Back to Journals » Journal of Pain Research » Volume 15
The Effect of Ultrasound-Guided Low Serratus Anterior Plane Block on Analgesia and Quality of Recovery After Robot-Assisted Thymectomy via Subxiphoid Approach: Study Protocol for a Randomized Controlled Trial
Authors Fu Y , Fu H, Lu Y, Lv X
Received 1 February 2022
Accepted for publication 26 March 2022
Published 5 April 2022 Volume 2022:15 Pages 939—947
DOI https://doi.org/10.2147/JPR.S359638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Yu Fu,1,* Huimin Fu,2,* Yugang Lu,1 Xin Lv1
1Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Anesthesiology, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yugang Lu; Xin Lv, Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, No. 507 Zhengmin Road, Shanghai, 200433, People’s Republic of China, Tel/Fax +86 021 65115006, Email [email protected]; [email protected]
Purpose: It is pivotal to optimize perioperative analgesia in order to fit a transition in the surgical approach for removing mediastinal tumors, from sternotomy to trans-subxiphoid robotic thymectomy (TRT). Serratus anterior plane block (SAPB) is a safe, effective and easy to perform analgesic technology, which could provide analgesia in thoracic and upper abdominal surgery. However, the efficacy of SAPB analgesia in the patients undergoing TRT is unclear and has never been described in scientific literature. Therefore, this study aimed to determine the effect of ultrasonic-guided low SAPB on analgesia and the quality of recovery (QoR) following the TRT.
Study Design and Methods: In this prospective double-blind, randomized controlled design trial, 40 adults scheduled for TRT will be randomly allocated to the low SAPB group (Group S) and placebo control group (Group C). The patient of Group S will be performed SAPB bilaterally at the level of T8–T9 under ultrasound guidance with 40 mL 0.375% ropivacaine after anesthesia induction. Group C will be administered normal saline at the same volume and timing. The primary study outcome is the global Quality of Recovery-40 (QoR-40) score on postoperative days (POD) 1. The secondary endpoints are numeric rating scale (NRS) scores and sufentanil consumption at different time points after surgery, QoR-40 scores on POD 2, 30 and 90, chronic pain at POD 90, and the incidence of perioperative adverse events.
Discussion: This is the first prospective trial to shed light on the efficacy of ultrasonic-guided low SAPB on postoperative pain and recovery after TRT. The findings will provide a new strategy of perioperative pain management for TRT.
Keywords: serratus anterior plane block, robot-assisted thoracic surgery, subxiphoid, thymectomy, postoperative analgesia
Introduction
In recent years, advancements in operative technology have rendered the surgical approach for thymectomy to develop rapidly. Compared with median sternotomy, the traditional standard surgical approach, minimally invasive approaches have the characteristics of less trauma, fewer surgical complications and rapider postoperative recovery.1–4 Within those approaches, the robotic subxiphoid approach is a promising technique since it can provide an advanced visualization of the bilateral phrenic nerves, the upper pole of the thymus, the anterior and superior mediastinum.5,6 What’s more, the advantages of robotic-assisted surgical system, including a three-dimensional, high-definition view, articulating endo-wristed instruments, and filtration of physiologic hand tremors, which is crucial for removing mediastinal tumors in a narrow area.7,8
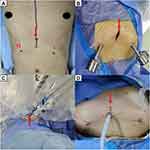
During trans-subxiphoid robotic thymectomy (TRT), a 3-cm subxiphoid incision is created to insert into a camera, and two additional trocars are inserted bilaterally below the costal margin (Figure 1).9,10 Despite the degree of trauma and postoperative pain in TRT is far lower than that in open surgery,10 the above-mentioned incisions can still cause postoperative pain in patients. Inadequate pain management after thoracic surgery is associated with increased risk of postoperative complications and length of stay.11 In addition, it has an impact on quality of recovery, patient satisfaction and chronic post-surgical pain.12,13
It is pivotal to optimize perioperative analgesia in order to fit a transition in the surgical approach to thymectomy, from sternotomy to minimally invasive surgery (MIS). Serratus anterior plane block (SAPB) is a kind of fascial plane block, which can provide analgesia to the T2 and T9 thoracic dermatomes via blocking the lateral cutaneous branches of the intercostal nerves passing through these planes.14 The SAPB is a safe, effective and easy to perform analgesic technology.15 The application of low SAPB has been demonstrated to reduce opioid consumption and increase patient’s satisfaction with postoperative analgesia in liver surgery and laparoscopic cholecystectomy.16,17 However, the efficacy of low SAPB analgesia in the patients undergoing TRT is unclear and has never been described in scientific literature.
Therefore, we have decided to conduct a randomized controlled trial to evaluate the analgesic efficacy of low SAPB for TRT in our institution, and to determine the impact of low SAPB on the quality of recovery (QoR) after TRT.
Materials and Methods
Study Design
This is a single-center, prospective double-blind, randomized controlled trial, which is designed following the SPIRIT 2013 statement.18 The trial was approved by the Clinical Research Ethics Committee of Shanghai Pulmonary Hospital and has been registered at chictr.org.cn on 6 January 2022 (identifier: ChiCTR2200055315). During the whole period of this study, all procedures will be conducted in accordance with the “Declaration of Helsinki.” The flowchart diagram of the study is illustrated in Figure 2, and the SPIRIT figure of enrolment, interventions, and assessments is presented in Table 1.
 |
Table 1 Trial Schedule |
 |
Figure 2 Flowchart of the study design. Abbreviations: ASA, American Society of Anesthesiologists; SAPB, serratus anterior plane block; TRT, trans-subxiphoid robotic thymectomy. |
Study Objective
The aim of our study is to evaluate the efficacy of low SAPB on postoperative pain and QoR in patients undergoing TRT.
Participants
Patients will be sifted according to the inclusion/exclusion criteria. Subjects eligible to participate in this study should meet all the following criteria: (1) age ≥18 years, (2) American Society of Anesthesiologists (ASA) physical status classification I-III, and (3) scheduled for elective TRT.
The exclusion criteria were as follows: (1) contraindication to regional anesthesia, (2) bleeding disorder, (3) history of opiate abuse, (4) infection or eczema at the injection site, (5) known psychiatric and neurologic disorders, (6) pre-existing chronic pain, and (7) the condition of the patients with myasthenia gravis was not stable.
Informed Consent
All suitable participants will be approached one day before surgery on the ward. The investigator will invite the patients to participate according to inclusion/exclusion criteria. The objective, procedure, methods of follow-up, benefits and potential risks of this trial will be explained to the patient by understandable language. Every participant will sign an informed consent document after decides to participate in the study voluntarily. Moreover, patients who participate in the study will have the right to obtain all relevant information, and they will have the opportunity to withdraw from the study at any time during the study.
Randomization and Blinding
Once informed consent has been received and participants will be randomized to the low SAPB group (Group S) and placebo control group (Group C) at a 1:1 ratio. Random numbers will be generated by an independent research investigator using IBM SPSS statistical software package V.26 (SPSS). The patient study code number and group allocation were placed in the envelopes and sealed. A 40-mL syringe labeled “research solution” consisting of 0.5% ropivacaine or normal saline will be prepared by an independent research investigator according to the group allocation. There was no difference in color between the ropivacaine and normal saline. Allocation is blinded for the anesthesiologist, participants, and statisticians, which will be maintained until completion of the final analyses.
Interventions
General Anesthesia and Postoperative Analgesia
Patients will receive standard perioperative care according to our institutional protocol. All patients will be undergoing right internal jugular vein catheterization and be monitored with electrocardiogram (ECG), heart rate (HR), pulse oximetry (SpO2), and non-invasive blood pressure (BP). General anesthesia induction will be performed using standard doses of midazolam, propofol, sufentanil, and rocuronium bromide. When the patient loses consciousness, double-lumen tracheal intubation will be performed. After intubation, all blocks will be performed according to the randomized grouping allocation by experienced anesthesiologists who are familiar with the low SAPB. Anesthesia will be maintained with total intravenous anesthesia to maintain the bispectral index monitor at 40–60. Muscle relaxation will be maintained by intermittent injections of rocuronium bromide as needed. The anesthetic, fluid volume, infusion speed and transfusion will be adjusted according to hemodynamic monitoring conditions to maintain the hemodynamic parameters within 20% of the preoperative baseline values. The tidal volume during the single-lung ventilation will be set to 5 to 6 mL/kg and the breathing frequency was adjusted to maintain PETCO2 at 35 to 45 mm Hg. Surgery will be performed by a fixed surgical team using the da Vinci S system (Surgical Intuitive, Mountain View, CA, USA) with three arms. Neuromuscular blockade will be reversed at the end of surgery with neostigmine (0.04 mg/kg) and atropine (0.02 mg/kg). After extubation, all patients will be transferred to the post-anesthesia care unit (PACU) for continuous monitoring of vital signs. Patients will be discharged to a surgical ward with a full evaluation of conscious state and vital signs.
Patients will receive intravenous analgesia with patient-controlled intravenous analgesia (PCIA) during the first 48 hours after surgery. The PCIA regimen is consisted of administration of a total of 100 mL of 1 μg/mL sufentanil in saline. The PCIA device will be programmed to provide 2 μg boluses on demand, with a lockout period of 15 minutes and a background infusion at the rate of 2 mL/hour. If a patient feels pain, the PCIA button could be push repeatedly until feeling relief. In the case that the pain relief is inadequate after a top-up dose (NRS score >4), a rescue analgesic will be provided via intravenous administration of 50 mg flurbiprofen, and it could be repeated if necessary. The maximum daily dose of flurbiprofen should not exceed 200 mg. Decisions about all other aspects of patient postoperative care, including administration of fluids, antibiotic use and enteral nutrition will be made by the attending physician according to the expertise and routine clinical practice.
Low Serratus Anterior Plane Block
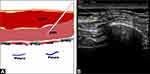
Low SAPB (T8–T9) will be performed bilaterally on each patient by experienced anesthesiologists under ultrasound guidance after anesthesia induction.16 The linear ultrasound transducer (8–12 MHz) will be placed over the mid-clavicular region of the thoracic cage in a sagittal plane, with the patients in the supine position. The ribs will be counted inferiorly and laterally. Then the scanning will be performed in the transverse plane starting at anterior axillary line at the level of 8–9th intercostal space and then moving posteriorly toward the midaxillary lines to identify the serratus anterior muscle. Supplementary Doppler mode was used to differentiate the nerve from vascular structures. If no blood is retracted during aspiration, a blinded solution of 20 mL 0.375% ropivacaine or 20 mL 0.9% saline (placebo) will be injected in the plane deep to serratus anterior muscle through the needle (22G, 80mm, UniPlex®, NanoLine®, Germany) using an in-plane approach (Figure 3). The procedure will be repeated bilaterally. Thus, the total amount of ropivacaine injected will be 150 mg. This amount will be reduced in patients weighing less than 50 kg so as not to exceed the dose of 3 mg/ kg. A successful spread of local anesthetic is defined as a hypoechoic ellipsoid with a well-defined margin beneath the serratus anterior muscle on ultrasonic view. Conversely, local anesthetic deposition primarily within muscle will be considered a block failure.
Follow-Up
The follow-up will be completed by an investigator blinded to the group allocation. All patients will be visited from the day before the operation to 90 postoperative days (POD). The pain intensity at rest and during coughing will be measured using an 11-point numeric rating scale (NRS).
Primary Endpoint
The primary study outcome is the global Quality of Recovery-40 (QoR-40) score on POD 1. The QoR-40 contains 40 items assessing five dimensions of recovery: emotions, physical comfort, psychological support, physical independence, and pain,19 which scores range from 40 to 200. The QoR-40 has been widely validated for both clinical and research use in different surgical types and anesthetic techniques.20,21
Secondary Endpoints
The secondary outcomes will be as follows:
- NRS scores at rest and with movement at 6 h, 12 h, 24 h and 48 h postoperatively.
- Cumulative opioid consumption at 6h, 12h, 24h and 48h postoperatively.
- Total number of times that patients pressed the PCIA button at POD 1 and POD 2, including effective presses and ineffective presses.
- Total consumption of analgesic drugs (type and dosage) except opioid within POD 1 and POD 2.
- QoR-40 scores at POD 2, 30, and 90.
- Assessment of chronic pain at POD 90.
Other Endpoints
The other outcomes include:
- Complications related to SAPB, such as respiratory depression and wound infection.
- Opioid-related adverse effects such as postoperative nausea and vomiting (PONV), sedation, and mental status changes.
- Other adverse effects, including but not limited to dizziness, urinary retention, incision infection, pneumothorax, pleural effusion, atelectasis, the injury of thoracic and abdominal organs.
Safety Consideration
This study will strictly adhere to the operation process and all study-related adverse events will be closely monitored. In particular, the occurrence of local anesthetic systemic toxicity is related to large volumes of local anesthetic agents. The regional nerve block will be administered under the closely monitoring of vital signs in the operating room, which enables detection and treatment of adverse events immediately. Administration of ropivacaine will be immediately ceased in cases when any adverse events associated with the ropivacaine set in. Furthermore, any unexpected adverse events that arise from this trial will be carefully managed and documented, it also will be reported to the principal investigator, the surgical consultant and the relevant hospital patient safety board immediately.
Sample Size Calculation
The established minimum clinically important difference in the global QoR-40 score after surgery is 10.22,23 Based on our pilot study, the mean ± SD QoR-40 score in a population of patients undergoing trans-subxiphoid thoracoscopic thymectomy was 168 ± 10. Assuming α = 0.05 and β = 0.20 for a 10-point difference in global QoR between groups, the calculated sample size was 17 patients per group. The sample size was calculated with the free software G*Power (Available at: https://stats.oarc.ucla.edu/other/gpower/). This figure will be increased to 20 in each group to compensate for possible dropouts. Therefore, 40 patients will be enrolled in this study.
Statistical Analysis
All statistical analyses will be performed using Statistical Product and Service Solutions (SPSS) version 26.0 (IBM SPSS Inc., Chicago, IL, USA). The data will be expressed as frequency (percentage) for qualitative data, as mean (standard deviation, SD) for normally distributed quantitative data, or as median (interquartile range, IQR) for non-normally distributed quantitative data, as appropriate. Normally distributed data will be analyzed with an independent sample t-test (Student’s t-test) or one-way ANOVA between groups or repeated measures ANOVA within groups. Post hoc multiple comparisons will be conducted using the Bonferroni method when significant interactions are detected using ANOVA. Non-normally distributed data will be analyzed using the Mann-Whitney U-test. Categorical data will be compared by the chi-square test or Fisher’s exact test. Two-tailed p values of less than 0.05 will be considered statistically significant.
Data Management and Monitoring
All original data will be recorded in case report forms, which will be filed in numerical order and kept in a secure filing cabinet. The study supervisor (Yugang Lu) will supervise the conduct of the trial conduction and perform monthly audits of the trial. If necessary, the datasets will be available from the chief investigator upon reasonable request.
Discussion
With its advantages of remarkable surgical exposures, precise dissection and well ergonomics for the operator, robot assisted thymectomy via the subxiphoid approach increasingly gain attention.6,10 Surgical incisions of this approach can be seen as focused on the upper abdominal wall. Firstly, an approximately 3 cm skin incision is made below the xiphoid process, where the camera port is inserted into. Then, two additional trocars are put bilaterally below the costal arch, where the da Vinci arms are fitted. Anatomically, these involved incisions range from the T6 to T10 intercostal nerves. The technology of TRT has been performed in our institution since February 2021, while the improvement of analgesic management appropriate for it is a challenging and basic requirement. Pain after robot-assisted thoracic surgery (RATS) may cause the inhibition of deep respiration, coughing and clearing of excess secretions, which will promote the development of atelectasis and pneumonia.24
The traditional analgesic method for thoracic and abdominal surgeries is epidural analgesia (EA), but application of which is limited owing to potential multitude of complications and strict requirements on coagulation function.25 Besides, as MIS becomes more pervasive for the treatment of thoracic tumors, the necessity for EA in MIS has been questioned.26 SAPB is thought to be a promising muscle plane block due to its easy performance and low requirement to the function of blood coagulation.14,27
The serratus anterior muscle is a superficial and easily identified muscle, originating from the anterolateral surfaces of the 1st to 9th ribs and terminates at the spinal margin of the scapula.28 The SABP was first put forward by Blanco et al14 which could provide analgesia to the anterolateral chest and abdominal wall by block the lateral cutaneous branches of the thoracic intercostal nerves (T2–T9). Daga et al29 performed SAPB at the level of 4–5th intercostal space to seven embalmed cadavers, the observed spread of ultrasound contrast ranged from T3 to subcostal space in the most cases. Owing to easy to perform, the low probability of pneumothorax and local anesthetic poisoning, the SAPB has been gradually applied in clinical practice.30 Given that innervation of incisions of TRT is derived from T6 to T10, low bilateral SAPB at T8–T9 levels be used for analgesia after TRT. Based on our previous studies, the patient who received the low SAPB with ropivacaine 0.375% had superior pain relief at 24 hours after surgery. Taking this evidence further, the quality of recovery of patients in the low SAPB group are expected to be higher than those in the placebo control group.
Therefore, we are conducting this prospective, randomized control trial among patients undergoing TRT, to evaluate the effect of low SAPB on analgesia and QoR following the TRT. To the best of our knowledge, this study will be the first to investigate the efficacy of low SAPB for postoperative pain and recovery of patients undergoing TRT. What’s more, this study will explore the effect of low SAPB for chronic pain after the surgery. Our results may promote the application of SAPB in patients who undergo TRT acting as an appropriate method to improve postoperative pain management and promote recovery.
Trial Status
This study is currently at the patient enrolment and data collection stage. The current version of the study protocol is version 1.1 and was approved on 6 January 2022. The first participant was recruited on 10 January 2022, and the anticipated completion date will be 1 January 2024.
Acknowledgments
The authors acknowledge Professor Deping Zhao and his surgical team in the Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, for his help in this project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Yu Fu and Huimin Fu contributed equally to this work.
Funding
This work was supported by grants from the following: Clinical Research Plan of SHDC (SHDC2020CR3044B), Shanghai “Rising Stars of Medical Talent” Youth Development Program: Outstanding Youth Medical Talents, and Program of Shanghai Academic Research Leader No.21XD1402800.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Erşen E, Kılıç B, Kara HV, et al. Comparative study of video-assisted thoracoscopic surgery versus open thymectomy for thymoma and myasthenia gravis. Wideochir Inne Tech Maloinwazyjne. 2018;13(3):376–382. doi:10.5114/wiitm.2018.75835
2. Imielski B, Kurihara C, Manerikar A, et al. Comparative effectiveness and cost-efficiency of surgical approaches for thymectomy. Surgery. 2020;168(4):737–742. doi:10.1016/j.surg.2020.04.037
3. Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg. 2015;4(6):495–508. doi:10.3978/j.issn.2225-319X.2015.08.01
4. O’sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg. 2019;8(2):174–193. doi:10.21037/acs.2019.02.04
5. Lu Q, Zhao J, Wang J, et al. Subxiphoid and subcostal arch “Three ports” thoracoscopic extended thymectomy for myasthenia gravis. J Thorac Dis. 2018;10(3):1711–1720. doi:10.21037/jtd.2018.02.11
6. Zhang H, Chen L, Zheng Y, et al. Robot-assisted thymectomy via subxiphoid approach: technical details and early outcomes. J Thorac Dis. 2018;10(3):1677–1682. doi:10.21037/jtd.2018.03.07
7. Ashrafian H, Clancy O, Grover V, et al. The evolution of robotic surgery: surgical and anaesthetic aspects. Br J Anaesth. 2017;119:i72–i84. doi:10.1093/bja/aex383
8. Zirafa C, Melfi F. Robot-assisted surgery for posterior mediastinal mass. J Thorac Dis. 2017;9(12):4929–4931. doi:10.21037/jtd.2017.10.160
9. Kang C, Na K, Song J, et al. The robotic thymectomy via the subxiphoid approach: technique and early outcomes. Eur J Cardiothorac Surg. 2020;58:i39–i43. doi:10.1093/ejcts/ezaa006
10. Chen K, Zhang X, Jin R, et al. Robot-assisted thoracoscopic surgery for mediastinal masses: a single-institution experience. J Thorac Dis. 2020;12(2):105–113. doi:10.21037/jtd.2019.08.105
11. Elmore B, Nguyen V, Blank R, et al. Pain management following thoracic surgery. Thorac Surg Clin. 2015;25(4):393–409. doi:10.1016/j.thorsurg.2015.07.005
12. Bayman E, Lennertz R, Brennan T. Pain-related limitations in daily activities following thoracic surgery in a United States population. Pain Physician. 2017;20(3):E367–E378. doi:10.36076/ppj.2017.E378
13. Zhang W, Cong X, Zhang L, et al. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med. 2020;10(3):e38. doi:10.1002/ctm2.38
14. Blanco R, Parras T, Mcdonnell J, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi:10.1111/anae.12344
15. Mayes J, Davison E, Panahi P, et al. An anatomical evaluation of the serratus anterior plane block. Anaesthesia. 2016;71(9):1064–1069. doi:10.1111/anae.13549
16. Wu Y, Yang W, Cai Z, et al. The effect of ultrasound-guided low serratus anterior plane block on laparoscopic cholecystectomy postoperative analgesia: a randomized clinical trial. Medicine. 2021;100(44):e27708. doi:10.1097/MD.0000000000027708
17. Tao K, Xu H, Zhu C, et al. Serratus anterior plane block catheter for hepatectomy: a method to decrease opioid use perioperatively. J Clin Anesth. 2020;61:109682. doi:10.1016/j.jclinane.2019.109682
18. Chan A, Tetzlaff J, Gøtzsche P, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
19. Myles P, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
20. Gornall B, Myles P, Smith C, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi:10.1093/bja/aet014
21. Leslie K, Troedel S, Irwin K, et al. Quality of recovery from anesthesia in neurosurgical patients. Anesthesiology. 2003;99(5):1158–1165. doi:10.1097/00000542-200311000-00024
22. Myles P, Hunt J, Nightingale C, et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg. 1999;88(1):83–90. doi:10.1213/00000539-199901000-00016
23. Abdallah F, Morgan P, Cil T, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120(3):703–713. doi:10.1097/ALN.0000436117.52143.bc
24. Loop T. Does thoracic epidural anaesthesia constitute over-instrumentation in video-and robotic-assisted thoracoscopic lung parenchyma resections? Curr Opin Anaesthesiol. 2021;34(2):199–203. doi:10.1097/ACO.0000000000000975
25. Pöpping D, Elia N, Van Aken H, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259(6):1056–1067. doi:10.1097/SLA.0000000000000237
26. Zeltsman M, Dozier J, Vaghjiani R, et al. Decreasing use of epidural analgesia with increasing minimally invasive lobectomy: impact on postoperative morbidity. Lung Cancer. 2020;139:68–72. doi:10.1016/j.lungcan.2019.11.001
27. Abdallah FW, Cil T, Maclean D, et al. Too deep or not too deep?: a propensity-matched comparison of the analgesic effects of a superficial versus deep serratus fascial plane block for ambulatory breast cancer surgery. Reg Anesth Pain Med. 2018;43(5):480–487. doi:10.1097/AAP.0000000000000768
28. Lung K, Lucia K, Lui F. Anatomy, thorax, serratus anterior muscles. In: StatPearls. Treasure Island (FL):StatPearls Publishing; 2022.
29. Daga V, Narayanan M, Dedhia J, et al. Cadaveric feasibility study on the use of ultrasound contrast to assess spread of injectate in the serratus anterior muscle plane. Saudi J Anaesth. 2016;10(2):198–201. doi:10.4103/1658-354X.168825
30. Liu X, Song T, Xu H, et al. The serratus anterior plane block for analgesia after thoracic surgery: a meta-analysis of randomized controlled trails. Medicine. 2020;99(21):e20286. doi:10.1097/MD.0000000000020286
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.