulmonary contusion: an open chest model in the rat. J Trauma. 2006;61(1):32–44. doi:10.1097/01.ta.0000224141.69216.aa
19. Kelly ME, Miller PR, Greenhaw JJ, Fabian TC, Proctor KG. Novel resuscitation strategy for pulmonary contusion after severe chest trauma. J Trauma. 2003;55(1):94–105. doi:10.1097/01.TA.0000029042.37577.A6
20. Knöferl MW, Liener UC, Seitz DH, et al. Cardiopulmonary, histological, and inflammatory alterations after lung contusion in a novel mouse model of blunt chest trauma. Shock. 2003;19:519–525. doi:10.1097/01.shk.0000070739.34700.f6
21. Strohmaier W, Trupka A, Pfeiler C, et al. Bilateral lavage with diluted surfactant improves lung function after unilateral lung contusion in pigs. Crit Care Med. 2005;33(10):2286–2293. doi:10.1097/01.CCM.0000182819.11807.16
22. Kucuk A, Yaylak F, Cavunt-Bayraktar A, et al. The protective effects of dexmedetomidine on hepatic ischemia reperfusion injury. Bratisl Lek Listy. 2014;115(11):680–684. doi:10.4149/bll_2014_132
23. Baltalarli A, Ozcan V, Bir F, et al. Ascorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of rats. Ann Vasc Surg. 2006;20:49–55. doi:10.1007/s10016-005-9284-0
24. Jiang L, Li L, Shen J, Qi Z, Guo L. Effect of dexmedetomidine on lung ischemia-reperfusion injury. Mol Med Rep. 2014;9(2):419–426. doi:10.3892/mmr.2013.1867
25. Shen J, Fu G, Jiang L, Xu J, Li L, Fu G. Effect of dexmedetomidine pretreatment on lung injury following intestinal ischemia-reperfusion. Exp Ther Med. 2013;6(6):1359–1364. doi:10.3892/etm.2013.1317
26. Yang C-L, Tsai P-S, Huang C-J. Effects of dexmedetomidine on regulating pulmonary inflammation in a rat model of ventilator-induced lung injury. Acta Anaesthesiol Taiwan. 2008;46(4):151–159. doi:10.1016/S1875-4597(09)60002-3
27. Meng PZ, Liu J, Hu PS, Tong F. Protective effect of dexmedetomidine on endotoxin- induced acute lung injury in rats. Med Sci Monit. 2018;24:4869–4875. doi:10.12659/MSM.908887
28. Geze S, Cekic B, Imamoğlu M, et al. Use of dexmedetomidine to prevent pulmonary injury after pneumoperitoneum in ventilated rats. Surg Laparosc Endosc Percutan Tech. 2012;22(5):447–453. doi:10.1097/SLE.0b013e31826183df
29. Wang Y, Wang C, Zhang D, Wang H, Bo L, Deng X. Dexmedetomidine protects against traumatic brain injury-induced acute lung injury in mice. Med Sci Monit. 2018;17(24):4961–4967. doi:10.12659/MSM.908133
30. Küçükebe ÖB, Özzeybek D, Abdullayev R, Ustaoğlu A, Tekmen I, Küme T. Effect of dexmedetomidine on acute lung injury in experimental ischemia-reperfusion model. Braz J Anesthesiol. 2017;67:139–146. doi:10.1016/j.bjan.2016.09.004
31. Suliburk JW, Helmer KS, Gonzalez EA, Robinson EK, Mercer DW. Ketamine attenuates liver injury attributed to endotoxemia: role of cyclooxygenase-2. Surgery. 2005;138(2):134–140. doi:10.1016/j.surg.2005.03.024
32. Helmer KS, West SD, Shipley GL, et al. Gastric nitric oxide synthase expression during endotoxemia: implications in mucosal defense in rats. Gastroenterology. 2002;123:173–186. doi:10.1053/gast.2002.34178
33. Lai YC, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating endotoxin-induced up- regulation of inflammatory molecules in murine macrophages. J Surg Res. 2009;154:212–219. doi:10.1016/j.jss.2008.07.010
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.