Back to Journals » Drug Design, Development and Therapy » Volume 15
The Effectiveness and Safety of Intravenous Dexmedetomidine of Different Concentrations Combined with Butorphanol for Post-Caesarean Section Analgesia: A Randomized Controlled Trial
Authors Liu S, Peng P , Hu Y, Liu C, Cao X, Yang C, Gao M
Received 19 October 2020
Accepted for publication 3 February 2021
Published 18 February 2021 Volume 2021:15 Pages 689—698
DOI https://doi.org/10.2147/DDDT.S287512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Shijiang Liu,* Peipei Peng,* Youli Hu, Cunming Liu, Xiaofei Cao, Chun Yang, Mei Gao
Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei Gao
Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, No. 300, Guangzhou Road, Nanjing, People’s Republic of China
Tel +86-25-68303569
Email [email protected]
Purpose: The present study aimed to determine the effectiveness of intravenous dexmedetomidine of different concentrations and to evaluate its maternal and neonatal safety when combined with butorphanol in parturients undergoing cesarean section.
Patients and Methods: A total of 114 parturients between 24 and 43 years of age, with singleton pregnancy who underwent elective cesarean section under epidural anesthesia, were randomly allocated to four groups: group C received 0.9% sodium chloride after delivery, followed by butorphanol (3 μg·kg− 1·h− 1); patients in groups D1, D2, and D3 received 0.5 μg·kg− 1·h− 1 dexmedetomidine after delivery, followed by butorphanol (3 μg·kg− 1·h− 1) combined with dexmedetomidine 0.03, 0.05, and 0.08 μg·kg− 1·h− 1, respectively. The primary outcome was the visual analogue scale (VAS) score at 6 h after delivery when patients were at rest. Secondary outcome measures included VAS after delivery when patients were on movement and uterine cramping, Ramsay sedation scale (RSS), relative infant dose (RID) of dexmedetomidine, satisfaction with analgesia after surgery and symptoms of CNS depression in neonates.
Results: There were no significant differences in patient characteristics among the groups (P > 0.05). The VAS at all timepoints after delivery in groups D2 and D3 were significantly lower than in groups C and D1 (P < 0.001). RSS scores were clearly higher in group D3 than in the other three groups at 6 h and 12 h (P < 0.0001). RID in groups D1, D2, and D3 was 0.171%, 0.197%, and 0.370%, respectively. Compared with group D1, RID was higher in group D3 (P = 0.0079). Degree of satisfaction with analgesia was higher in groups D2 and D3 (P < 0.005).
Conclusion: Continuous intravenous infusion of 0.05 μg·kg− 1·h− 1 dexmedetomidine combined with 3 μg·kg− 1·h− 1 butorphanol could be safely applied in healthy parturients with satisfactory analgesia after cesarean section without changes in sedation.
Keywords: cesarean section, dexmedetomidine, analgesia, relative infant dose, anesthesia
Introduction
Postoperative pain can exacerbate the body’s stress response, which is induced by surgery, and adversely affect both endocrine and immune functions.1 The intensity of acute postoperative pain is a predictor of chronic pain.2 Several studies have demonstrated that inadequate postoperative pain management is associated with persistent pain, greater opioid use, delayed functional recovery, and increased postpartum depression.3,4
The combination of different analgesics that act by different mechanisms (ie, multimodal analgesia) to enhance clinical outcome is a common strategy in pain management.5 Butorphanol, a totally synthetic opioid, exerts partial agonist and antagonist activity at the μ-opioid receptor, and agonist activity at the κ-opioid receptor. In addition, κ-opioid receptor agonists have been suggested to be more effective in females than in males.6 Dexmedetomidine (DEX) is a potent and highly selective α2-adrenoreceptor (α2-AR) agonist, exhibiting hypnotic, sedative, anxiolytic, sympatholytic, and analgesic properties.7–9 Several studies have indicated that postoperative intravenous opioid-DEX combined with patient-controlled analgesia (PCIA) strategies lead to superior analgesia, significant opioid sparing, fewer opioid-related side effects, fewer chills, and greater overall patient satisfaction.5,10,11 Furthermore, poor sleep quality is strongly associated with increased pain scores post-cesarean delivery,12 while DEX could share similarities with natural sleep.13 However, only a small number of studies have focused on the use of DEX in parturients.14–17 Two previous studies investigated the safety of lactation with DEX; however, the sample sizes were small.18,19 Nevertheless, the optimal dosage and safety of DEX used in combination with butorphanol for post-cesarean analgesia remain unclear.
The present prospective, randomized, double-blind controlled study was designed to investigate whether the administration of DEX could decrease postoperative pain intensity after delivery and during PCIA.
Patients and Methods
Ethics Approval
This study was registered at www.ClinicalTrials.gov (NCT03065530). The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (Nanjing, Jiangsu province, China). Written consent was obtained from all participants and they were informed the purpose of this research. This study was conducted at The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, between February and October 2017.
Patient Population
Parturients (>18 and <45 years of age) with a singleton pregnancy admitted to the authors’ institute, who underwent elective cesarean delivery under epidural anesthesia, were recruited for this study between February and October 2017. Parturients who had successfully breastfed a previous infant and planned to breastfeed after this delivery were screened for eligibility. Patients who became pregnant by assisted reproductive technologies were excluded. Other major exclusion criteria included: lack of informed consent; pregnancy-induced hypertension syndrome; HELLP syndrome (hemolysis, elevated liver enzymes, and a low platelet count); hypertension; ischemic heart disease; long-term use of non-steroidal anti-inflammatory drugs (NSAIDs); addiction to alcohol, opioid(s), or sedative-hypnotics; psychiatric disorders; preoperative heart rate (HR) <50 beats/min with/without cardiac conduction or rhythm abnormalities; neuromuscular and endocrine diseases or allergic reactions to α2-AR agonists; or any previous abdominal surgery. Individuals were excluded from the study if epidural anesthesia was unsuccessful, or blood transfusion for hemorrhage required a second operation or inadvertent PCIA was suspended. Parturients in whom surgery ended after 11:00 were excluded so as to not to disturb their rest. Before beginning the procedure, parturients were trained on how to use the PCIA pump and instructed on how to use the 10 cm visual analog scale (VAS: 0 = no pain, 10 = worst pain imaginable).
Randomization and Masking
A computer-generated randomization table was used to equally allocate parturients into 4 groups (n = 30 per group) before the study. A total of 120 subjects were divided into four groups, which were treated with group C, group D1, group D2 and group D3, respectively. The practice is as follows: (1) draw up in advance the serial number of 120 subjects is 1–120; (2) use Excel to generate random numbers; (3) stipulate that random numbers are arranged from small to large, the smallest is group C, then group D1, then group D2, and then group D3, divided into 4 groups, each group of 30 cases. A research nurse, who was not blinded to the study, opened opaque envelopes at the time of request for study, which concealed the group allocations in sequential number. The drugs for treatment were prepared by pharmacy staff who were also not involved in the study. After the research nurse obtained the intravenous solution and the PCIAs, the original contents were labelled “study drug”. All other study staff, including parturients, obstetrician and anesthesiologist, were blinded to group allocation. To ensure patients and neonates safety, each had a treatment plan within the sealed envelope, which could guide emergency treatment if the experiment was terminated due to serious adverse events [eg, circulatory failure, severe respiratory depression, coma, or hemorrhage, among others. Symptoms of CNS depression (eg, drowsiness, cyanosis, or difficult breathing, feeding, and latching) and paradoxical effects (eg, unusual excitement and irritability) in any neonates].
Patients were randomly allocated to one of four groups immediately after delivery of the newborn and cord clamping. Patients in group C received 30 mL 0.9% sodium chloride within 15 minutes, whereas patients in group D1, D2, and D3 received 0.5 μg·kg−1 intravenous DEX diluted to 30 mL with 0.9% sodium chloride within the same time. Based on the previous studies,15,20–23 the PCIA protocol was programmed with 3 μg·kg−1·h−1 butorphanol in group C, while with 3 μg·kg−1·h−1 butorphanol combined with 0.03, 0.05, and 0.08 μg·kg−1·h−1 DEX in groups D1, D2, and D3, respectively. The settings for PCIA were a basal infusion at a rate of 2 mL·h−1 and 0.5 mL of boluses with a lock-out interval of 15 min (butorphanol and DEX in these PCIA protocols were calculated based on patient weight and infusion rate).
None of the parturients received any medication before the induction of anesthesia. On arrival to the operating room, a 20-gauge intravenous cannula was inserted into a peripheral vein on the arm, and five-lead electrocardiogram (ECG), noninvasive blood pressure (NIBP), and oxygen saturation on pulse oximetry (SpO2) were continuously monitored. NIBP was measured every 2 min during the operation. The parturients were positioned in the lateral decubitus position with knees bent toward the chest and the epidural space was identified at the L2 to L3 interspace. After loss-of-resistance confirmed that the tip of the epidural needle was in the epidural space, the epidural catheter was inserted into the space and 3 mL 1.5% lidocaine combined with 5 μg·mL−1 epinephrine was administered via the epidural catheter as a test. All parturients were administered 0.75% ropivacaine with 2 μg·mL−1 fentanyl, and were in supine to the left lateral position. Surgery commenced when T4 to T6 sensory block was achieved.
Oxygen was administered at 5 L·min−1 via facemask, hypotension (systolic blood pressure [SBP] ≤90 mmHg or >20% decline from baseline) was treated with fluid loading, intravenous ephedrine or phenylephrine. When it comes to hypotension occurs with HR>50 bpm, a loading dose of 100~200 μg phenylephrine was considered as the first choice. The next dose was determined according to the patient’s blood pressure response or other vasoconstrictor will be considered. Whereas hypotension with HR<50 bpm, 5~10 mg ephedrine was chosen for application. Parturients received the “study drug” immediately, which was intravenously administered for 20 min when the umbilical cord was clamped. When the obstetrician closed the peritoneum, 50 mg flurbiprofen axetil and 10 mg azasetron hydrochloride was injected in every parturient as a loading dose. No other analgesics were administered post-cesarean section except the study drugs. Immediately after surgery, the PCIA pumps were attached at a rate of 2 mL·h−1, which was 0.5 mL per demand with lock-out intervals of 15 min, and the mother was transferred to the ward after a 1 h stay in the recovery room. NIBP was measured every 30 min for the first 6 h, and every 1 h until 48 h after the operation, with continuous HR and SpO2 monitoring.
Side effects, such as hypotension (SBP < 90 mmHg), bradycardia (HR < 60 beasts/min), hypoxemia (SpO2 < 90%), respiratory rate (RR, < 10 breaths/min, lasting > 10 min), and nausea and vomiting were recorded during the period starting from the end of surgery until 48 h after surgery. Respiratory depression was treated with oxygen and naloxone until RR reached >15 breaths/min. Severe nausea and vomiting were treated with azasetron or dexamethasone, whereas bradycardia was treated with atropine.
Breast milk samples were collected on primary lactation (the start of which was from delivery to when >5 mL of breast milk was expressed by massaging and compressing both breasts) for 48 h, and the time was also recorded. After collection, colostrum samples were frozen at −30°C until used. The samples were then transported to the State Key Laboratory of Natural Medicines, Department of Natural Medicinal Chemistry, China Pharmaceutical University. Sample analysis was performed on an high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) system consisting of a SHIMADZU LC-20AD series HPLC system (Shimadzu, Tokyo, Japan) and an API 4000 triple quadrupole mass spectrometer (Applied Biosystems Sciex, Ont., Canada). A VP-ODS column (2.0 × 150 mm, 5 μm, Shimadzu, Tokyo, Japan) was used for separation. The mobile phase was composed of A (0.2% formic acid-water, v/v) and B (acetonitrile) at a flow rate of 0.3 mL/min with the following optimal gradient elution condition: 0–0.5 min, 10–10% B; 0.5–1.0 min, 10–90% B; 1.0–4.0 min, 90% B; 4.0–4.5 min 90–10% B; 4.5–6 min, 10% B. The column temperature was maintained at 35°C and the injection volume was 10 μL. The total time for each injection was 6.0 min. An aliquot of 1 mL of each colostrum sample was mixed with 20 μL of working internal standard solution (100 ng/mL of erlotinib solution). Diethyl ether (5 mL) was then added for liquid-liquid extraction. After vortexing for 5 min and centrifuging at 8000 rpm for 10 min, 4.8 mL of the supernatant was transferred to a new tube and was evaporated to dryness at 50°C in a centrifugal vacuum evaporator (LABCONCO Corp., USA). The residue was then reconstituted in 100 μL mobile phase. After centrifuging at 14,000 rpm for 5 min, 10 μL of the supernatant was injected for HPLC–MS/MS analysis.18
Outcome Measures
Pain was evaluated using the VAS at rest (VAS-R), movement (VAS-M), and during uterine cramping (VAS-C). VAS-R was assessed when the patient was supine, VAS-M was assessed when patients changed from supine to lateral position, and VAS-C was assessed when the patient required oxytocin after surgery in supine position. Sedation was assessed using the Ramsay sedation scale (RSS) as follows: 1, Awake; agitated or restless or both; 2, Awake; cooperative, oriented, and tranquil; 3, Awake but responds to commands only; 4, Asleep; brisk response to light glabellar tap or loud auditory stimulus; 5, Asleep; sluggish response to light glabellar tap or loud auditory stimulus; and 6, Asleep; no response to glabellar tap or loud auditory stimulus.24 The degree of satisfaction (0, very satisfied; 1, satisfied; 2, moderately satisfied; and 3, not satisfied) was evaluated 48 h after surgery. VAS and RSS were recorded at 6, 12, 24 and 48 h after surgery. The dose (infant) in mg·kg−1 was calculated by multiplying the concentration of the drug in breast milk by the volume of breast milk consumed daily (approximately 150 mL·kg−1). Relative infant dose (RID) = dose (infant, mg·kg−1·day−1)/dose (mother, mg·kg−1·day−1) μg·kg−1·h−1. Detailed results of this study have been uploaded to ClinicalTrials.gov PRS (www.ClinicalTrials.gov, registration number: NCT03065530). The other way is that data made available to all interested researchers upon request (The Ethics Committee of The First Affiliated Hospital of Nanjing Medical University).
Statistical Analysis
The primary outcome was the VAS-R at 6 h after delivery. When designing the study, the sample size was calculated on the basis of an initial pilot study measuring VAS-R 6 h after surgery in 20 patients, and the standard deviation (SD) among the four groups was 1.4. The authors hypothesized that differences in VAS among the four groups and the SDs would be 15%. A power analysis suggested that 80% power would be required to detect differences at an α level of 0.05 (two-tailed), including 24 individuals per treatment group. Considering an anticipated attrition rate of 25%, 30 parturients were eventually recruited for each group. Secondary outcomes included VAS-M, VAS-C, RSS, RID, and satisfaction with analgesia after surgery.
GraphPad Prism version 7 (GraphPad, La Jolla, California, USA) and STATA version 15.1 (Stata Corp, USA) were used to perform statistical analysis. All continuous data that were normally distributed are reported as means and standard deviation (SD). Continuous covariates were assessed for normality using the Shapiro–Wilk test (STATA version 15.1), if the test indicated a P value>0.05, then the data were normally distributed. Patient characteristics were analyzed using one-way analysis of variance (ANOVA). Patient satisfaction was analyzed using the chi-squared test and Kruskal–Wallis rank sum test. RID, VAS, and RSS were analyzed using the Kruskal–Wallis rank sum test. Dunn’s multiple comparison tests were also used for multiple comparisons (post hoc test). P < 0.05 was considered to be statistically significant (P<0.0083 was considered to be statistically significant when the post hoc test was used).
Results
Between February and October 2017, a total of 120 patients were recruited for this study; 6 parturients withdrew from among all four groups. The flow of the study participants is shown in Figure 1. Ultimately, 114 patients completed the study. Patient characteristics showed no significant differences between the 4 groups (Table 1).
 |
Figure 1 Flow chart of study. Abbreviations: DEX, dexmedetomidine; PCIA, patient-controlled intravenous analgesia. |
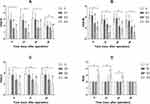
The VAS-R after delivery in groups D2 and D3 were significantly lower than in groups C and D1 at 6 h (P < 0.001), and at 12 and 24 h (P < 0.0001). At 48 h, VAS-R in group D3 was lower than groups C and D1 (P = 0.002 and 0.004, respectively). There were no differences between groups C and D1, nor groups D2 and D3 at all timepoints (Figure 2A).
There were no significant differences in VAS-M and VAS-C between groups C and D1 at all time-points. At 6 and 24 h, VAS-M in group D2 (P < 0.001) and group D3 (P < 0.0001) was lower than in groups C and D1. Moreover, VAS-M in group D3 was lower than in group D2 at 12 h (P = 0.0007) (Figure 2B). When uterine cramping occurred, the VAS-C in group D3 was lower than in groups C and D1 (P < 0.0001) (Figure 2C).
The RSS of group D3 was obviously increased at 6 and 12 h after surgery (P<0.0001) (Figure 2D). In addition, the RIDs of neonate in all patients were far <10%. There were no differences in RID between groups D1 and D2, nor groups D2 and D3. Compared with group D1, the RID in group D3 was clearly higher (P = 0.0079) (Figure 3).
The incidence of “over-satisfied” (ie, satisfied and very satisfied) patients was significantly higher in groups D2 and D3 than in groups C and D1 (P < 0.005) (Figure 4). During the 48 h after surgery, one patient in group C, D2 and D3, and two patients in group D1 experienced nausea, but there were no significant differences in nausea, nor vomiting recorded in any of the four groups. Side effects, such as hypotension, bradycardia and hypoxemia were not observed in parturients. Symptoms of CNS depression (eg, drowsiness, cyanosis, or difficult breathing, feeding, and latching) and paradoxical effects (eg, unusual excitement and irritability) were also not observed in any neonates.
Discussion
The major findings of this prospective study were that intravenous injection of a loading dose of DEX after delivery, followed by continuous intravenous infusion of DEX along with butorphanol in PCIA, led not only to pain reduction (according to VAS) at rest, movement and uterine cramping, but also enhanced the analgesic effect and improved maternal satisfaction. We also found that RIDs were far below 10%, suggesting that there was no central nervous system (CNS) depression observed in neonates after maternal DEX intravenous infusion.25,26
The considerable pharmacological action of DEX is due to the excitement of α2-ARs. DEX can activate presynaptic α2-ARs, inhibit norepinephrine release through a negative feedback mechanism, and stop pain signal transduction. The unique “conscious sedation” of DEX is primarily associated with the nucleus coeruleus in the brain. When compared with remifentanil, it has superior properties, particularly in wake-up sedation, mild analgesia,27 and a lower risk for respiratory depression. In this study, as an adjuvant to opioids, DEX exhibited enhanced analgesia and improved maternal satisfaction after cesarean section. The loading dose of 0.5 μg·kg−1 was chosen during cesarean section under epidural anesthesia, which proved to be beneficial to parturients.15,21,28 We then administered 0.03, 0.05, and 0.08 μg·kg−1·h−1 DEX to determine the dose-dependent effect and optimal dosage in analgesic management along with PCIA.29
Postoperative pain and side effects of analgesic treatment, in particular those of opioids, need to be minimized. Uterine cramping pain is distinct from incision pain in both pathophysiological mechanism and pharmacological responses to analgesic agents; thus, making post-cesarean pain generally different from other postoperative pains.22 Studies have shown that butorphanol can also relieve visceral pain, but butorphanol reduces visceral pain in a dose-dependent manner.30,31 Furthermore, there were insufficient evidence to make conclusions regarding the effectiveness of opioids at relieving pain from uterine cramping.32 Previous studies have found that DEX is effective in controlling visceral pain.33,34 In our study, when uterine cramping occurred, the VAS-C in group D3 was lower than in groups C and D1. We thought that DEX as an adjuvant combined with butorphanol, with an infusion rate of 0.08 μg·kg−1·h−1 seems to play a role in inhibiting visceral pain, and had the potential benefits of reducing the side effects of high-dose butorphanol.
In addition, having an awake parturient able to have skin-to-skin contact with her newborn soon after delivery is recommended in several hospitals, also the American Academy of Pediatrics. We here surmised that appropriate sedation may improve the comfort of the parturient receiving intervertebral anesthesia and skin-to-skin will not be affected. A large proportion of women scheduled for cesarean section experience poor-quality sleep before surgery, in which poor sleep quality significantly increased the risk for severe peak pain upon movement.12 One study found that DEX exhibited a hypercapnic arousal phenomenon similar to what has been described during natural sleep. The characteristic of mimic natural sleep caused that DEX clinically considered apart from other sedatives, such as gamma-aminobutyric acid-related sedation, including propofol and benzodiazepines.13 Adequate sedation without respiratory depression reduced the pain after cesarean section. However, with 0.08 μg·kg−1·h−1 DEX infusion, the mothers’ RSS was significantly higher (RSS > 3) than the other three groups at 6 h and 12 h after surgery. Over-sedation could attenuate mother’s ability to interact with infants. In fact, it is not uncommon for mothers to fall asleep while feeding their infants. The American Academy of Pediatrics believe that a mother falling asleep can increases the risk of Sudden infant death syndrome (SIDS).35,36 Breastfeeding appears to have an independent protective effect against SIDS.37 Therefore, we speculated that this higher dose of DEX was not beneficial for baby care and lactation, because over-sedation could attenuate the mother’s ability for interaction with infants.
On the other hand, due to CNS depression that often occurs (up to 24%) in breastfed infants when mothers use drugs, such as codeine, morphine, oxycodone and benzodiazepines, CNS depression in neonates should be closely considered when the safety of DEX intravenous infusion is assessed during puerperium medication.38,39 In this study, symptoms of CNS depression (eg, drowsiness, cyanosis, or difficult breathing, feeding, and latching) and paradoxical effects (eg, unusual excitement and irritability) were not observed in any neonates, regardless of group. None of the patients experienced high blood pressure, lower blood pressure, bradycardia, respiration depression, or hypoxemia. Even in the 0.08 μg·kg−1·h−1 group with several cases, in which RSS > 3, no one required naloxone, atropine, ephedrine, or phenylephrine treatment.
We then further measured the RID of DEX to evaluate the safety of DEX during breastfeeding of the infants by mothers who received the DEX infusion for post-cesarean analgesia. Several studies have demonstrated that breastfeeding and breast milk are superior to formula in immediate and long-term health. Cesarean section is believed to expose neonates to significantly more medication. The majority of medications are relatively safe for breastfeeding mothers; however, the safety of many newer medications used during and after cesarean section has not yet been studied. DEX is one such example of these newer medications. The RID provides an estimate of the weight-normalized dose relative to the mother’s dose, which is more meaningful to clinicians.25 In general, a RID < 10% is considered to be acceptable in a healthy postnatal infant, a RID>25% may have a therapeutic effect on the infant if absorbed, which may be unacceptable.26 In our study, we found that all RIDs were in the safe range. The oral bioavailability of drug(s) in breastfeeding mothers should also be considered. Given that DEX demonstrates an oral bioavailability value of 16%, the DEX concentration in the neonate plasma absorbed from DEX-containing breast milk should be especially low.40
There were several limitations to our study. Oral analgesics (such as NSAIDs) are usually used as part of a multimodal analgesia regimen for post-cesarean section. In our study, no other narcotics were administered for post-cesarean section. When the obstetrician closed the peritoneum, only 50 mg flurbiprofen axetil was injected in every parturient as a loading dose, which was probably insufficient, and even lacked remediation. A further limitation of the current study was that we did not include nulliparous women because we needed to collected breast milk to calculate the RID for DEX. We selected parous parturients, who had a successful breastfeed experience to minimize variability, which could possibly influence breastfeeding. Although we cannot conclude that intravenous DEX has no effect on breastfeed in primipara, theoretically, in pharmacodynamics and pharmacokinetics, it would be unlikely to occur according the results of our study. Thirdly, simple random is not optimal for this study, Stratified Blocked Randomization is more appropriate. This is the deficiency of this study. Finally, the present study lacked a standardized scale for evaluating infant CNS function and relied simply on clinical observation; as such, further research is warranted.
Conclusion
Continuous intravenous infusion of a high dose of DEX combined with butorphanol in PCIA not only enhanced analgesic effect and reduced VAS score but also improved parturient satisfaction compared with butorphanol PCIA alone. Maternal DEX used during cesarean delivery was safe for the breastfed neonate. In terms of consideration of maternal analgesia efficiency and lactation, and safety of the neonate, a 0.5 μg·kg−1 loading dose with 0.05 μg·kg−1·h−1 intravenous infusion of DEX in PCIA should be considered as an optimal regimen for post-cesarean section analgesia.
Acknowledgments
The authors acknowledge the people who contributed to this study and those who provided care for study patients: Yin Yin, Ziyan Jiang, and Li Chen (Department of Gynecology and Obstetrics, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu province, China). We sincerely thank Qingwang Liu in the State Key Laboratory of Natural Medicines, Department of Natural Medicinal Chemistry, China Pharmaceutical University, for his relentless support during this study, and we are indebted to Kai Zhang in the Pancreas Institute of Nanjing Medical University, and the Pancreatic Center and Department of General Surgery at The First Affiliated Hospital of Nanjing Medical University for his guidance in statistical analysis.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–117. doi:10.1093/bja/85.1.109
2. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–1133. doi:10.1097/00000542-200010000-00038
3. Kainu JP, Halmesmäki E, Korttila KT, Sarvela PJ. Persistent Pain After Cesarean Delivery and Vaginal Delivery: a Prospective Cohort Study. Anesth Analg. 2016;123(6):1535–1545. doi:10.1213/ANE.0000000000001619
4. Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140(1):87–94. doi:10.1016/j.pain.2008.07.011
5. Lin TF, Yeh YC, Lin FS, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102(1):117–122. doi:10.1093/bja/aen320
6. Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2(11):1248–1250. doi:10.1038/nm1196-1248
7. Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine for long-term sedation investigators. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1160. doi:10.1001/jama.2012.304
8. Schnabel A, Meyer-Friessem CH, Reichl SU, et al. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154(7):1140–1149. doi:10.1016/j.pain.2013.03.029
9. Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacokinetic-pharmacodynamic modeling in healthy volunteers: 1. Influence of arousal on bispectral index and sedation. Br J Anaesth. 2017;119(2):200–210. doi:10.1093/bja/aex085
10. Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98(1):153–158. doi:10.1213/01.ANE.0000093225.39866.75
11. Ren CG, Chi MY, Zhang YW, et al. Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy: a consort-prospective, randomized, controlled trial. Medicine. 2015;94(32):e1348. doi:10.1097/MD.0000000000001348
12. Zinger SO, Fireman S, Haroush AB, et al. Preoperative sleep quality predicts postoperative pain after planned caesarean delivery. Eur J Pain. 2017;21(5):787–794. doi:10.1002/ejp.980
13. Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine Pharmacodynamics: part I: crossover Comparison of the Respiratory Effects of Dexmedetomidine and Remifentanil in Healthy Volunteers. Anesthesiology. 2004;101(5):1066–1076. doi:10.1097/00000542-200411000-00005
14. El-Tahan MR, Mowafi HA, Al Sheikh IH, Khidr AM, AI-Juhaiman RA. Efficacy of dexmedetomidine in suppressing cardiovascular and hormonal responses to general anaesthesia for caesarean delivery: a dose-response study. Int J Obstet Anesth. 2012;21(3):222–229. doi:10.1016/j.ijoa.2012.04.006
15. Nie YY, Liu YQ, Luo QY, Huang SQ. Effect of dexmedetomidine combined with sufentanil for post-section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2014;31(4):197–203. doi:10.1097/EJA.0000000000000011
16. Sia AT, Sng BL. Intravenous dexmedetomidine for obstetric anaesthesia: converting a challenge into an opportunity? Int J Obstet Anesth. 2009;18(3):204–206. doi:10.1016/j.ijoa.2009.02.008
17. Abu-Halaweh SA, Al Oweidi AK, Abu-Malooh H, et al. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur J Anaesthesiol. 2009;26(1):86–87. doi:10.1097/EJA.0b000e000000f3fb
18. Nakanishi R, Yoshimura M, Suno M, et al. Detection of dexmedetomidine in human breast milk using liquid Chromatography-tandem mass spectrometry: application to a study of drug safety in breastfeeding after Cesarean section. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1040:208–213. doi:10.1016/j.jchromb.2016.11.015
19. Yoshimura M, Kunisawa T, Suno M, et al. Intravenous dexmedetomidine for cesarean delivery and its concentration in colostrum. Int J Obstet Anesth. 2017;32:28–32. doi:10.1016/j.ijoa.2017.05.002
20. Peng K, Liu HY, Wu SR, Cheng H, Ji FH. Effects of combining dexmedetomidine and opioids for postoperative intravenous patient controlled analgesia: a systematic review and meta-analysis. Clin J Pain. 2015;31:1097–1104. doi:10.1097/AJP.0000000000000219
21. Gao YT, Deng XM, Yuan HB, et al. Patient-controlled Intravenous Analgesia With Combination of Dexmedetomidine and Sufentanil on Patients After Abdominal Operation: a Prospective, Randomized, Controlled, Blinded, Multicenter Clinical Study. Clin J Pain. 2018;34(2):155–161. doi:10.1097/AJP.0000000000000527
22. Cai Q, Gong HL, Fan MB, Chen W, Cai L. The analgesic effect of tramadol combined with butorphanol on uterine cramping pain after repeat caesarean section: a randomized, controlled, double-blind study. J Anesth. 2020;34(6):825–833. doi:10.1007/s00540-020-02820-9
23. Wang FZ, Shen XF, Liu YS, Xu SQ, Guo XR. Continuous infusion of butorphanol combined with intravenous morphine patient-controlled analgesia after total abdominal hysterectomy: a randomized, double-blind controlled trial. Eur J Anaesthesiol. 2009;26(1):28–34. doi:10.1097/EJA.0b013e32831a6aa2
24. Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. doi:10.1136/bmj.2.5920.656
25. Newton ER, Hale TW. Drugs in Breast Milk. Clin Obstet Gynecol. 2015;58(4):868–884. doi:10.1097/GRF.0000000000000142
26. Bennett PN. Use of the Monographs on Drugs. Drugs and Human Lactation.
27. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90(3):699–705. doi:10.1097/00000539-200003000-00035
28. Jiang W, Wang Q, Xu M, et al. Assessment of different loading doses of dexmedetomidine hydrochloride in preventing adverse reaction after combined spinal-epidural anaesthesia. Exp Ther Med. 2017;13(6):2946–2950. doi:10.3892/etm.2017.4335
29. Chen ZL, Tang RD, Zhang R, et al. Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J Clin Anesth. 2017;36:118–122. doi:10.1016/j.jclinane.2016.10.022
30. Houghton KJ, Rech RH, Sawyer DC, et al. Dose-response of intravenous butorphanol to increase visceral nociceptive threshold in dogs. Proc Soc Exp Biol Med. 1991;197(3):290–296. doi:10.3181/00379727-197-43258
31. Fu HM, Zhong CC, Fu Y, Gao YT, Xu XG. Perioperative Analgesic Effects of Preemptive Ultrasound-Guided Rectus Sheath Block Combined with Butorphanol or Sufentanil for Single-Incision Laparoscopic Cholecystectomy: a Prospective, Randomized, Clinical Trial. J Pain Res. 2020;25(13):1193–1200. doi:10.2147/JPR.S252952
32. Deussen AR, Ashwood P, Martis R. Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev. 2011;11(5):CD004908.
33. Ulger F, Bozkurt A, Bilge SS, et al. The antinociceptive effects of intravenous dexmedetomidine in colorectal distension-induced visceral pain in rats: the role of opioid receptors. Anesth Analg. 2009;109(2):616–622. doi:10.1213/ane.0b013e3181a9fae2
34. Jiang ZM, Zhou GZ, Song QL, Bao CY, Wang H, Chen ZH. Effect of Intravenous Oxycodone in Combination With Different Doses of Dexmedetomidine on Sleep Quality and Visceral Pain in Patients After Abdominal Surgery: a Randomized Study. Clin J Pain. 2018;34(12):1126–1132. doi:10.1097/AJP.0000000000000645
35. Task Force On Sudden Infant Death Syndrome SIDS and Other Sleep-Related Infant Deaths: updated 2016 Recommendations for a Safe Infant Sleeping Environment. Pediatrics. 2016;138(5):e20162938. doi:10.1542/peds.2016-2938
36. Kellams A. Breastfeeding without bed-sharing. In: Moon RY, editor. Infant Safe Sleep: A Pocket Guide for Clinicians. Springer; 2020:
37. Hauck FR, Thompson JM, Tanabe KO, Moon RY, Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128(1):103–110. doi:10.1542/peds.2010-3000
38. Kelly LE, Chaudhry SA, Rieder MJ, et al. A Clinical Tool for Reducing Central Nervous System Depression among Neonates Exposed to Codeine through Breast Milk. PLoS One. 2013;8(7):e70073. doi:10.1371/journal.pone.0070073
39. Chow CK, Koren G. Sedating drugs and breastfeeding. Can Fam Physician. 2015;61(3):241–243.
40. Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56(6):691–693. doi:10.1046/j.1365-2125.2003.01944.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.