Back to Journals » Journal of Pain Research » Volume 14
The Effect of Ultrasound-Guided Erector Spinae Plane Block versus Thoracic Epidural Block on Postoperative Analgesia After Nuss Surgery in Paediatric Patients: Study Protocol of a Randomized Non-Inferiority Design Trial
Authors Ren Y, Zheng T, Hua L, Zhang F, Ma Y, Zhang J
Received 4 August 2021
Accepted for publication 23 September 2021
Published 28 September 2021 Volume 2021:14 Pages 3047—3055
DOI https://doi.org/10.2147/JPR.S332078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Yi Ren, Tiehua Zheng, Lei Hua, Fuzhou Zhang, Yangwei Ma, Jianmin Zhang
Department of Anesthesiology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
Correspondence: Jianmin Zhang
Department of Anesthesiology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, No. 56, South Lishi Road, Beijing, 100045, People’s Republic of China
Tel +861059616415
Fax +861059616429
Email [email protected]
Purpose: The Nuss procedure is a recognized treatment for adolescent pectus excavatum that results in severe postoperative pain. Erector spinae plane block (ESPB) is a novel technique that provides postoperative analgesia and reduces opioid consumption. Our aim is to explore whether ESPB produces analgesia similar to thoracic epidural anaesthesia (TEA) in paediatric patients undergoing Nuss procedure.
Study Design and Methods: This randomized, controlled, non-inferiority trial will enrol 300 paediatric patients undergoing Nuss surgery. Participants will be randomly assigned 1:1 to receive ESPB or TEA preoperatively. The primary, joint endpoint is the average numeric rating scale (NRS) score and cumulative sufentanil consumption. The secondary endpoints are pain scores and sufentanil consumption at different time points after surgery, analgesia-related side effects, and other postoperative complications. Data will be analysed by the intention-to-treat principle.
Discussion: This study investigates the effect of ESPB on postoperative opioid consumption and pain scores and intend to provide a new strategy of analgesia management for Nuss procedure in paediatric patients.
Keywords: postoperative pain, erector spinae plane block, pectus excavatum, Nuss procedure, opiates
Background
Pectus excavatum (PE) is characterized by depression of the anterior chest wall with pulmonary and cardiac compression. It is one of the most common congenital chest-wall deformities in children and adolescents, with an incidence of 1 in 1000 births.1,2 The Nuss procedure is the modern surgical treatment for the correction of PE, which provides an immediate correction of the chest wall defect by a convex bar placed under the sternum through two lateral thoracic incisions and guided across the mediastinum with thoracoscopy3 and has favourable outcomes in children and young adults.4 Although it is “minimally” invasive, immediate remodelling of the chest wall causes severe and prolonged postoperative pain, which is usually the most important issue during the patient’s postoperative hospital course.5 Thus, optimized perioperative pain management of patients undergoing the Nuss procedure is a challenge.
Thoracic epidural analgesia (TEA) has long been the gold standard of analgesia for thoracic surgeries6 because of its consistent superiority in analgesia and opioid sparing, as demonstrated by many studies.7–9 However, as an invasive procedure, TEA carries risks of failure and severe complications, such as neurological deficits or epidural haematoma, and has contraindications, including coagulation disorders and spinal deformities.10,11 Taking these issues into consideration, many institutions have utilized alternative regional anaesthesia techniques instead of epidural techniques.12 However, the evidence demonstrating the superiority of one technique compared to another for pain management is controversial.13
Erector spinae plane block (ESPB) is a newly defined fascial plane block technique.14 In this procedure, local anaesthetic injection is performed beneath the erector spinae muscle but superficial to the transverse process. A cadaveric study showed that the spread of the dye involved both the ventral and dorsal rami of the spinal nerves, causing a sensory blockade over the anterolateral thorax.15 The block is capable of covering dermatomes T2 to T10 depending on the dermatomal level placement and the volume injected.6 A retrospective chart review reported the efficacy of ESPB for a broad spectrum of surgeries involving incisions from T1 to L4.16 ESPB is not performed as closely towards the pleura, spinal cord, nerves, or major blood vessels, and thus there are no structures at risk of needle injury in the immediate vicinity.14 It is also safer in terms of anticoagulation than neuraxial techniques and avoids potential epidural haematoma formation.17
In a systematic review and meta-analysis, ESPB was found to be more effective than systemic analgesia for controlling acute pain following breast surgery.18 Two randomized trials reported that single-injection ESPB provided effective analgesia management and enhanced the quality of recovery after VATS.19,20 Compared with TEA, ESPB provided a comparable pain score after adult cardiac surgery.21 However, published evidence for the efficacy of ESPB in paediatric patients is relatively insufficient. Apart from case reports and small series,22–26 the literature regarding paediatric use is currently limited. Although one randomized trial reported that in paediatric patients undergoing splenectomy, ESPB reduced postoperative pain scores compared with those of sham controls,27 there is a gap of evidence in directly comparing the analgesic efficacy between ESPB and the gold standard TEA in Nuss surgery. Therefore, we have decided to conduct a non-inferiority trial to compare the effect of ESPB and TEA in our institution, where large number of PE patients come for NUSS surgery. The primary outcome will be a joint endpoint of opioid consumption and pain NRS scores assessed in a joint hypothesis-testing framework.
Method
Objective
The primary objective is to test the hypothesis that ESPB is non-inferior to TEA in paediatric patients undergoing Nuss surgery. Non-inferiority will be assessed in terms of the NRS and sufentanil consumption.
Study Design
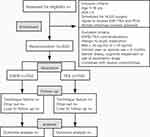
This is a prospective, randomized controlled non-inferiority trial. The study design was completed in accordance with the SPIRIT 2013 statement. The trial was approved by the Clinical Research Ethics Committee of Beijing Children’s Hospital (IEC-C-008-A08-V.05.1) and has been registered in the Chinese Clinical Trials registry (identifier: ChiCTR2100046210) with the latest version 1.1. During the whole period of the study, we will strictly adhere to Good Clinical Practice guidelines and the Declaration of Helsinki. The flowchart diagram of the study is illustrated in Figure 1, and the SPIRIT figure of enrolment, interventions, and assessments is presented in Table 1.
 |
Table 1 Trial Schedule of Enrolment, Interventions, and Assessments |
Randomization and Blinding
After assignments, participants will be randomly divided into the following two groups before induction of anaesthesia: (1) ESPB group and (2) TEA group, according to a 1:1 ratio. Random numbers will be generated by a third-party biostatistician using the SAS 9.3 software package (SAS Institute, Cary, NC, USA). The anaesthesiologists who will perform epidural or nerve blocks and the researchers designated for postoperative follow-up are independent individuals. After the data collection, the allocation will be revealed for statistical analysis. However, it is not possible to conduct a double-blinded design, as the differences between the ESPB and TEA will be readily obvious during the study period.
Participants
Patients will be screened according to the inclusion/exclusion criteria. Subjects eligible to participate in this study should meet all the following criteria: (1) age between 7 and 18 years of age, (2) American Society of Anesthesiologists (ASA) physical status classification I–II, (3) scheduled for elective Nuss surgery, (4) agreement to receive ESPB/TEA and parent-controlled intravenous analgesia (PCIA), and (5) written informed consent obtained before enrolment.
The exclusion criteria were as follows: (1) regional block or epidural analgesia contraindications, including abnormal coagulation status, haemorrhagic diseases, local infection, pre-existing neurological deficits of the torso or lower limbs, and spinal disease; (2) allergy to local anaesthetics or other study medication; (3) BMI > 30 kg/m2 or < 15 kg/m2); (4) chronic pain or opioid use over 3 months; (4) mental illness, cognitive impairment or use of psychiatric drugs; (5) severe comorbidities, including but not limiting to renal dysfunction, liver dysfunction and heart failure; and (6) inability to use a postoperative analgesia pump.
Intervention
General Anaesthesia and Postoperative Analgesia
Patients will receive standard perioperative care according to our institutional protocol. After the establishment of standard monitoring (non-invasive blood pressure, electrocardiogram, and oxygen saturation) and intravenous access, general anaesthesia will be induced with 0.4–0.5 μg/kg sufentanil, 2.0–3.0 mg/kg propofol and 0.1 mg/kg cisatracurium. After endotracheal intubation, TEA or ESPB will be performed according to the randomized grouping allocation by experienced anaesthesiologists who will not take part in the follow-up. Anaesthesia will be maintained by sevoflurane inhalation to maintain the bispectral index monitor at 40–60. Muscle relaxation will be maintained by intermittent injections of cisatracurium as needed. The anaesthetic, fluid volume, infusion speed and transfusion will be adjusted according to haemodynamic monitoring conditions to maintain the haemodynamic parameters within 20% of the preoperative baseline values. Surgical procedures will be performed by a fixed surgical team. At the end of the procedure, residual neuromuscular blockade will be antagonized with 40 μg/kg neostigmine and 20 μg/kg atropine. After removal of the tracheal tube, all patients will be admitted to the post-anaesthesia care unit (PACU). Patients will be discharged to a surgical ward with a full evaluation of conscious state and vital signs. After the surgery, all participants in the two groups will receive PCIA. Electronic PCIA pumps (CPE-101, Fornia Medical Equipment Co., Ltd, Zhuhai, China) will be used within 48 h after surgery. The PCIA regimen is as follows: sufentanil 0.04 μg/kg/mL, 1 mL of loading volume, 0.02 μg/kg/h of background infusion, a bolus of 0.04 μg/kg on demand, with a 15 min lock-out interval, and 0.1 mg/kg tropisetron for prophylaxis of postoperative nausea and vomiting. Parents will be trained to use the analgesia pump. Pain will be assessed by the nurse via the NRS.
Erector Spinae Plane Block
ESPB will be performed under ultrasound guidance after anaesthesia induction. Patients in this group will be placed in the lateral position and scanned by a high-frequency (4–15 MHz) linear transducer (Labat SP; Wisonic, Shenzhen, China), placed longitudinally 2–3 cm lateral to the T5 spinous process to achieve visualization of the erector spinae, rhomboid muscle and trapezius muscle. Under aseptic conditions, a 21-gauge block needle (5-cm, Hakko disposable monopolar nerve blockage needle, Hakko Co., Ltd, Nagano, Japan) will be inserted in-plane into the ultrasound beam from the cranial-to-caudal direction until the tip of the needle contacts the tip of the T5 transverse process. Once the correct location is confirmed by hydrodissection of the interfascial plane with 2 mL of saline solution, a bolus of 0.3% ropivacaine in the volume of 0.5 mL/kg (max 20 mL each side) will be injected into the block, with aspiration every 5 mL per injection in case of accidental puncture of the vessel or pleura. The procedure will be repeated bilaterally. A successful injection is defined as a hypoechoic ellipsoid with a well-defined margin beneath the erector spinae muscle on ultrasonic view.
Thoracic Epidural Analgesia
The thoracic epidural will be performed by two experienced consultant anaesthesiologists using the conventional landmark-guided technique in line with currently accepted practice. Under strict aseptic precautions, the epidural space will be administered at the T6/T7 intervertebral space; the exact level will be determined by the anaesthesiologist. After confirming the absence of blood or cerebrospinal fluid upon epidural catheter aspiration, a 0.1 mL/kg (maximum: 3 mL) test dose of lidocaine (1.5%) will be administered to rule out intravascular injection and unintentional intrathecal injection. Then, a loading dose of ropivacaine 0.3% in a volume of 0.5 mL/kg (maximum: 20 mL) will be administered. Once the single injection of epidural is completed, the patient will be positioned for surgery.
Endpoints
Primary Endpoint
The primary outcome is the joint endpoint of pain intensity and postoperative cumulative opioid consumption. The pain intensity will be measured by a pain nurse (blinded to the study) using the 11-point NRS (0 = no pain and 10 = worst pain), which is one of the most widely used scales to assess self-reported pain intensity in children aged between 7 and 18 years.28 An average of pain scores at rest and with movement at 24 h will be calculated for each patient. The 24 h cumulative opioid consumption data expressed as millilitres will be extracted from the electronic PCIA pump.
Secondary Endpoint
The secondary outcomes include: (1) Cumulative opioid consumption at 1 h, 3 h, 6 h, 12 h, 24 h and 48 h postoperatively; the numbers of required and administered boluses from the PCIA pump at 1 h, 3 h, 6 h, 12 h, 24 h and 48 h postoperatively. (2) NRS scores at rest and with movement at 0 h (extubation), 1 h, 3 h, 6 h, 12 h, 24 h and 48 h postoperatively.
The other endpoints are defined as follows:
(1) Overall incidence of postoperative complications (from the end of surgery to discharge), including the following:
a. Complications related to analgesic techniques, including respiratory depression, wound infection, epidural abscess, and neurological complications.
b. Opioid-related side effects such as nausea, vomiting, sedation, mental status changes/hallucinations, respiratory distress (recorded as respiratory rate <10 breaths/minute or oxygen saturation <90%) and need for supplemental oxygen.
c. Surgical complications, including thoracic organ injury, incision infection, pneumothorax, pleural effusion, atelectasis, subcutaneous emphysema and bar displacement.
d. Other complications, including but not limited to fever, pulmonary and extrapulmonary infection, and gastrointestinal symptoms.
(2) Time to first mobilization (hours).
(3) Return of gastrointestinal function measured as time to first a. flatus (hours); b. bowel movement (hours); c. liquid ingestion (hours); and d. solid-food ingestion (hours).
(4) Quality of postoperative recovery, as measured by the Quality of Recovery-15 (QoR-15) score on postoperative days (POD) 1 and 2.29
(5) Hospital length of stay (days).
(6) Total cost.
Safety Consideration
We will strictly adhere to the operation process to minimize the risk of adverse events that might be caused by epidural or regional nerve block. Complications of neuraxial anaesthesia include total spinal, subdural injection, nerve injury, spinal–epidural haematoma, and infection. One of the most important complications of ESPB is local anaesthetic systemic toxicity, as large volumes of local anaesthetic agents are injected. The dosing of 0.3% ropivacaine at a volume of 0.5 mL/kg (maximum: 3 mg/kg) is the current standard practice in our institution. The operation will be conducted under the extensive monitoring of vital signs and the direct presence of experts in the field who are able to provide immediate support if required. All adverse events that occur will be carefully managed, and severe adverse events will be reported to the Clinical Research Ethics Committee as soon as possible.
Data Collection
Patient demographics (age, sex, weight and height), comorbidities, important laboratory tests, instrumental examination, Haller index and prior pectus repair will be collected. Documented intraoperative data will include the number of bars, number of subperichondrial resections, duration of surgery (incision to closure), duration of anaesthesia (from induction to exit from the operating room), intraoperative opioid and other anaesthesia medication, ventilation parameters and fluid balance. Outcome data will be evaluated and recorded according to the follow-up plan at all time points. Data will be collected into the case report forms, and entry will be performed via EmpowerDataWeb ((X&Y Solutions, Inc., Boston, Massachusetts), a password-protected, secure, web-based, electronic data-capture tool. The data will be kept timely and correctly and monitored by the Clinical Research Ethics Committee, and the database will be locked after the electronic data are checked. After data entry and verification as required are completed, the case report forms will be filed in numerical order and kept in a secure filing cabinet.
Statistical Analysis
Sample Size Calculation
Our primary endpoint is the combination of the average NRS score at 24 h postoperatively and cumulative 48 h opioid consumption. The sample size was calculated based on the primary endpoint according to the non-inferiority hypothesis. In a pilot investigation of our patients, the mean (± standard deviation) NRS scores were 3.1 (± 2.2) with TEA and 3.3 (±2.4) with ESPB. A 1-point difference in NRS scores is usually considered acceptable subjective pain discrimination.30 When the non-inferiority margin of NRS was set to 1 point on the 11-point scale, 109 samples were estimated per group to achieve 90% power to detect non-inferiority using a one-sided, two-sample t-test. The mean (± standard deviation) of the cumulative 48 h sufentanil consumption was 25.1 (± 19.1) mL (each millilitre contained 0.04 μg/kg sufentanil) with TEA and 27.2 (±16.8) mL with ESPB. The non-inferiority margin (δ) was set at 30% (ie, 7.5 mL of sufentanil), which is treated as an acceptable difference in clinical practice).31 With a significance level of α = 0.05 and a power of 1-β = 90%, the sample size required to detect differences was 138 patients in each group. Hence, a greater sample of 138 participants per group will be used. Considering a 10% dropout rate, we have decided to enrol 150 participants per group. The sample size was estimated by PASS software (version 15.0; NCSS PASS, UT, USA).
Endpoint Analysis
The primary endpoint is the joint cumulative opioid consumption and average pain NRS score. The hypothesis is that (1) analgesia as measured on an NRS would be non-inferior with ESPB, and (2) opioid consumption would be non-inferior with ESPB. Both hypotheses have to involve at least non-inferiority so that we can claim that ESPB is non-inferior to TEA based on a joint hypothesis-testing framework.32 We will calculate the 95% confidence interval (CI) of the median difference in NRS scores to test the non-inferiority of ESPB compared with TEA by the Wilcoxon test, and the result will be expressed as the effect size (difference) (95% CI). As mentioned above, the non-inferiority margin of the VAS is set to 1. If the lower limit of the 95% CI for median average NRS pain scores is smaller than 1, the ESPB group will also be considered non-inferior. The non-inferiority of ESPBs regarding opioid consumption will be similarly tested by comparing the limits of a 95% CI to the non-inferiority margin of 30%, which is 7.5 mL of sufentanil. If the effect size upper limit of the one-sided 95% CI is smaller than 7.5 mL in cumulative 48 h opioid consumption, we will conclude that ESPB is non-inferior to TEA. If non-inferiority is determined for the primary outcome, the superiority of the corresponding comparison will be evaluated for each outcome using an overall α of 0.025 with Holm-Bonferroni correction for testing both outcomes (an upper limit of 97.5% CI smaller than the predefined margin for the most significant outcome and an upper limit of 95% CI for the other outcome). If superiority is detected on at least either opioid consumption or pain NRS scores, the ESPB group will be claimed to be better than TEA group.
All analyses will be performed in the intention-to-treat (ITT) population, ie, all patients will be analysed in the group to which they are randomized and will receive at least part of the study intervention. A per-protocol (PP) analysis will also be performed for the primary endpoint. All conclusions will be based on the original data.
Descriptive and Analytical Statistics
For continuous data, the Shapiro–Wilk test will be used for quantitative analysis of the data distribution. Data with a normal distribution will be expressed as the mean ± standard deviation (SD). Non-normally distributed data will be presented as the median (interquartile range, IQR). Categorical data will be reported as numbers (percentages). For comparing baseline data and outcome variables with a normal distribution, an independent sample t-test (Student’s t-test) will be used. For those with a non-normal distribution, the Mann–Whitney U-test will be used. Categorical data will be compared by the chi-square test or Fisher’s exact test. Time-to-event data will be analysed by the Kaplan-Meier estimator, with the difference between groups tested by the log-rank method. Two-tailed p values of less than 0.05 will be regarded as statistically significant. Additionally, a two-way repeated-measures analysis of variance using a Bonferroni correction for multiple comparisons will be used to evaluate postoperative pain scores up to 48 h after surgery. For the cumulative opioid consumption at different times, the numbers of required and administered boluses from the PCIA pump and the pain NRS scores at 1 h, 6 h, 18 h, 24 h and 48 h after surgery, repeated-measures two-factor analysis of variance (ANOVA) will be performed if normality and homogeneity of variance and sphericity hypotheses (Mauchly’s test) are met. If not, one-way ANOVA and its correction (Greenhouse-Geisser coefficient correction and Huynh-Feldt coefficient correction) as well as a generalized estimated equation (GEE) model will be performed. Post hoc multiple comparisons will be conducted using the Bonferroni method when significant interactions are detected using ANOVA.
The statistical analyses will be performed with the SPSS 25.0 statistical package (IBM SPSS Inc., Chicago, IL, USA).
Missing Values
Missing data will not be replaced. Mixed models will be used in the analysis of repeated data to avoid deleting subjects with any missing values.
Discussion
The Nuss procedure is incorporated by high-volume centres around the world, including our own. However, as opposed to most minimally invasive versions of an operation, the immediate recovery period has been associated with significant and prolonged postoperative pain.33 In the past, our lack of knowledge on postoperative analgesia and serious shortage of medical personnel with relevant qualifications have caused great deficiencies in the field of postoperative analgesia for Chinese paediatric patients, seriously affecting their physical and mental recovery. Now, we are gradually realizing that pain management following the Nuss procedure is a challenging and basic requirement for the clinical care of youths.
ESPB is thought to be a promising interfascial block due to its easy performance.17 The preliminary results of ESPB in terms of analgesic effects are promising.34–37 However, there have been limited studies of its clinical efficacy compared with the traditional gold standard of TEA in paediatric patients undergoing Nuss surgery.
Therefore, we are conducting this prospective, randomized control trial among paediatric patients to compare the effectiveness of ESPB to that of TEA for postoperative analgesia following the Nuss procedure. The study is designed as a non-inferiority trial, as the analgesic effectiveness of successful TEA is generally acknowledged. Considering that a reduction in pain scores itself may not equate to an analgesic improvement, a joint endpoint of pain score and opioid consumption, which contributes equally to pain management, will be used.
Our study is expected to contribute data in support of the effect of ESPB on the Nuss procedure in paediatric patients, whereas ESPB is currently mostly (90.5%) placed on adult patients.38 If the non-inferiority of ESPB is proven, it could be a relatively safe and convenient replacement for TEA. In addition, the use of intravenous opioids is associated with an increased risk of respiratory depression, nausea, vomiting, and intolerance, which has aroused social concern.39 We believe that our research will support the effect of ESP on opioid sparing and contribute to reducing the use of opioids among paediatric patients. Furthermore, many aspects, including the anatomy, mechanism of action, analgesic duration and complications, remain controversial.40 Our results may provide clinical evidence relevant to these aspects of ESPB and promote its application in specific populations.
Trial Status
This study is currently at the patient enrolment and data collection stage. The current version of the study protocol is version 1.1 and was approved on 16 July 2021. Patient recruitment started on 1 August 2021 and is expected to be finished by 31 January 2023.
Acknowledgments
This work was supported by the Wu Jieping Medical Foundation Special Fund for Clinical Research, grant number 320.6750.19089-102.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work”.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goretsky MJ, Kelly RE
2. Holcomb GW, Murphy JP, Ostlie DJ, eds. Ashcraft’s Pediatric Surgery.
3. Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Ann Cardiothorac Surg. 2016;5(5):422–433. doi:10.21037/acs.2016.08.05
4. Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. 2010;252(6):1072–1081. doi:10.1097/SLA.0b013e3181effdce
5. Densmore JC, Peterson DB, Stahovic LL, et al. Initial surgical and pain management outcomes after Nuss procedure. J Pediatr Surg. 2010;45(9):1767–1771. doi:10.1016/j.jpedsurg.2010.01.028
6. Marshall K, McLaughlin K. Pain management in thoracic surgery. Thorac Surg Clin. 2020;30(3):339–346. doi:10.1016/j.thorsurg.2020.03.001
7. Tseng WC, Lin WL, Lai HC, Huang TW, Chen PH, Wu ZF. Fentanyl-based intravenous patient-controlled analgesia with low dose of ketamine is not inferior to thoracic epidural analgesia for acute post-thoracotomy pain following video-assisted thoracic surgery: a randomized controlled study. Medicine. 2019;98(28):e16403. doi:10.1097/MD.0000000000016403
8. Man JY, Gurnaney HG, Dubow SR, et al. A retrospective comparison of thoracic epidural infusion and multimodal analgesia protocol for pain management following the minimally invasive repair of pectus excavatum. Paediatr Anaesth. 2017;27(12):1227–1234. doi:10.1111/pan.13264
9. Veneziano G, Iliev P, Tripi J, et al. Continuous chloroprocaine infusion for thoracic and caudal epidurals as a postoperative analgesia modality in neonates, infants, and children. Paediatr Anaesth. 2016;26(1):84–91. doi:10.1111/pan.12807
10. Kamiyoshihara M, Nagashima T, Ibe T, Atsumi J, Shimizu K, Takeyoshi I. Is epidural analgesia necessary after video-assisted thoracoscopic lobectomy? Asian Cardiovasc Thorac Ann. 2010;18(5):464–468. doi:10.1177/0218492310381817
11. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional Anesthesia in the Patient Receiving Antithrombotic or Thrombolytic Therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263–309. doi:10.1097/AAP.0000000000000763
12. Sujka JA, Dekonenko C, Millspaugh DL, et al. Epidural versus PCA pain management after pectus excavatum repair: a multi-institutional prospective randomized trial. Eur j Pediatric Surg. 2020;30(5):465–471. doi:10.1055/s-0039-1697911
13. Stroud AM, Tulanont DD, Coates TE, Goodney PP, Croitoru DP. Epidural analgesia versus intravenous patient-controlled analgesia following minimally invasive pectus excavatum repair: a systematic review and meta-analysis. J Pediatr Surg. 2014;49(5):798–806. doi:10.1016/j.jpedsurg.2014.02.072
14. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi:10.1097/aap.0000000000000451
15. Nielsen MV, Moriggl B, Hoermann R, Nielsen TD, Bendtsen TF, Borglum J. Are single-injection erector spinae plane block and multiple-injection costotransverse block equivalent to thoracic paravertebral block? Acta Anaesthesiol Scand. 2019;63(9):1231–1238. doi:10.1111/aas.13424
16. Holland EL, Bosenberg AT. Early experience with erector spinae plane blocks in children. Paediatr Anaesth. 2020;30(2):96–107. doi:10.1111/pan.13804
17. Chin KJ. Thoracic wall blocks: from paravertebral to retrolaminar to serratus to erector spinae and back again - A review of evidence. Best Pract Res Clin Anaesthesiol. 2019;33(1):67–77. doi:10.1016/j.bpa.2019.02.003
18. Leong RW, Tan ESJ, Wong SN, Tan KH, Liu CW. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76(3):404–413. doi:10.1111/anae.15164
19. Ciftci B, Ekinci M, Celik EC, Tukac IC, Bayrak Y, Atalay YO. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: a Prospective Randomized Study. J Cardiothorac Vasc Anesth. 2020;34(2):444–449. doi:10.1053/j.jvca.2019.04.026
20. Yao Y, Fu S, Dai S, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth. 2020;63:109783. doi:10.1016/j.jclinane.2020.109783
21. Nagaraja PS, Ragavendran S, Singh NG, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):323–327. doi:10.4103/aca.ACA_16_18
22. Balaban O, Koculu R, Aydin T. Ultrasound-guided lumbar erector spinae plane block for postoperative analgesia in femur fracture: a pediatric case report. Cureus. 2019;11(7):e5148. doi:10.7759/cureus.5148
23. Nardiello MA, Herlitz M. Bilateral single shot erector spinae plane block for pectus excavatum and pectus carinatum surgery in 2 pediatric patients. Rev Esp Anestesiol Reanim. 2018;65(9):530–533. doi:10.1016/j.redar.2018.04.006. Bloqueo bilateral del plano del musculo erector de la columna espinal para cirugia de pectus excavatum y pectus carinatum en 2 pacientes pediatricos.
24. De la Cuadra-fontaine JC, Concha M, Vuletin F, Arancibia H. Continuous Erector Spinae Plane block for thoracic surgery in a pediatric patient. Paediatr Anaesth. 2018;28(1):74–75. doi:10.1111/pan.13277
25. Ciftci B, Ekinci M. Ultrasound-guided single shot preemptive erector spinae plane block for thoracic surgery in a pediatric patient. Agri. 2020;32(1):58–59. doi:10.14744/agri.2019.57778. Pediatrik hastada preemtif erector spina plan blogunun toraks cerrahisi sonrasi analjezik etkinligi.
26. Adhikary SD, Pruett A, Forero M, Thiruvenkatarajan V. Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: a case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth. 2018;62(1):75–78. doi:10.4103/ija.IJA_693_17
27. Mostafa SF, Abdelghany MS, Abdelraheem TM, Abu Elyazed MM, Polaner D. Ultrasound‐guided erector spinae plane block for postoperative analgesia in pediatric patients undergoing splenectomy: a prospective randomized controlled trial. Pediatric Anesthesia. 2019;29(12):1201–1207. doi:10.1111/pan.13758
28. Castarlenas E, Jensen MP, von Baeyer CL, Miro J. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents: a systematic review. Clin J Pain. 2017;33(4):376–383. doi:10.1097/AJP.0000000000000406
29. Myles PS. Measuring quality of recovery in perioperative clinical trials. Curr Opin Anaesthesiol. 2018;31(4):396–401. doi:10.1097/ACO.0000000000000612
30. Kang R, Chin KJ, Gwak MS, et al. Bilateral single-injection erector spinae plane block versus intrathecal morphine for postoperative analgesia in living donor laparoscopic hepatectomy: a randomized non-inferiority trial. Reg Anesth Pain Med. 2019:
31. Althunian TA, de Boer A, Klungel OH, Insani WN, Groenwold RH. Methods of defining the non-inferiority margin in randomized, double-blind controlled trials: a systematic review. Trials. 2017;18(1):107. doi:10.1186/s13063-017-1859-x
32. Mascha EJ, Turan A. Joint hypothesis testing and gatekeeping procedures for studies with multiple endpoints. Anesth Analg. 2012;114(6):1304–1317. doi:10.1213/ANE.0b013e3182504435
33. Elmore B, Nguyen V, Blank R, Yount K, Lau C. Pain management following thoracic surgery. Thorac Surg Clin. 2015;25(4):393–409. doi:10.1016/j.thorsurg.2015.07.005
34. Horth D, Sanh W, Moisiuk P, et al. Continuous erector spinae plane block versus intercostal nerve block in patients undergoing video-assisted thoracoscopic surgery: a pilot randomized controlled trial. Pilot Feasibility Stud. 2021;7(1):56. doi:10.1186/s40814-021-00801-7
35. Zhao H, Xin L, Feng Y. The effect of preoperative erector spinae plane vs. paravertebral blocks on patient-controlled oxycodone consumption after video-assisted thoracic surgery: a prospective randomized, blinded, non-inferiority study. J Clin Anesth. 2020;62:109737. doi:10.1016/j.jclinane.2020.109737
36. Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med. 2019;7(8):174. doi:10.21037/atm.2019.03.53
37. Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med. 2019. doi:10.1136/rapm-2019-100827
38. Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29–34. doi:10.1016/j.jclinane.2018.09.036
39. Kolettas A, Lazaridis G, Baka S, et al. Postoperative pain management. J Thorac Dis. 2015;7(Suppl 1):S62–72. doi:10.3978/j.issn.2072-1439.2015.01.15
40. Saadawi M, Layera S, Aliste J, Bravo D, Leurcharusmee P, Tran Q. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68:110063. doi:10.1016/j.jclinane.2020.110063
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.