Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 8
The effect of weight, body mass index, age, sex , and race on plasma concentrations of subcutaneous sumatriptan: a pooled analysis
Authors Munjal S , Gautam A, Rapoport A, Fisher D
Received 22 March 2016
Accepted for publication 26 May 2016
Published 1 September 2016 Volume 2016:8 Pages 109—116
DOI https://doi.org/10.2147/CPAA.S108966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Arthur E. Frankel
Sagar Munjal,1 Anirudh Gautam,2 Alan M Rapoport,3 Dennis M Fisher4
1Department of Neurology Clinical Development and Medical Affairs, Dr. Reddy’s Laboratories Ltd, Princeton, NJ, USA; 2Pharmacokinetics, Modeling and Simulation & Bioanalysis, Dr. Reddy’s Laboratories Ltd, Hyderabad, India; 3Department of Neurology, The David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, 4P Less Than, San Francisco, CA, USA
Objective/background: Factors such as body size (weight and body mass index [BMI]), age, sex, and race might influence the clinical response to sumatriptan. We evaluated the impact of these covariates on the plasma concentration (Cp) profile of sumatriptan administered subcutaneously.
Methods: We conducted three pharmacokinetic studies of subcutaneous sumatriptan in 98 healthy adults. Sumatriptan was administered subcutaneously (236 administrations) as either DFN-11 3 mg, a novel 0.5 mL autoinjector being developed by Dr. Reddy’s Laboratories; Imitrex® (Sumatriptan) injection 3 mg or 6 mg (6 mg/0.5 mL); or Imitrex STATdose 4 mg or 6 mg (0.5 mL). Blood was sampled for 12 hours to determine sumatriptan Cp. Maximum Cp (Cmax), area under the curve during the first 2 hours (AUC0–2), and total area under the curve (AUC0–∞) were determined using noncompartmental methods. Post hoc analyses were conducted to determine the relationship between these exposure metrics and each of body weight, BMI, age, sex, and race (categorized as white, black, or others).
Results: Both weight and BMI correlated negatively with each exposure metric for each treatment group. Across all treatment groups, AUC0–2 for subjects with BMI less than or equal to median value was 1.03–1.12 times the value for subjects with BMI more than median value. For subjects with BMI less than or equal to median value receiving DFN-11, median AUC0–2 was slightly less than that for subjects with BMI more than median value receiving Imitrex 4 mg and larger than that for subjects with BMI more than median value receiving Imitrex 3 mg. Results were similar for the other exposure metrics and for weight. Exposure was higher in women than in men, which can be attributed in part to differences in weight. There was no relationship between exposure and age. For DFN-11, AUC0–2 and AUC0–∞ were lower in non-whites compared with whites; the ratio of median values was 0.84 and 0.89, respectively. A similar, nonstatistically significant, trend was observed in the other products (ratio of median values ranging from 0.84 to 0.89).
Conclusion: Weight and BMI appear to be important covariates for sumatriptan exposure: subjects with lower values for either metric of body size have higher systemic exposure compared with subjects with higher values. Additional studies are required to determine if doses of subcutaneous sumatriptan may be adjusted based on BMI for comparable efficacy and a potentially improved tolerability profile.
Keywords: sumatriptan plasma concentration, migraine, body size, BMI, obesity, age, sex, race
Introduction
Migraine is a chronic, disabling neurological disorder with episodic clinical findings of headache, often associated with nausea and vomiting. In the US, ~17% of women and 6% of men experience migraine.1 Sumatriptan is one of the most commonly used triptans for treatment of migraine attacks. Oral, intranasal (IN), and subcutaneous (SC) formulations of sumatriptan are approved by the Food and Drug Administration and are marketed in the US and several other countries.2–4 SC sumatriptan has a better efficacy profile than oral and IN sumatriptan products.2–4 Absorption is more rapid with SC administration (time to reach maximum observed [peak] plasma concentration [tmax] for SC Imitrex® (Sumatriptan) is 12 minutes [range: 5–20 minutes]), significantly shorter than that for the oral (2–2.5 hours)2–4 and nasal routes (range: 60–90 minutes).5 As a result, onset of action with SC sumatriptan (10 minutes) is faster than that with IN (30–45 minutes) or oral (45–60 minutes) delivery.2–4 There are several published pharmacokinetic (PK) studies for sumatriptan; however, few of these examine whether sumatriptan PK metrics (ie, Cmax [maximum plasma concentration {Cp}] and AUC [area under the Cp vs time curve]) are affected by covariates such as weight, body mass index (BMI), age, sex, and race. In addition, sample size for many of these studies is small.
Currently, SC sumatriptan is available in 4 mg and 6 mg dosage forms (Imitrex STATdose, 0.5 mL) and as an injectable solution (Imitrex injection, 6 mg/0.5 mL). A low-dose (3 mg) sumatriptan product for SC injection, known as DFN-11 (ZEMBRACE™SymTouch™, Dr. Reddy’s Laboratories, Hyderabad, Telangana, India), is now approved for sale in the US. DFN-11 is a single-dose, 0.5 mL prefilled syringe as a ready-to-use disposable autoinjector. Efficacy of this 3 mg autoinjector is expected to be same as existing sumatriptan injection 3 mg products.
We conducted three clinical pharmacology studies (unpublished) to support a PK bridge between DFN-11 and several Imitrex products; data from these studies were pooled as the target populations and study methodologies were similar. To understand the impact of covariates on systemic exposure of sumatriptan, we examined the relationships between sumatriptan PK metric for each product and each of weight, BMI, age, sex, and race. Results of this analysis could provide insights into treatment outcomes. In addition, we evaluated whether subsets of subjects who received DFN-11 3 mg have systemic exposure comparable to that in subsets of subjects who received larger doses of Imitrex.
Methods
We conducted three PK studies of SC sumatriptan in 98 healthy adults. The data from three studies were combined for pooled data analysis. The protocols were approved by the Chesapeake Research Review, Inc., Institutional Review Board, and the studies were conducted in compliance with good clinical practice at Celerion Research (Tempe, AZ and Lincoln, NE). Before the studies began, their nature was explained to the subjects, and subjects provided written informed consent. All studies used a single-dose, open-label, randomized crossover design to determine relative bioavailability following SC administration of sumatriptan succinate in healthy, fasted adults. The randomization sequences were generated by Celerion as per their Standard Operating Procedure. Each study included DFN-11 (3 mg/0.5 mL) vs either Imitrex injection (3 mg/0.25 mL or 6 mg/0.5 mL) or Imitrex STATdose system (4 mg/0.5 mL or 6 mg/0.5 mL). All three studies were conducted from July 2014 to February 2015. Brief descriptions of the three unpublished studies are as follows: study 001 (DFN-11-CD-001) was a two-way crossover study comparing DFN-11 with Imitrex injection 3 mg; subjects were randomized to one of two treatment sequences. Study 002 (DFN-11-CD-002) was a three-way crossover study that compared DFN-11 with Imitrex injection 3 mg and 6 mg; subjects were randomized to one of six treatment sequences. Study 003 (DFN-11-CD-003) was a three-way crossover study comparing DFN-11 with Imitrex 4 mg STATdose system and 6 mg STATdose system; subjects were randomized to one of six treatment sequences. For all three studies, in each period, a single dose was administered SC over ~5 seconds. PK samples were obtained predose and at 5 minutes, 10 minutes, 15 minutes, 20 minutes, 30 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours postdose. Subjects were confined from 8 hours before the first dose through the last sample after the final dose. Doses were separated by at least 2 days.
Cps of sumatriptan were determined using high-performance liquid chromatography with mass spectrometric detection assay, sensitive to 0.5 ng/mL. PK parameters for each dosing session were determined using noncompartmental methods with WinNonlin Version 6.3 (Certara, St Louis, MO, USA) or SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA). The following exposure parameters were determined:
- Cmax: the maximum measured Cp, determined by examination of the data.
- AUC0–2: area under the Cp vs time curve from the time of drug administration to the 2-hour sample.
- AUC0–∞: area under the concentration–time curve from the time of drug administration extrapolated to infinity. AUC0–∞ is calculated as the sum of AUC0–t (where t is the last measured nonzero concentration) plus the ratio of the last measurable Cp to the elimination rate constant.
AUCs were calculated by the linear trapezoidal method.
Graphics were prepared to examine the relationship between each of AUC0–2, AUC0–∞, and Cmax and the following covariates: weight (kg), BMI (kg/m2), age (years), sex, and race (categorized as white, black, or other [two subjects listed as “multiple” race were characterized as “other”]). For continuous covariates, the relationship between each metric and covariate was evaluated by linear regression and a smoother (Supersmoother, Stanford, CA, USA). In addition, the median value for each continuous covariate (weight: 72.2 kg; BMI: 26.33 kg/m2; and age: 30.5 years) was identified, and the median value for the exposure metric for subjects below and above that median covariate value was determined; the ratio of these values is reported. For example, we compared exposure metrics for subjects weighing >72.2 kg with those weighing less. For categorical covariates, the mean for each group was identified; groups were compared by t-test. The ratio of median values for female/male and non-white/white was determined. In addition, we conducted multiple regression analyses including each of weight or BMI plus either race (Caucasian vs non-Caucasian) or sex (race and sex included in the model as factors) to determine whether race or sex contributed to differences in exposure after accounting for body size.
Safety
Subjects were monitored for injection site reactions including pain, tenderness erythema/redness, and induration/swelling before dosing and at 6 hours and 12 hours after study drug administration in all periods.
Results
Subject disposition
Demographics characteristics are summarized in Table 1. All subjects were healthy and none used tobacco. One subject in Study 001 was found to be pregnant before Period 2 and was discontinued from the study. In Study 001, an incorrect procedure was applied for injection of Imitrex 3 mg; as a result, data from those sessions were excluded from the PK analysis. All remaining sessions were included in the PK analyses; all sessions were included in safety analyses. Thus, the total number of sessions included in the PK analysis was 97 for DFN-11, 35 for Imitrex injection 3 mg, 36 for Imitrex STATdose 4 mg, and 68 for Imitrex STATdose 6 mg. For one session with DFN-11, AUC0–2 could not be determined due to a missing sample at 2 hours; however, AUC0–∞ could be determined since tmax was much earlier than 2 hours.
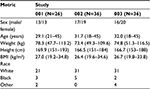  | Table 1 Demographic characteristics of the subjects in the three studies Note: Values for sex and race are counts; for the other metrics, values are mean (range). Abbreviation: BMI, body mass index. |
Effect of weight
For each treatment group, increasing weight was associated with decreasing sumatriptan exposure (Figures 1–3 and Table 2) for all three metrics (P<0.003 [linear regression] for each treatment group within each metric). Across all treatment groups, AUC0–2 for subjects weighing less than or equal to median weight was ~1.2 times the value for subjects with weight more than median value. For subjects with weight less than or equal to median value receiving DFN-11, median AUC0–2 was similar to that for subjects with weight more than median value receiving Imitrex 4 mg and larger than that for subjects with weight more than median value receiving Imitrex 3 mg. AUC0–∞ for subjects with weight less than or equal to median value was 1.12–1.19 times the value for subjects with weight more than median value. For subjects with weight less than or equal to median value receiving DFN-11, median AUC0–∞ was slightly less than that for subjects with weight more than median value receiving Imitrex 4 mg and markedly larger than that for subjects with weight less than or equal to median value receiving Imitrex 3 mg. Cmax for subjects with weight less than or equal to median value was ~1.18–1.30 times the value for subjects with weight more than median value. For subjects with weight less than or equal to median value receiving DFN-11, median Cmax was slightly more than that for subjects with wieght more than median value receiving Imitrex 4 mg and markedly larger than that for subjects with weight more than median value receiving Imitrex 3 mg.
In the multiple regression analyses, after incorporating the effect of weight, Cmax for DFN-11 was 5.8 ng/mL higher in women and AUC0–2 for DFN-11 was 3.0 ng/mL h higher in women (Table 3). After incorporating the effect of weight, AUC0–2 was 3.3 ng/mL h lower in non-Caucasians with DFN-11 and 6.1 ng/mL lower with Imitrex 4 mg. AUC0–∞ was 3.6 ng/mL h lower with DFN-11 and 9.5 ng/mL h lower with Imitrex 4 mg (Table 4).
Effect of BMI
For most treatment groups, increasing BMI was associated with decreasing sumatriptan exposure (Table 5) for all three metrics (P<0.05 [linear regression] for each treatment group within each metric); exceptions were AUC0–∞ for Imitrex 3 mg (P=0.052) and Cmax for Imitrex 4 mg (P=0.061). Across all treatment groups, AUC0–2 for subjects with BMI less than or equal to median value was 1.03–1.12 times the value for subjects with BMI more than median value. For subjects with BMI less than or equal to median value receiving DFN-11, median AUC0–2 was slightly less than that for subjects with BMI more than median value receiving Imitrex 4 mg and larger than that for subjects with BMI more than median value receiving Imitrex 3 mg. AUC0–∞ for subjects with BMI less than or equal to median value was 1.03–1.15 times the value for subjects with BMI less than or equal to median value. For subjects with BMI less than or equal to median value receiving DFN-11, median AUC0–∞ was less than that for subjects with BMI more than median value receiving Imitrex 4 mg and larger than that for subjects with BMI more than median value receiving Imitrex 3 mg. Cmax for subjects with BMI less than or equal to median value was 1.02–1.14 times the value for subjects with BMI more than median value. For subjects with BMI less than or equal to median value receiving DFN-11, median Cmax was less than that for subjects with BMI more than median value receiving Imitrex 4 mg and larger than that for subjects with BMI more than median value receiving Imitrex 3 mg.
In the multiple regression analyses, after incorporating the effect of BMI, Cmax was 9.0 ng/mL higher in women with DFN-11, 8.9 ng/mL higher with Imitrex 4 mg, and 14.8 ng/mL higher with Imitrex 6 mg. AUC0–2 was 3.7–8.6 ng/mL h higher in women across the four products. AUC0–∞ was 6.1 ng/mL h higher in women with DFN-11 and 8.3 ng/mL h higher with Imitrex 6 mg. After incorporating the effect of BMI, Cmax was 10.9 ng/mL lower in non-Caucasians with Imitrex 4 mg, AUC0–2 was 5.1–8.7 ng/mL h lower in non-Caucasians for DFN-11 and Imitrex 4 mg and 6 mg. AUC0–∞ was 5.7–12.3 ng/mL h lower in non-Caucasians for DFN-11 and Imitrex 4 mg and 6 mg.
Effect of age
There was no relationship between sumatriptan exposure and age.
Sex
For most treatment groups, sumatriptan exposure in women was higher than in men, the ratio typically being ~1.2. This ratio results in part from the difference in weight between men and women.
Effect of race
For DFN-11, AUC0–2 and AUC0–∞ were lower in non-whites compared with whites; the ratio of median values was 0.84 and 0.89, respectively. A similar, nonstatistically significant trend was observed in the other products (ratio of median values ranging from 0.84 to 0.892).
Safety
In 98 subjects exposed to SC sumatriptan, all reported adverse events were among those currently described for marketed sumatriptan products. There were no deaths or related serious adverse events. Only one study (Study 003) included a head-to-head comparison of DFN-11 to the two autoinjector products, Imitrex STATdose 4 mg and Imitrex STATdose 6 mg. In that study, the incidence of injection site pain with DFN-11 was 39%, lower than that observed with Imitrex STATdose 6 mg (53%) and similar to Imitrex STATdose 4 mg (42%). The incidence of injection site erythema with DFN-11 was 28%, higher than with Imitrex STATdose 6 mg (19%) and similar to Imitrex STATdose 4 mg (25%). All these events were mild in nature and resolved without medical intervention. The percentage of subjects experiencing at least one adverse event was 56% for DFN-11, 72% for Imitrex STATdose 4 mg, and 67% for Imitrex STATdose 6 mg.
Discussion
These post hoc exploratory analyses demonstrate a statistically significant impact of body size, assessed by weight and BMI, on systemic exposure to sumatriptan administered as DFN-11 or Imitrex. For each treatment group, subjects with lower BMI or weight had higher systemic exposure compared with subjects with higher BMI or weight. For both products, exposure was higher in women than in men, with ratios as high as 1.29; this sex effect is largely a function of weight (women weighing less than men).
In addition to evaluating Cmax and AUC0–∞ as exposure metrics, we evaluated AUC0–2. This metric was selected (and prespecified in the analysis plan) because sumatriptan’s efficacy window is typically within the first 2 hours; therefore, systemic exposure during this period is more relevant than systemic exposure well after efficacy is expected to wane.
For continuous covariates, the median value of the covariate was used to divide subjects into two groups; the median value for each metric was then compared between these groups. If the ratio were markedly less than unity, it would suggest that subjects weighing more or having a larger BMI experienced lower exposure for that metric. In turn, it might imply different dosing requirements as a function of that covariate.
These post hoc analyses demonstrate that sumatriptan exposure was higher in lighter subjects and those with BMI less than or equal to the median value. Several studies have reported that body size affects migraine response. Saracco et al6 pooled ITT data from 346 subjects in three double-blind, placebo-controlled randomized studies with frovatriptan and other triptans. Pain-free rates at 2 hours were lower in obese subjects (27%) compared with nonobese subjects (34%). Visser et al7 mailed a survey questionnaire to 869 migraine patients, of whom 735 (85%) replied. To determine risk factors for nonresponders to sumatriptan, the investigators compared clinical characteristics, demographics, and migraine-associated symptoms: non responders to SC sumatriptan had a higher BMI. In a post hoc analysis from a double-blind randomized, placebo-controlled study with rizatriptan 10 mg oral tablets in children aged 12–17 years, weight <40 kg was associated with higher pain-free rates (34.6%) compared with weight ≥40 kg (30.2%).8 One of the limitations of this study was that the sample size for the <40 kg group was much smaller than that for the ≥40 kg group (26 vs 258). In contrast, the post hoc analysis of a combination product (sumatriptan 85 mg/aproxen 500 mg) by Winner et al9 found no correlation between BMI and treatment response. Cosson and Fuseau10 demonstrated a relationship between apparent clearance and weight and, consequently, an inverse relationship between AUC and weight. However, these investigators did not report the coefficient of that relationship so we were unable to compare their results with ours.
Two prospective efficacy studies have compared sumatriptan SC 3–6 mg. In a pilot study of SC sumatriptan, Landy et al11 reported that subjects preferred 3 mg over 6 mg: 66.7% of subjects administered 3 mg achieved a combined end point at 2 hours of being pain-free with no significant side effects compared with 50% of subjects administered with 6 mg. The difference between groups was larger at 24 hours: 63.3% vs 33.3%. Preference for the smaller dose in both studies suggests that larger doses, despite their potential for improved efficacy, may cause adverse effects limiting their utility.
Mathew et al12 reported similar 1-hour pain relief rates with 3 mg (57%) and 4 mg (50%) and a higher rate with 6 mg (73%). Although both studies demonstrate that SC 3 mg provides pain relief to a significant number of migraineurs, major limitations of both are that sample sizes were small (30 subjects per group) and efficacy data were not stratified by BMI. The same study also reported few of certain triptan-related adverse events – flushing, nausea, tingling, warm/hot sensation, numbness, and dizziness – with sumatriptan injection 3 mg compared with 6 mg. Prospective studies are required to understand if BMI impacts efficacy of low-dose sumatriptan injection or other triptans for episodic migraineurs. In addition, smaller doses may be preferred by certain subgroups of patients if they provide comparable efficacy with fewer adverse effects.
Conclusion
Weight and BMI appear to be important covariates for sumatriptan exposure: subjects with lower values for either metric of body size have higher systemic exposure compared with subjects with higher values. Additional studies are required to determine if doses of SC sumatriptan may be adjusted based on BMI for comparable efficacy and a potentially improved tolerability profile.
Acknowledgment
This study was supported and funded by Dr. Reddy’s Laboratories Ltd.
Disclosure
Doctor Sagar Munjal and Anirudh Gautam are employed by and own stock of Dr. Reddy’s Laboratories Ltd. Doctor Alan Rapoport serves as a consultant, advisory board member, and/or has received compensation from Autonomic Technologies Inc, Avanir Pharmaceuticals, Depomed Inc, Dr. Reddy’s Laboratories, ElectroCore®, Impax Laboratories Inc, Pernix Therapeutics, Teva Pharmaceutical Industries, and Zosano Pharma Inc. Doctor Dennis Fisher is a full-time employee of P Less Than and is a paid consultant for Dr. Reddy’s Laboratories. The authors report no other conflicts of interest in this work.
References
Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53(8):1278–1299. | ||
Imitrex® (sumatriptan succinate) injection [prescribing information] [webpage on the Internet]. Research Triangle Park: GlaxoSmithKline. 2015. Available from: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Imitrex_Injection/pdf/IMITREX-INJECTION-PI-PPI-PIL-COMBINED.PDF. Accessed October 24, 2015. | ||
Imitrex® (sumatriptan succinate) tablets [prescribing information] [webpage on the Internet]. Research Triangle Park: GlaxoSmithKline. 2015. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Imitrex_Tablets/pdf/IMITREX-TABLETS-PI-PIL.PDF. Accessed October 24, 2015. | ||
Imitrex® (sumatriptan succinate) nasal spray [prescribing information] [webpage on the Internet]. Research Triangle Park: GlaxoSmithKline. 2015. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Imitrex_Nasal_Spray/pdf/IMITREX-NASAL-SPRAY-PI-PIL.PDF. Accessed October 24, 2015. | ||
Moore KH, Hussey EK, Shaw S, Fuseau E, Duquesnoy C, Pakes GE. Safety, tolerability, and pharmacokinetics of sumatriptan in healthy subjects following ascending single intranasal doses and multiple intranasal doses. Cephalalgia. 1997;17(4):541–550. | ||
Saracco MG, Allais G, Tullo V, et al. Efficacy of frovatriptan and other triptans in the treatment of acute migraine of normal weight and obese subjects: a review of randomized studies. Neurol Sci. 2014;35(suppl 1):S115–S119. | ||
Visser WH, de Vriend RH, Jaspers NH, Ferrari MD. Sumatriptan-nonresponders: a survey in 366 migraine patients. Headache. 1996;36(8):471–475. | ||
United States Food and Drug Administration [webpage on the Internet]. Clinical Pharmacology Review for MAXALT-MLT®; 2011. Available from: http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm289415.pdf. Accessed October 24, 2015. | ||
Winner PK, Brandes JL, Lener SE, Derosier F, White J, McDonald SA. Evaluation of the relationship body mass index (BMI) to response and tolerability after treatment with a single fixed-dose tablet of sumatriptan 85mg formulated with RT technology/naproxen sodium 500mg (SumaRT/Nap) for the acute treatment of migraine. Presented at the 50th Annual Scientific Meeting of the American Headache Society (Poster S19). Headache. 2008;48(suppl 1):S47. | ||
Cosson VF, Fuseau E. Mixed effect modeling of sumatriptan pharmacokinetics during drug development: II. From healthy subjects to phase 2 dose ranging in patients. J Pharmacokinet Biopharm. 1999;27(2):149–171. | ||
Landy SH, McGinnis JE, McDonald SA. Pilot study evaluating preference for 3-mg versus 6-mg subcutaneous sumatriptan. Headache. 2005;45(4):346–349. | ||
Mathew NT, Dexter J, Couch J, et al. Dose ranging efficacy and safety of subcutaneous in the acute treatment of migraine. Arch Neurol. 1992;49(12):1271–1276. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







