Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
The effectiveness and safety of amisulpride in Chinese patients with schizophrenia who switch from risperidone or olanzapine: a subgroup analysis of the ESCAPE study
Received 20 January 2017
Accepted for publication 30 March 2017
Published 21 April 2017 Volume 2017:13 Pages 1163—1173
DOI https://doi.org/10.2147/NDT.S132363
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Ying Liang, Xin Yu
On behalf of the ESCAPE Study Group
Peking University Sixth Hospital, Peking University Institute of Mental Health, Key Laboratory of Mental Health, Ministry of Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, People’s Republic of China
Introduction: Second-generation antipsychotics show significant interpatient variability in treatment response and side-effect profiles, and the majority of patients with schizophrenia require multiple treatment changes. This subgroup analysis of the ESCAPE study evaluated the efficacy and safety of amisulpride in Chinese patients with schizophrenia who switched from risperidone or olanzapine.
Methods: ESCAPE was a prospective, open-label, multicenter, single-arm Phase IV study in which Chinese patients with an ICD-10 diagnosis of schizophrenia received amisulpride for 8 weeks. This analysis included 109 patients who switched to amisulpride from risperidone (n=68) or olanzapine (n=41) and 59 treatment-naïve patients for reference. The primary effectiveness outcome was a ≥50% decrease in Positive and Negative Syndrome Scale (PANSS) Total score from Baseline to Week 8. The study was registered at ClinicalTrials.gov (NCT01795183).
Results: Of the patients who switched from risperidone and olanzapine, 77.9% and 56.1% achieved ≥50% reduction in PANSS Total score from Baseline to Week 8 and 57.4% and 46.3% achieved ≥20% reduction in PANSS score from Baseline to Week 2, respectively; these end points were achieved by 66.1% and 61.0% of treatment-naïve patients, respectively. No unexpected adverse events (AEs) were reported. Of the most common AEs, extrapyramidal side effects occurred in 32.4% and 14.6%, blood prolactin increase in 32.4% and 39.0%, and ≥7% increase in body weight in 4.4% and 12% of patients switching from risperidone and olanzapine, respectively.
Conclusion: The results of this subgroup analysis suggest that switching to amisulpride from risperidone and olanzapine is effective and generally well tolerated in Chinese patients with schizophrenia.
Keywords: schizophrenia, amisulpride, risperidone, olanzapine, China
Introduction
Schizophrenia is a chronic condition that can affect personal, occupational, and social functioning. A large epidemiological study conducted in 2013 found that the overall point prevalence of schizophrenia in China was 4.62 per 1,000 (95% confidence interval [95% CI]: 4.18–5.06) with an overall lifetime prevalence of 5.44 per 1,000 (95% CI: 5.01–5.88).1 The current prevalence of schizophrenia-related functional disability among noninstitutionalized Chinese adult patients is estimated to be 0.41%.2 In addition, the suicide attempt rate among Chinese patients with schizophrenia is estimated to be 9.2%.3 Schizophrenia-related functional disability greatly affects the quality of life of patients and their families and places a burden on society. This psychiatric disorder is therefore an important public health issue.4,5
Antipsychotics are recommended for the acute management of schizophrenia in all treatment guidelines.6 Of the second-generation antipsychotics, amisulpride, clozapine, olanzapine, and risperidone have been associated with a greater improvement of both positive and negative symptoms of schizophrenia compared with first-generation drugs.7 Furthermore, second-generation antipsychotics are generally associated with a reduced incidence of extrapyramidal side effects in comparison to first-generation therapeutics.7 However, among the second-generation antipsychotics, there is significant interpatient variability in treatment response and side-effect profiles.8 This suggests that there may be no single antipsychotic drug that is suitable for all patients.9 Therefore, for patients with schizophrenia, switching therapy due to lack of improvement or worsening of symptoms or intolerable side effects or toxicity is often necessary.10 Finding a tolerable management strategy may require multiple switches of treatment to suit the individual patient.
Amisulpride (Sanofi, data on file, 2016), risperidone,11 and olanzapine12 are second-generation, atypical, antipsychotic drugs commonly used in the treatment of schizophrenia. In China, risperidone and olanzapine are among the most commonly prescribed antipsychotics.13 Amisulpride is effective for the improvement of both positive and negative symptoms of schizophrenia and possesses a safety profile that compares favorably with other second-generation antipsychotics.14–16 In particular, amisulpride is associated with less weight gain, which is an important consideration given the reported contribution of weight gain and metabolic syndrome to mortality and morbidity in patients with schizophrenia.17–19 In addition, amisulpride has a low risk of drug–drug interactions with other antipsychotics, which makes switching to this treatment relatively straightforward.12,20,21 Indeed, the available clinical data suggest that switching to amisulpride from other antipsychotics is possible without adverse effects in the majority of patients.20,22
While several previous studies have evaluated switching between second-generation antipsychotics, clinical data for Asian patients with schizophrenia who switch to amisulpride from other atypical antipsychotics are scarce, particularly for Chinese patients.23,24 The Phase IV ESCAPE study showed that amisulpride is effective and relatively well tolerated in Chinese patients with schizophrenia and found no difference in the effectiveness or safety profile of amisulpride for patients who switched to amisulpride vs treatment-naïve patients.25 As risperidone and olanzapine are among the most commonly prescribed antipsychotics in China, this subgroup analysis of the ESCAPE study was conducted to compare the effectiveness and safety of amisulpride for Chinese patients with schizophrenia who switched from treatment with either risperidone or olanzapine.
Methods
Study design
The ESCAPE study was an 8-week, prospective, open-label, multicenter, single-arm Phase IV trial conducted at 13 psychiatric-specialist Tier 1 hospitals in China between October 30, 2012 and December 3, 2013. The study consisted of a screening phase followed by 8 weeks of amisulpride treatment, with clinic visits at Baseline and Weeks 2, 4, and 8. The primary objective was to evaluate the effectiveness and safety of amisulpride (Solian®; Sanofi, Gentilly, France) for Chinese patients with schizophrenia. The ESCAPE study was conducted in accordance with the principles of the 1964 Declaration of Helsinki and all subsequent amendments, and received ethical approval from the following institutional ethics review boards: the Ethics Committee of Peking University Institute of Mental Health; Mental Health Center of Wuhan City Ethics Committee; Beijing Anding Hospital of Capital Medical University Ethics Committee; Beijing Huilongguan Hospital Ethics Committee; Hangzhou Seventh People’s Hospital Ethics Committee; Nanjing Brain Hospital Ethics Committee; the Second Xiangya Hospital of Central South University Ethics Committee; Guangzhou City Mental Hospital Ethics Committee; Shenzhen Kangning Hospital Ethics Committee; The First Hospital of Hebei Medical University Ethics Committee; Tianjin Anding Hospital Mental Health Center of Tianjin City Ethics Committee; Shandong Mental Health Center Ethics Committee; Harbin City, the First Specialist Hospital Ethics Committee; Shanghai Mental Health Center Ethics Committee; and Huaxi Hospital Clinical Trials and Biomedical Ethics Committee. Written informed consent was obtained from all study subjects. The study was registered at ClinicalTrials.gov (NCT01795183). The primary results of the ESCAPE study have been previously published.25
Patients
This analysis included data from adults (aged 18–65 years) included in the ESCAPE study, who met the ICD-10 criteria for schizophrenia, had a Positive and Negative Syndrome Scale (PANSS) Total score ≥60, and had received previous treatment with either risperidone or olanzapine before switching to amisulpride due to suboptimal treatment response or unacceptable tolerability.26,27 Treatment-naïve patients from the ESCAPE study were also included in this analysis to allow a comparison of the effectiveness and safety of amisulpride to be made compared with the pretreated patients. Few previous studies have allowed such a comparison to be made. The ESCAPE study included patients treated as both inpatients and outpatients. Patients were defined as having predominantly negative symptoms if they had a PANSS-Negative subscale score >20 and scored higher in the PANSS-Negative subscale than the PANSS-Positive subscale. A patient was considered to have predominantly positive symptoms if they scored ≥4 in any of the two items of the PANSS-Positive subscale, regardless of the PANSS-Negative subscale score.
Key exclusion criteria included refractory schizophrenia or failure to respond to a full-dose and full-duration treatment with clozapine, contraindications to amisulpride as described in the Chinese package insert, previous or current use of amisulpride, receiving treatment with clozapine in the previous month or long-acting antipsychotic agents in the previous 2 months, receipt of electric convulsion therapy or modified electric convulsion therapy in the previous month, and unsuitability for participation in a clinical trial due to follow-up compliance or safety issues, as assessed by the investigators.
Treatment
All patients received amisulpride tablets (50 mg/tablet) administered orally for 8 weeks, in accordance with the approved Chinese labeling, accessed October 2015). The dose of amisulpride was titrated based on a patient’s individual response 1 week after initiation of treatment, up to a maximum dose of 1,200 mg/day. Doses >400 mg were administered using a twice-daily dosing schedule. In order to limit withdrawal reactions in patients switching from risperidone and olanzapine due to unsatisfactory treatment response or tolerability, a cross-titration scheme was used whereby the dose of the previous medication was gradually reduced as the amisulpride dose was up-titrated, with the aim of complete discontinuation of prior medication within 1 week.
In order to control insomnia, the use of zolpidem (≤10 mg/day), zopiclone (≤7.5 mg/day), or zaleplon (≤10 mg/day) was permitted. For agitation and anxiety, benzodiazepines could be used for <1 week continuously and <2 weeks, respectively. Anticholinergic agents were permitted for the treatment of extrapyramidal side effects. No other concomitant medications or therapies for the treatment of schizophrenia were permitted, and all approved concomitant medications were given ≥12 hours prior to any of the effectiveness or safety assessments.
Study end points
This subgroup analysis assessed the end points used in the primary analysis of the ESCAPE study. In brief, the primary end point of the ESCAPE study was ≥50% reduction in PANSS Total score from Baseline to Week 8 and secondary end points included early response (≥20% reduction in PANSS Total score from Baseline to Week 8), change in PANSS Total, Positive, and Negative and Clinical Global Impression-Severity (CGI-S) scores from Baseline, and safety end points. In addition, this subgroup analysis included an investigation of the association between patients’ demographics and baseline characteristics and treatment response at Week 8 (≥50% reduction in PANSS Total score from Baseline).
All investigators received standardized training before study initiation on the use of all scoring systems utilized in this study to ensure consistency of scoring. All investigators were experienced psychiatric health care professionals, and the majority were members of the Chinese Society of Psychiatry. In addition, all assessments were rater-blinded, with at least one clinical coordinator at each study center responsible for performing the evaluations.
Safety assessment
Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 14.0. Laboratory abnormalities related to the drug were included as AEs. Safety data were continuously monitored and recorded by investigators at each visit or consultation, from Baseline to the end of the study. Electrocardiograms and laboratory testing data were also collected at Baseline and at the end of the study. Side effects were categorized by system organ class. The Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale was also used to rate side effects.28
Blood samples for prolactin level testing were taken from patients in the morning before breakfast, and each study site had one allocated staff member responsible for prolactin measurement who had received standardized training to ensure consistency of testing between sites.
Statistical methods
The effectiveness analysis included all patients who received at least one dose of study medication and had at least one assessment of the primary effectiveness variable available. The safety analysis included all patients who received at least one dose of study medication. The last-observation-carried-forward method was used to estimate values for patients with missing data for the primary effectiveness assessments. Categorical variables were described using frequency, percentage, and 95% CI. Continuous variables were described using mean and standard deviation (SD), unless otherwise stated. Differences between patients switching from risperidone or olanzapine were analyzed using a Student’s t-test (for nonnormally distributed data, the Wilcoxon signed-rank test was used). Safety end points were analyzed as continuous variables, and a Wilcoxon signed-rank test was used to compare values between patients switching from risperidone or olanzapine. All statistical analyses and treatment effects were tested at a two-sided significance level of 0.05. Statistical Analytic System software version 9.2 (SAS Institute, Cary, NC, USA) was used to perform all statistical analyses.
A Student’s t-test or Wilcoxon signed-rank test was used to compare demographics and baseline characteristics for patients achieving ≥50% reduction in PANSS Total score from Baseline to Week 8 vs those who did not achieve this end point.
Results
Patients
Of the 295 patients included in the primary ESCAPE analysis, 109 treatment-experienced and 59 treatment-naïve Chinese patients were eligible for inclusion in this subgroup analysis (Figure 1). Of the pretreated patients, 68 had received prior treatment with risperidone and 41 had received olanzapine. The reasons for switching to amisulpride were not routinely collected as part of the ESCAPE study. All three groups of patients had similar demographics and baseline characteristics (Table 1). The mean age (33.9, 31.7, and 31.5 years), body mass index (BMI) (23.8, 23.2, and 21.9 kg/m2), PANSS Total score (89.5, 89.1, and 90.7) and CGI-S score (5.4, 5.3, and 5.5) were comparable between patients who switched to amisulpride from risperidone and olanzapine and treatment-naïve patients.
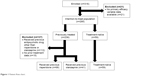  | Figure 1 Patient flow chart. |
Amisulpride dose and concomitant medication use
Patients switching from risperidone and olanzapine initiated amisulpride at similar average Week 1 doses (413.5 and 412.2 mg/day, respectively) (Table 2). Over the first 2 weeks of amisulpride treatment, patients who switched from both risperidone and olanzapine received a similar mean dose of amisulpride, which had increased from the average Week 1 dose (543.7 and 549.3 mg/day, respectively). Among treatment-naïve patients, the Weeks 1 and 2 doses of amisulpride were similar to those received by the pretreated patients. At the end of the study period, the average Week 8 dose of amisulpride was highest in patients who switched from olanzapine (822.7 mg/day), followed by patients who switched from risperidone (747.8 mg/day) and treatment-naïve patients (700.0 mg/day). Although the differences in mean Week 8 dose of amisulpride between the groups were not statistically significant, the difference between patients who switched from olanzapine and treatment-naïve patients approached significance (P=0.052).
Concomitant medications were received by 75.0%, 53.7%, and 44.1% of patients who switched to amisulpride from risperidone and olanzapine and treatment-naïve patients, respectively (Table 2). A higher proportion of patients who switched from risperidone received concomitant trihexyphenidyl, promethazine, and diphenhydramine due to extrapyramidal side effects compared with patients switching from olanzapine or treatment-naïve patients (42.6% vs 26.8% and 30.5%).
Treatment effectiveness
A significantly greater proportion of patients who switched from risperidone achieved a ≥50% reduction in PANSS Total score from Baseline to Week 8, compared with those who switched from olanzapine (77.9% vs 56.1%; P=0.047) (Figure 2). In comparison to the pretreated patients, 66.1% of treatment-naïve patients achieved a ≥50% reduction in PANSS Total score from Baseline to Week 8. The proportion of early responders (≥20% reduction in PANSS Total score from Baseline to Week 2) was 57.4% for patients who switched from risperidone, 46.3% for those who switched from olanzapine, and 61.0% for treatment-naïve patients, and none of the intergroup differences reached statistical significance.
Patients switching from risperidone and olanzapine experienced a similar trend in the reduction of mean PANSS Total, Positive, and Negative scores and CGI-S scores from Baseline to Weeks 2, 4, and 8 (Figure 3A–D). However, patients who switched from risperidone appeared to have a numerically greater reduction in PANSS Total, Positive, and Negative scores at Weeks 2, 4, and 8. In addition, the mean reduction in PANSS Total score from Baseline to Week 8 was greater among patients who switched from risperidone vs olanzapine (30.0 vs 38.8).
Baseline characteristics vs treatment outcomes for patients switching antipsychotics
Patients switching from risperidone who achieved ≥50% reduction in PANSS Total score from Baseline to Week 8 had a significantly higher mean baseline PANSS Positive score (24.7 vs 21.2; P=0.0143), excitability score (14.4 vs 11.7; P=0.0292), and CGI-S (5.5 vs 5.1; P=0.0369) compared with those who did not achieve ≥50% reduction in PANSS Total score from Baseline to Week 8 (Table 3). No other significant differences in baseline characteristics were observed between patients who did or did not achieve ≥50% reduction in PANSS Total score from Baseline to Week 8, either for patients switching from risperidone or olanzapine.
Safety
For patients switching to amisulpride from risperidone and olanzapine, the proportion who experienced ≥1 AE over 8 weeks of treatment was 70.6% and 65.9%, respectively (Table 4). In comparison, of the treatment-naïve patients, 61.0% experienced ≥1 AE during the 8-week study.
The most commonly reported AEs among all patients were extrapyramidal side effects, prolactin increase, increase in body weight, and hyperprolactinemia (Table 4). Among patients switching from risperidone vs those switching from olanzapine, extrapyramidal side effects were more frequent (32.4% and 14.6%) and prolactin increase less frequent (32.4% and 39.0%). Furthermore, a >7% increase in body weight from Baseline was observed in 4.4% of patients who switched from risperidone and 12.2% of those who switched from olanzapine, and hyperprolactinemia was reported in 13.2% of patients switching from risperidone and 0% of patients switching from olanzapine.
There was an overall reduction in side effects for all patients who switched to amisulpride, as demonstrated by a reduction in total UKU scores from Baseline to Week 8. However, patients switching from risperidone showed a numerically greater total reduction from Baseline to Week 8 compared with those switching from olanzapine (−1.4 vs −0.7). The Baseline to Week 8 UKU psychiatric scores exhibited a greater decrease for the patients switched from olanzapine, compared with those switched from risperidone (−2 vs −1.6). In addition, the patients switching from risperidone showed no change in UKU neurologic or autonomic scores from Baseline to Week 8, which was in contrast to the patients switched from olanzapine who showed an increase in these scores.
Discussion
Significant interpatient variability in treatment response and tolerability to second-generation antipsychotics necessitates multiple changes of therapy for the majority of patients, and intolerability or side effects can jeopardize the success of treatment.8,29 This subgroup analysis of the ESCAPE study showed that switching Chinese patients with schizophrenia who are achieving suboptimal treatment response or tolerability with risperidone or olanzapine to amisulpride is effective and safe. This result has great clinical value, as risperidone and olanzapine are among the most commonly prescribed antipsychotic medications in China. The results from this analysis support findings showing that switching to amisulpride is effective and safe in Western20,22 and also Korean and Chinese patients.23,24 In particular, the Korean study showed that patients switching to amisulpride from various antipsychotics, including 38% from olanzapine and 5.4% from risperidone, achieved a clinical benefit in terms of effectiveness and tolerability, with over half of patients experiencing an improvement in symptoms as indicated by improved CGI-CB score.23 However, making a more detailed comparison between this analysis and a previous Korean study is difficult as effectiveness was evaluated using different end points in the two studies.
This study showed that a ≥50% reduction in PANSS score from Baseline to Week 8 was achieved by 77.9% and 56.1% of patients who had previously received risperidone and olanzapine, respectively (P=0.047). In comparison, 66.1% of the treatment-naïve patients included in the primary ESCAPE study analysis achieved this end point.25 These results are broadly in agreement with previous findings; a 6-month study of amisulpride conducted in European patients reported that 65.3% of study participants achieved a ≥50% reduction in PANSS over the 6-month study duration, with most of the reduction in symptoms occurring over the first 2 months.30 Similarly, the mean decrease in PANSS Total score in this analysis for patients switching from risperidone and olanzapine (38.8 vs 30.0, respectively) was comparable to reductions following 6 months of treatment with amisulpride in a European patient population (32.2)30 and higher than reductions observed in a 6-week study of amisulpride in patients from Taiwan (24.1).31
The greater effectiveness of amisulpride in patients switching from risperidone vs olanzapine observed in this study is difficult to explain, particularly because baseline PANSS Total score was similar in both groups of patients. Furthermore, a large meta-analysis has shown comparable efficacy for risperidone, olanzapine, and amisulpride.8 However, it should be noted that patients switching from risperidone and olanzapine due to poor tolerability often do so for different reasons (risperidone due to extrapyramidal side effects and olanzapine due to concerns about weight gain) and that the two groups of pretreated patients in this study may have an inherent selection bias based on each group’s profile of reasons for switching medication, which was unfortunately not recorded as part of the ESCAPE study.10,32 In addition, the analysis included a higher number of patients who switched from risperidone vs olanzapine, and the small patient number overall may have influenced this result. Interestingly, the mean Week 8 dose of amisulpride was lower among patients who switched from risperidone compared with patients who switched from olanzapine, even though these patients experienced better treatment effectiveness, although this difference was not statistically significant. However, this likely reflects greater titration of amisulpride for patients who were not achieving an optimal treatment response. It is also interesting to note that the average Week 2 dose of amisulpride was similar in all patient groups.
Switching antipsychotic therapy to find an optimally tolerated treatment is an important part of the schizophrenia treatment strategy, as treatment compliance is associated with better long-term quality of life.29 Therefore, it is encouraging that patients who switched to amisulpride from risperidone and olanzapine had a comparable incidence of AEs (70.6% vs 65.9%), which was also broadly comparable to the incidence for treatment-naïve patients (61.0%). In addition, although the incidence of AEs in the subgroups included in this analysis was higher than that observed in the overall ESCAPE study population (59.2%),25 it was still in line with previous European studies (69%–76%).30,33,34 Importantly, no unexpected AEs were observed.
Amisulpride has been associated with relatively low weight gain compared with risperidone30 and olanzapine.12,35 In this study, patients in both pretreated groups had a similar mean BMI at Baseline and experienced a relatively low incidence of weight gain (≥7% increase in body weight from Baseline) during amisulpride treatment; 4.4% for patients switched from risperidone and 12.2% for those switched from olanzapine. The incidence of weight gain was also similar among treatment-naïve patients (5.1%). While there appears to be no directly comparable previously published data available for weight gain after 8 weeks of amisulpride treatment, after 6 months, a lower incidence of weight gain was reported for amisulpride vs risperidone (18% vs 34%) and olanzapine (21% vs 35%).16 Despite the difference in incidence of weight gain between the two groups of pre-treated patients included in this analysis, the results confirm that weight gain is relatively low for patients treated with amisulpride who switch from risperidone or olanzapine.
Although the incidence of extrapyramidal side effects is generally lower with second-generation antipsychotics compared with first-generation drugs, this class of side effects is still a relevant safety concern for the newer class of therapeutics.7 In this analysis, extrapyramidal side effects were more common in the patients who switched from risperidone to amisulpride compared with those switching from olanzapine and the treatment-naïve patients (32.4% vs 14.6% and 25.4%). Furthermore, the incidence of extrapyramidal side effects in patients switching from risperidone was slightly higher than previous reports from two studies of amisulpride conducted in France by Carrière et al36 (23%) and Colonna et al33 (13%). However, it is known that patients more frequently discontinue risperidone due to extrapyramidal side effects and olanzapine due to concerns about weight gain.8,10,32 Therefore, it may be that patients switching from risperidone were already experiencing extrapyramidal side effects before initiation of amisulpride, which is also evidenced by the higher use of concomitant therapy for this side effect in these patients vs patients switching from olanzapine or the treatment-naïve patients.
There are several limitations of this subgroup analysis that should be mentioned. First, the patient numbers in the study are relatively low and there were unequal numbers of patients in the two pretreated patient groups; 62.4% of the pretreated patients had switched from risperidone. Additionally, the lack of data on the reasons for patients switching to amisulpride, and other data such as length of time on previous treatment, may introduce bias into the data analysis and limit the strength of conclusions that can be drawn. However, given the high interpatient heterogeneity of treatment response to antipsychotic drugs, which necessitates multiple switches of treatment, and the common use of risperidone and olanzapine in China, this analysis provides a valuable source of high-quality clinical data on the effectiveness and safety of amisulpride in patients who have poor tolerability or treatment response with risperidone or olanzapine. In addition, this analysis also enabled comparison of the effectiveness and safety of amisulpride in pretreated patients and treatment-naïve patients from the same study.
Conclusion
The results of this subgroup analysis of the ESCAPE study showed that switching to amisulpride from risperidone or olanzapine was effective and generally well tolerated in Chinese patients with schizophrenia.
Acknowledgments
The authors thank the members of the ESCAPE Study Group: Changan Cao (Department of Psychiatry, Shenzhen Kangning Hospital, Shenzhen, China), Cheng Zhu (Department of Psychiatry, The Seventh People Hospital of Hangzhou, Hangzhou, China), Chuanyue Wang (Department of Psychiatry, Beijing Anding Hospital, Capital Medical University, Beijing, China), Congpei Zhang (Department of Psychiatry, The First Special Hospital of Harbin, Harbin, China), Fang Dong (Department of Psychiatry, Beijing Anding Hospital, Capital Medical University), Fude Yang (Department of Psychiatry, Beijing Huilongguan Hospital, Beijing, China), Hong Deng (Department of Psychiatry, West China Hospital, Sichuan University, Chengdu, China), Jingjie Yu (Department of Psychiatry, Tianjin Anding Hospital, Tianjin, China), Jisheng Tang (Department of Psychiatry, Mental Health Centre of Shandong Province, Jinan, China), Lei Su (Department of Psychiatry, Mental Health Centre of Shandong Province), Limin Xin (Department of Psychiatry, Beijing Huilongguan Hospital), Ling Hong (Department of Psychiatry, West China Hospital, Sichuan University), Minglong Gao (Department of Psychiatry, The First Hospital of Hebei Medical University, Shijiazhuang, China), Muni Tang (Department of Psychiatry, Guangzhou Brain Hospital, Guangzhou, China), Shiping Xie (Department of Psychiatry, Nanjing Brain Hospital, Nanjing, China), Shuiping Lu (Department of Psychiatry, Nanjing Brain Hospital), Tiebang Liu (Department of Psychiatry, Shenzhen Kangning Hospital), Xiaojin Xu (Department of Psychiatry, Mental Health Center of Wu Han City, Wuhan, China), Xijin Wang (Department of Psychiatry, The First Special Hospital of Harbin), Xuanzi Li (Department of Psychiatry, Guangzhou Brain Hospital), Xueyi Wang (Department of Psychiatry, The First Hospital of Hebei Medical University), Yi Li (Department of Psychiatry, Mental Health Center of Wu Han City), Yong Zhang (Department of Psychiatry, Tianjin Anding Hospital), and Zhiyu Chen (Department of Psychiatry, The Seventh People Hospital of Hangzhou). Sanofi (China) provided funding for this study and developed and approved the study protocol. No financial or other incentives were given to study participants. Editorial assistance for this manuscript was provided by Jake Burrell of Adelphi and paid for by Sanofi. The authors received sponsorship (AMISU_L_06155) from Sanofi (China) to conduct this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Long J, Huang G, Liang W, et al. The prevalence of schizophrenia in mainland China: evidence from epidemiological surveys. Acta Psychiatr Scand. 2014;130(4):244–256. | ||
Liu T, Song X, Chen G, Paradis AD, Zheng X. Prevalence of schizophrenia disability and associated mortality among Chinese men and women. Psychiatry Res. 2014;220(1–2):181–187. | ||
Zhang XY, Al Jurdi RK, Zoghbi AW, et al. Prevalence, demographic and clinical correlates of suicide attempts in Chinese medicated chronic inpatients with schizophrenia. J Psychiatr Res. 2013;47(10):1370–1375. | ||
Kirov GK, Murray RM, Seth RV, Feeney S. Observations on switching patients with schizophrenia to risperidone treatment. Risperidone Switching Study Group. Acta Psychiatr Scand. 1997;95(5):439–443. | ||
Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116(5):317–333. | ||
Saddichha S, Chaturvedi SK. Clinical practice guidelines in psychiatry: more confusion than clarity? A critical review and recommendation of a unified guideline. ISRN Psychiatry. 2014;2014:828917. | ||
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. | ||
Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. | ||
Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–179. | ||
Davis JM, Leucht S. Commentary on strategies for switching antipsychotics. BMC Med. 2008;6:18. | ||
Sampson S, Hosalli P, Furtado VA, Davis JM. Risperidone (depot) for schizophrenia. Cochrane Database Syst Rev. 2016;4:CD004161. | ||
Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;3:CD006654. | ||
Li Q, Xiang YT, Su YA, et al. Antipsychotic polypharmacy in schizophrenia patients in China and its association with treatment satisfaction and quality of life: findings of the third national survey on use of psychotropic medications in China. Aust N Z J Psychiatry. 2015;49(2): 129–136. | ||
Leucht S, Wagenpfeil S, Hamann J, Kissling W. Amisulpride is an “atypical” antipsychotic associated with low weight gain. Psychopharmacology. 2004;173(1–2):112–115. | ||
Loo H, Poirier-Littre MF, Theron M, Rein W, Fleurot O. Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry. 1997;170:18–22. | ||
McKeage K, Plosker GL. Amisulpride: a review of its use in the management of schizophrenia. CNS Drugs. 2004;18(13):933–956. | ||
Andrade C. Cardiometabolic risks in schizophrenia and directions for intervention, 1: magnitude and moderators of the problem. J Clin Psychiatry. 2016;77(7):e844–e847. | ||
Coulouvrat C, Dondey-Nouvel L. Safety of amisulpride (Solian): a review of 11 clinical studies. Int Clin Psychopharmacol. 1999;14(4):209–218. | ||
Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1–93. | ||
Burns T, Chabannes JP, Demyttenaere K. Switching antipsychotic medications: general recommendations and switching to amisulpride. Curr Med Res Opin. 2002;18(4):201–208. | ||
Pani L, Villagran JM, Kontaxakis VP, Alptekin K. Practical issues with amisulpride in the management of patients with schizophrenia. Clin Drug Investig. 2008;28(8):465–477. | ||
Peuskens J. Switching to amisulpride. Curr Med Res Opin. 2002;18(suppl 3):s23–s28. | ||
Kim Y, Wang SM, Kwak KP, et al. Amisulpride switching in schizophrenic patients who showed suboptimal effect and/or tolerability to current antipsychotics in a naturalistic setting: an explorative study. Clin Psychopharmacol Neurosci. 2016;14(4):371–377. | ||
Wang YT, Chiu NY, Jou SH, et al. Effects of amisulpride on the cognitive function of patients with schizophrenia who switched from risperidone. Int J Psychiatry Clin Pract. 2008;12(3):180–186. | ||
Liang Y, Cao C, Zhu C, et al. The effectiveness and safety of amisulpride in Chinese patients with schizophrenia: an 8-week, prospective, open-label, multicenter, single-arm study. Asia Pac Psychiatry. 2016;8(3):241–244. | ||
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. | ||
WHO. The International Statistical Classification of Diseases and Related Health Problems 10th Revision. Geneva: World Health Organization; 2009. | ||
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. | ||
Hayhurst KP, Drake RJ, Massie JA, et al. Improved quality of life over one year is associated with improved adherence in patients with schizophrenia. Eur Psychiatry. 2014;29(3):191–196. | ||
Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y; Amisulpride Study Group. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology. 2002;27(6):1071–1081. | ||
Hwang TJ, Lee SM, Sun HJ, et al. Amisulpride versus risperidone in the treatment of schizophrenic patients: a double-blind pilot study in Taiwan. J Formos Med Assoc. 2003;102(1):30–36. | ||
Linden M, Eich FX, Pyrkosch L. Do differences in atypical antipsychotics matter in routine practice? Medication switch from olanzapine and risperidone to amisulpride. Int Clin Psychopharmacol. 2007;22(3):175–178. | ||
Colonna L, Saleem P, Dondey-Nouvel L, Rein W. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int Clin Psychopharmacol. 2000;15(1):13–22. | ||
Peuskens J, Bech P, Moller HJ, Bale R, Fleurot O, Rein W. Amisulpride vs. risperidone in the treatment of acute exacerbations of schizophrenia. Amisulpride study group. Psychiatry Res. 1999;88(2):107–117. | ||
Lecrubier Y, Quintin P, Bouhassira M, Perrin E, Lancrenon S. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand. 2006;114(5):319–327. | ||
Carriere P, Bonhomme D, Lemperiere T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group). Eur Psychiatry. 2000;15(5):321–329. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






