Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 11
The Effect of Smoking Habit on Apical Status of Adequate Endodontically Treated Teeth with and Without Periodontal Involvement
Authors Mahmood AA , AbdulAzeez AR , Hussein HM
Received 31 October 2019
Accepted for publication 3 December 2019
Published 30 December 2019 Volume 2019:11 Pages 419—428
DOI https://doi.org/10.2147/CCIDE.S236747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Athraa A Mahmood,1 Ali R AbdulAzeez,2 Hashim M Hussein3
1Department of Periodontology, College of Dentistry, Mustansiriyah University, Baghdad, Iraq; 2Department of Periodontology, College of Dentistry, Uruk University, Baghdad, Iraq; 3Department of Dentistry, Al-Rafidain University-College, Baghdad, Iraq
Correspondence: Hashim M Hussein
Department of Dentistry, Al-Rafidain University-College, Palestine Street, P.O., Box 46036, Baghdad, Iraq
Tel +964 780 710 1071
Email [email protected]
Background: The possible connection between apical periodontitis (AP), periodontal disease (PD) and the bad habit of smoking is among the most interesting aspects faced by the dental scientific community. The aim of this study was to pinpoint the effect of smoking on the status of the apical region, in properly root-filled teeth with and without periodontal involvement of Iraqi males.
Materials and methods: Total number of 75 patients were chosen, divided into 3 groups of 25’s, teeth were subdivided into 6 subgroups (G-a: Light smokers without Periodontal involvement, G-b: Heavy smokers without Periodontal involvement, G-c: Non-smokers without Periodontal involvement, G-d: Light smokers with Periodontal involvement, G-e: Heavy smokers with Periodontal involvement, G-f: Non-smokers with Periodontal involvement), examination involved: clinical periodontal parameters, coronal restoration fitness. Panoramic and periapical radiographs were used to judge the quality of periodontal tissue in the apical region (AP) of root canal treated teeth.
Results: Among all the examined teeth (1859), only (89) were found with fitted both coronal and endodontic restorations. Rates of AP were: (G-a: 11.1%, G-b: 25%, G-c: 0%, G-d: 18.8%, G-e: 35.7%, G-f: 4.8%). Statistically, in related to the AP, there was a highly significant difference between heavy smokers’ groups (G-b, G-e). Moreover, heavy smokers with PD (G-e) showed a significant difference with light smokers without PD (G-a) and non-smokers without PD (G-c), also, there was a significant difference between heavy smokers without PD (G-b) and non-smokers with PD (G-f). While, there was a non-significant difference relationship between non–smokers’ groups (G-c, G-f), and light smokers’ groups (G-a, G-d).
Conclusion: There is a noticeable negative effect of smoking on the severity and prognosis of AP and this negative effect worsens when it is accompanied by lateral periodontitis.
Keywords: smoking, apical periodontitis, root canal therapy, periapical index
Introduction
The infamous habit of smoking and its destructive effects on body tissues casts its shadow on various treatments and its outcomes in dentistry as well as in medicine. One of these treatments which we are going to tackle in this research is the endodontically treated teeth and its periodontal status.1
Clinical markers of a hygienic periodontium include the absence of the cardinal signs of inflammation, gingival recession, and loss of attachment. Diseased periodontium is related to long-standing inflammation, produced by different types of subgingival bacteria negatively affecting the periodontium.2 Yet the periodontal disease (PD) is altered by multiple etiological factors like age, gender, smoking, drugs, alcohol consumption, and systemic diseases. The patient’s behavior modification and lifestyle improvement can contribute to the elimination or bringing the PDs under control.3
Healing the diseased periapical tissue and render it inflammation-free is the main purpose of endodontic treatment. Still, there is an ambiguity of the clinical signs that are supposed to point to pulpal or periapical inflammation since they are not highly specific to the disease.4
The fact that smoking imposes negative effects on periodontal bone and considered as a risk factor for periodontitis; relies on cross-sectional and longitudinal studies,5 therefore an assumption was made that it affects the apical periodontium of endodontically compromised teeth worsening the periapical bone destruction and increasing the size/and or number of periapical lesions due to its hindering effect on the repair and healing process that follows the root canal treatment.6 There is a proportional relationship between the amount of tobacco smoking and periodontal attachment loss,7 periodontitis elects heavy smokers more often than it does with non-smokers and to a great extent, the light smokers too.8
Generally speaking, Perio-endo lesions have two major sources of infection, either periodontal where the tooth is still vital, or endodontic origin where tooth is usually non-vital, still the vitality (pulp testing) alone is not enough sign to rule out the causative factor, especially in a multi-rooted teeth, so parameters like mobility, palpation, percussion, radiographs, and fistula tracking are used for more specific diagnosis. There are cases where there is a true conjugation between the two sources, but these are less frequent and known as true Perio-endo lesions.9 Whether primary or secondary, it is the decisive point depending on which the lesions are classified.10
It is hard to know beforehand the origin of the lesion, therefore the endodontic therapy should be delivered first and tooth to be put under observation before we attempt the periodontal therapy, since in most of the cases; bacteria that reside in the empty spaces of root canals can transfer to the periodontal tissue either by lateral canals or by apical foramen and cause destruction.11
Once the root canal is properly sealed with filling material and sealant, the attention will be accentuated towards the periodontal status, if the lesion persists, then its of a periodontal origin and routine periodontal treatment is delivered, the PDs; which the chronic periodontitis is the most common among them, have always been treated by disturbing the microbial plaque aggregation on tooth surfaces mechanically as well as by other adjective means such as chemical, photodynamic, etc.12
On rare occasions, there are true Perio-endo lesions where both endodontic and periodontal origins of infection are available simultaneously, still, the sequence of treatment will not change; endodontic first, periodontal intervention comes next.13,14
Apical periodontitis (AP), is an inflammatory process around the apex of a root, it is primarily a sequel to microbial infection of the pulp space. Thus, considered as a disease of bacterial infection.15 The AP is multifactorial and related to several risk factors, the source of which can be either intra-canal or extra-canal, so it can be looked at as a potential Perio-endo lesion. Among the risk factors that play a role in developing AP in endodontically treated teeth, is the quality of endodontic filling, and the coronal restoration.16 In Europe, the prevalence of AP is as high as (34–61%) of individuals and (2.8–4.2%) of the teeth.17,18
The present study aimed at exploring the effect of smoking health-related habits as a risk factor in the apical area status of adequate root-filled teeth with and without periodontal involvement in Iraqi males.
Materials and Methods
- The design of this study is a cross-sectional observational study. Randomly selected patients aged between 18 and 45 years reporting to the “Specialized dental center” in Baghdad in 2018 were enrolled. After their full medical, dental, and endodontic history was taken, they were examined clinically and radiographically.
Ethical Concerns
The study was carried out in accordance with the principles of the Declaration of Helsinki. All the patients read the information about the study and signed a letter of a written consent form for enrollment in research and data publishing. Research acquired the ethical research committee of Al-Mustansiriyah University’s approval (protocol 49; February 1st, 2018).
Inclusion Criteria
- Minimally one tooth with RCT.
- Male patients.
- Fitted RCT, and fitted coronal restoration teeth.
Exclusion Criteria
- Female patients to neutralize the effect of hormonal changes.
- Patients with systemic diseases, especially cardiovascular disease (CVD), diabetes mellitus (DM), chronic liver disease, blood disorders, and low bone mineral density (osteoporosis).19
- Patients with less than 8 teeth left.
- The 3rd molar teeth.
- Mentally retarded patients.
- Patients under 18 or over 45 years old, due to the age-related diminution of oral hygiene where aggregation of plaque cause introduction of microorganisms through the periodontium leading to PD.20,21
- Unfitted RCT teeth (over or under obturation) (using x-ray).
- Teeth with fitted RCT but with poor coronal restoration (using x-ray).
Patient Groups Configuration
At first, the total number of patients with a history of root canal therapy (RCT) was (205) and after application of historical and clinical exclusion criteria, we filtered only (155) patients at first. Then, after taking panoramic X-ray and application of radiographical exclusion criteria, the final included sample was 75 patients, which were divided into 3 groups (each group 25 patients) according to smoking habit:
- Group 1 (G1): 25 Light smokers (<10 cigarettes/day).22
- Group 2 (G2): 25 Heavy smokers (≥10 cigarettes/day).22
- Group 3 (G3): 25 Non-smokers (Control).
Then, the teeth in each group were subdivided into 2 subgroups (without and with PD) as follows:
- Group a (G-a): Light smoker without Periodontal involvement
- Group b (G-b): Heavy smoker without Periodontal involvement
- Group c (G-c): Control without Periodontal involvement
- Group d (G-d): Light smoker with Periodontal involvement
- Group e (G-e): Heavy smoker with Periodontal involvement
- Group f (G-f): Control with Periodontal involvement
Presence of RCT Teeth
After taking a history of RCT from the patient, the first x-ray type taking to patients is panoramic radiography (OPG) was taken by the “CarestreamR” device “model CS9000, USA”. The OPG was used to show all teeth in a single x-ray, easy of using OPG, in addition to decreasing the lost time of x-ray taken by intraoral periapical x-ray (PA x-ray) films to achieve complete mouth radiography (we need approximately 14 periapical x-rays at minimum to show all teeth while single OPG can give accepted information about RCT teeth).23,24 Viewing the radiography was standardized as all films were examined in a unified level of indoor-illumination/light intensity (darkened room), radiographs were shown on a computer screen in which the light could be controlled using “CarestreamR” software for the best possible contrast.
RCT Quality
A panoramic radiograph was used to identify teeth with RCT and primary assessment,25 then the quality of RCT later was re-assed with a second x-ray which was a PA x-ray to achieve on high degree evaluation due to the quality of PA x-ray is superior than OPG.23,26 Periapical x-ray was obtained using “CarestreamR RVG5200, USA” Sensor with “CarestreamR” software. The parallel technique was used, where digital film holders were used to refine the alignment of film/tooth to avoid elongation or shortening of images for all patients.
The (Orstavik et al 1986) method was used to classify RCT according to quality, only degree 1 (adequate root canal filling) where gutta-percha ends at 2mm away from the apex in x-ray film) was accepted in the study while degree 2 and 3 were excluded from the study subsequently (degree 2: gutta-percha ends shorter than 2mm away from radiographical apex, degree 3: over-filling as the filling material extends beyond the radiographic apex).25
Assessment of Coronal Filling Fit
Clinical examination by careful inspection using a dental mirror, sickle dental probe and aided with later on OPG was made to rule out the fitting of the coronal filling where the unified restoration included (overhang, open margin, recurrent caries, temporary filling or no filling).27
Assessment of Periodontal Parameters
Clinically, the teeth with RCT were considered as periodontally involved tooth if any of below statement was found, where a William’s periodontal probe (Hu-Friedy, UK) was used to measure indices of (PI, GI, PPD, CAL) for the selected teeth, while the furcation involvement was also measured by the Nabers probe (Hu-Friedy, UK).
- Probing pocket depth (PPD): The PPD was measured to the nearest mm from the base of the pocket to the free gingival margin. The tooth with PPD ≥ 4 mm was considered as a tooth with PD.28
- Clinical attachment loss (CAL): The CAL was determined from the junction (CEJ) to the base of the pocket. The tooth with CAL ≥ 2 mm was considered as a tooth with PD.28
- Gingival recession (GR): The GR was the distance from the CEJ to the gingival margin.29 The GR grades are (Grade I: < 3mm, Grade II: 3–4mm, Grade III: >4mm). The tooth with any of the above grades was considered as a tooth with PD.30
- Furcation involvement in posterior teeth (FI): The furcation degrees are (Degrees I: horizontal loss of periodontal tissue support less than 3mm, Degree II: horizontal loss of periodontal tissue support exceeding 3mm but not entirely passing the total width of furcation area, Degree III: horizontal through and through the destruction of periodontal tissue in the furcation). The tooth with any of the above degrees is considered as a tooth with PD.31
- Tooth mobility (TM): The mobility degrees are (Degree 1: 0.2–1 mm horizontal crown motion, Degree 2: greater than 1 mm horizontal crown motion, Degree 3: horizontal and vertical crown motion). The tooth with degrees 2 and 3 is considered as a tooth with PD.32
The severity of the above conditions was not taken into consideration, in a dichotomous 1/0 manner, once the tooth had any of PD signs, it was included as a tooth of PD.
Assessment of Periapical Tissue
Periapical status of each tooth was examined with PA x-ray using Periapical Index (PAI) for periapical status evaluation and measurement. According to PAI, grades 1 or 2 indicated healthy teeth and grades 3–5 indicated the presence of AP. In multi-rooted teeth, the worst grade of all roots was taken. When in doubt, a higher grade was assigned. The PAI index was classified by Orstavik et al in 1986 as follows:25
- Grade 1 (Gr1): Normal periapical structures.
- Grade 2 (Gr2): Small changes in periapical bone structure or bone structural changes indicating, but not pathognomonic for AP.
- Grade 3 (Gr3): Changes in periapical bone structure with some mineral loss characteristic of AP.
- Grade 4 (Gr4): Demineralization of periapical bone, periodontitis with the well-defined radiolucent area or well-defined radiolucency.
- Grade 5 (Gr5): Demineralization of periapical bone, severe periodontitis with exacerbating features or radiolucency with radiating expansions of bone structural changes.
Calibration of the Examiners
Two well skillful and trained observers with over 5 years of clinical experience assessed the radiographs and periodontal parameters (Inter-examiner agreement for scores of two observers).
Statistical Analysis
Using the computer software IBM® SPSS® version 21, descriptive statistics in the form of means were obtained in this study. A one-way analysis of variance (ANOVA) test was used to compare groups in general. In addition to the least significant difference (LSD) formula which was obtained to compare the means of the 6 subgroups with each other. Moreover, the level of significance (S) was accepted at P<0.05, highly significant (HS) at P≤0.01 and non-significant (NS) at P≥0. 05.
Results
The total sample of our study was 75 patients were divided into 3 groups of 25 patients each, the total number of examined teeth were 1859 (G1: 607, G2: 627, G3: 625), number of missing teeth were 241 (G1: 93, G2: 73, G3: 75), number of RCT were 235 teeth (12.64% from all examined teeth) Table 1.
 |
Table 1 Prevalence of Variables in All Three Main Groups |
Among those RCT teeth, we found only 96 teeth with fitted endodontically treated (G1: 27, G2: 35, G3: 34), 131 teeth with under root canal teeth (G1: 43, G2: 50, G3: 38), while 8 teeth had an over endodontic filling (G1: 0, G2: 6, G3: 2) Table 1.
Whereas the number of teeth with fitted coronal restorations were 89 (92.71% of all RCT teeth, 4.79% of all examined teeth) (G1: 25, G2: 32, G3: 32) as appose to 7 teeth with unfitted coronal restorations (G1: 2, G2: 3, G3: 2) Table 1.
We found that 24 teeth (26.97%) of fitted restoration were without periodontal involvement while 65 teeth (73.03%) were with periodontal involvement in Table 1.
We found that highest rate of periodontal parameters (PPD, CAL, GR, FI, TM) in G2, while the lessor rate of PPD and CAL was found in G1, and the lest rate of GR and TM was found in G3, whereas the lest rate of FI was found in both G1 and G3 in Table 2.
 |
Table 2 Percentages and No. of Periodontal Parameters in All Three Main Groups |
The worst grade Gr5 of AP did not appear in all groups. Also, the Grs4 was not appeared in all groups except in groups G-e, G-d with rates (7.1%, 6.3%), respectively, as shown in Table 3.
 |
Table 3 Percentages and No. of Grades of PAI in All Subgroups (G-a, G-b, G-c, G-d, G-e, G-f) |
In general, the rates of AP were (11.1%) for G-a, (25%) for G-b, (0%) for G-c, (18.8%) for G-d, (35.7%) for G-e, (4.8%) for G-f as shown in Table 3.
The Gr3 of AP did not appear in only one group which was the G-c group. Whereas the remaining groups showed different rates. The highest rates appeared in heavy smokers groups (G-e, G-b); (28.6%, 25%) respectively, while the light smokers groups (G-d, G-a) showed lessor rates of Gr3 than heavy smokers groups; (12.5%, 11.1%) respectively, while the least rate of Gr3 appeared in Gf (4.8%) Table 3.
The G-c showed a healthy apical area, where only contain on Grs1 and 2; (90.9%, 9.1%), respectively, Table 3.
The rates of Gr1 appeared in highest rate in non-smokers group (G-c, G-f); (90.9%, 81%) and to lessor rate in light smokers groups (G-a, G-d); (66.7%, 62.5%) respectively, while the least rate appeared in heavy smokers groups (G-b, G-e); (50%) Table 3.
Moreover, the rates of Gr2 appeared in highest rate in smokers without periodontal involvement groups (G-b, G-a,); (25%, 22.2%), and to lesser rate in periodontal involvement groups (G-d, G-e, G-f); (18.8%, 14.3%, 14.3%) respectively, while the least rate of Gr2 was appeared in G-c (9.1%) Table 3.
In Table 4, there is a (S) difference among groups according to the ANOVA test.
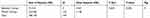 |
Table 4 ANOVA Test for Means of 6 Subgroups (G-a, G-b, G-c, G-d, G-e, G-f) |
As shown in Table 5, the LSD test showed a (NS) difference between light smokers without PD (G-a) and other groups (G-b, G-c, G-d, and G-f) except for a sole (S) difference between G-a and non-smoker without PD (G-c), Table 5.
 |
Table 5 LSD of 6 Subgroups (G-a, G-b, G-c, G-d, G-e, G-f) |
There was a (S) difference between heavy smokers without PD (G-b) and non-smoker with PD (G-f). Also, the same result obtained when compared the G-c with G-e as showed in Table 5.
Whereas comparing between two heavy smokers’ groups (G-b, G-e), the result showed (HS) difference Table 5.
While there was a non-significant difference between non-smokers groups (G-c, G-f), and between light smokers’ groups (G-a, G-d) Table 5.
While comparing of G-b with groups (G-c and G-d) the result gave (NS) difference, same result of the comparison between G-c with G-d, and too, when comparing the G-d with groups (G-e, G-f), and also when comparing G-e with G-f as shown in Table 5.
Discussion
In Iraq, most of the researchers care about the estimation of the effect of smoking on the periodontium, but there is little information about the AP itself. However, there are no data on this topic in the Iraqi population. Our results came in agreement with other authors like (Ali et al 2013, Correia-Sousaa et al 2015, and Peršić Bukmir et al 2016) regarding smoking negative role in AP,33–35 alongside with other authors; knowing that smoking alters the microvasculature and the function of immune system, the cadmium that is found in cigarettes also consumes the Super Oxide dismutase protective enzyme which is an anti-oxidant- in blood and saliva, by replacing the Manganese, copper, and Zinc leaving SOD in an inactive state opening the door to the free radicals generated from smoking to destroy periodontal tissue33 and this was obvious in the significant relationship between groups (G-c, Ge).
Although some authors (Bergström et al 2004, Bahammam 2012, Balto et al 2019) reached different results where the development of AP was irrelevant to smoking habit6,36,37 suggesting that the development of AP in RCT teeth is multifactorial.
Some authors like (Ray and Trop 1995) and (Estrela et al 2008) believed that coronal restoration is the crucial factor since failure of the proper coronal seal might cause seepage of infection towards deeper points apically even if the endodontic treatment was performed carefully,26,38 while other authors like (Hommez et al 2002) argued that endodontic treatment is the key factor in preventing AP regardless from the quality of apical restoration quality given that appropriate seal comes solely from good canal obturation,39 this literature controversy pushed us to exclude the 8 unfitted coronal restorations.
A key feature in this research is counting samples as per tooth not per patient, our opinion matches (Balto et al 2019) as this would give a truer picture of the disease distribution and severity, if we put in mind that a patient with three or more affected teeth is not equal to a person with only one tooth affected with the condition in question which is AP.37
The endodontic treatment itself is multifactorial too because it passes through instrumentation, irrigation, and lastly obturation, unfortunately, we cannot trace the first two procedures because its operator-dependent and un-documentable, so we depended on the quality of obturation obtained from OPG’s and later from PA X-ray to judge the quality of RCT.
Intentionally we only chose the endodontically properly filled teeth to neutralize the remaining bacterial infection in the canals factor and the insult caused by the excess/overfilling materials, this way we can observe the effect of smoking alone in the development of AP.
Assessment of periodontal condition included both radiographical and clinical measures, as AP cannot be seen with the naked eye, the PA x-ray proved to be an effective way for examination.23 The periodontal apparatus is an inter-linked system, it even manifests itself radiographically as a continuous thin radiolucency surrounding the tooth, so it is not uncommon to witness a communication between the lateral and peri-apical periodontal tissue of smokers, inflammation and/or infection in one region can affect the other and vice-versa, this was apparent in the results as teeth of smokers with lateral periodontal involvement (clinical attachment loss, active pocket) had AP too but this relationship was not significant in the absence of smoking; thus, there was a synergistic relationship between smoking and lateral periodontal involvement on the persistence of AP. In addition to the above, the connection between the pulp and the periodontium occurs firstly by exposing dentinal tubules, accessory, and lateral canals, and by apical foramen. The location of these accessories and lateral canals are in coronal, middle, furcation and apical third, so if any inflammation to periodontium due to PD like loss of attachment or actively inflamed pocket or GR can lead to advancement and pushing of bacteria through these routes to pulp space, so the inflammation inside pulp was spread to all areas and finally reach to periodontal tissue in the apical region and result in AP.40,41
The heavy smokers with periodontal involvement showed a higher rate of periodontal parameters than other groups due to the effect of smoking on periodontium as discussed in the past paragraph.
Not only that the cause of both AP and PD share the same agent (G – anaerobic bacteria) but the two diseases are involved in elevation cytokine levels as well as inflammatory mediators.42,43
Smoking, on the other hand, is not far from participating in developing AP, by affecting immunity and periodontal tissue integrity. It also disturbs the balance between the reactive oxidase species and its buffers; the anti-oxidants. Such a condition of imbalance will affect the lipids in membranes, intracellular proteins, and most importantly damage to DNA, leading inevitably into cellular death.33
Negative effects of smoking habit on apical periodontal tissue can be observed in provoking the release of bone de-calcification chemical signals, hinders the intake of Ca+2 by the GIT system, leading to bone destruction in peri-apical region, weakening of immunity in both of its compartments (humoral and cell-mediated), and even impaired wound healing in some studies.44
All that being said, the link between AP and smoking varies in significance level between studies and that is due to differences in methods of measuring AP by roentgenography type which may be periapical, OPG, CBCT. Also, it is mandatory to know the effects of smoking habit on AP which is proportionally related to the magnitude and frequency of tobacco smoking, which shifts from light, moderate to heavy smoking.45
Conclusion
The results showed a negative, harmful effect of smoking with or without periodontal involvement on the apical status of endodontically treated teeth.
The combination of smoking and periodontal effects was higher than the smoking effect alone.
The continuous daily consumption of cigarettes for a long period of more than 5 years increases the possibility of AP especially RCT teeth.
Acknowledgments
Gratitude and thanks to the staff of Al-Mustansiriyah University (www.uomustansiriyah.edu.iq) for their full support and help along with all the steps in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jang A, Lee J, Shin J, Lee H. Association between smoking and periodontal disease in Korean adults: the fifth Korea National Health and Nutrition Examination Survey (2010 and 2012). Korean J Fam Med. 2016;37(2):117–122. doi:10.4082/kjfm.2016.37.2.117
2. Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediators Inflamm. 2019;2019:1–14. doi:10.1155/2019/1029857
3. Madiba TK, Bhayat A. Periodontal disease – risk factors and treatment options. SADJ. 2018;73(9):571–575.
4. Hyman JJ, Cohen ME. The predictive value of endodontic diagnostic tests. Oral Surg Oral Med Oral Pathol. 1984;58(3):343–346. doi:10.1016/0030-4220(84)90065-3
5. Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71(8):1338–1347. doi:10.1902/jop.2000.71.8.1338
6. Bergstrom J, Babcan J, Eliasson S. Tobacco smoking and dental periapical condition. Eur J Oral Sci. 2004;112(2):115–120. doi:10.1111/eos.2004.112.issue-2
7. Martinez-Canut P, Lorca A, Magan R. Smoking and periodontal disease severity. J Clin Periodontol. 1995;22(10):743–749. doi:10.1111/cpe.1995.22.issue-10
8. Haber J, Wattles J, Crowley M, et al. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. 1993;64(1):16–23. doi:10.1902/jop.1993.64.1.16
9. Shenoy N, Shenoy A. Endo-perio lesions: diagnosis and clinical considerations. Indian J Dent Res. 2010;21(4):579–585. doi:10.4103/0970-9290.74238
10. Alquthami H, Almalik AM, Alzahrani FF, Badawi L. Successful management of teeth with different types of endodontic-periodontal lesions. Case Rep Dent. 2018;29(2018):7084245.
11. Oh S, Chung SH, Han JY. Periodontal regenerative therapy in endo-periodontal lesions: a retrospective study over 5 years. J Periodontal Implant Sci. 2019;49(2):90–104. doi:10.5051/jpis.2019.49.2.90
12. AbdulAzeez AR, Mahmood MS, Ali WM. Phototoxic effect of visible blue light on aggregatibacter actinomycetemcomitans and porphyromonas gingivalis in patients with chronic periodontitis (an in-vitro study). J Bagh College Dentistry. 2015;27(1):144–150.
13. Vishwanath V, Rao HM, Prasad BSK, Shashikala K. Successful endodontic management of endo-period lesions with different treatment modalities: case series. SRM J Res Dent 2019;10(2):105–109.
14. Khaled Y, Pahuja BK. Condition requiring endodontic treatment to maintain the integrity of periodontium. J Dent Maxillofacial Res. 2019;2(3):54–58.
15. Siqueira JRJF, Rocas IN. Present status and future directions in endodontic microbiology. Endodontic Topics. 2014;30:3–22.
16. Estrela C, Leles CR, Hollanda AC, Moura MS, Pécora JD. Prevalence and risk factors of apical periodontitis in endodontically treated teeth in a selected population of Brazilian Adults. Braz Dent J. 2008;19(1):34–39.
17. Jimenez-Pinzon A, Segura-Egea JJ, Poyato-Ferrera M, Velasco-Ortega E, Rios-Santos JV. Prevalence of apical periodontitis and frequency of root-filled teeth in an adult Spanish population. Int Endod J. 2004;37:167–173. doi:10.1111/j.0143-2885.2004.00759.x
18. Lopez-Lopez J, Jane-Salas E, Estrugo-Devesa A, et al. Frequency and distribution of root-filled teeth and apical periodontitis in an adult population of Barcelona, Spain. Int Dent J. 2012;62(1):40–46. doi:10.1111/j.1875-595X.2011.00087.x
19. Khalighinejad N, Aminoshariae MR, Aminoshariae A, Kulild JC, Mickel A, Fouad AF. Association between systemic diseases and apical periodontitis. J Endod. 2016;42(10):1427–1434. doi:10.1016/j.joen.2016.07.007
20. Rao S, Thanikachala S, Sathiyasekaran B, Vamsi L, Balaji T, Jagannathan R. Prevalence and risk indicators for attachment loss in an urban population of South India. OHDM. 2014;13(1):1–5.
21. Hussein HM, Mahmood AA, Alberaqdar FA. The prevalence and relationship of root caries depth and gingival recession among different Iraqi groups. MDJ. 2015;12(1):144–155.
22. Maffei G, Brouwer N, Dolman KM, Van der Velden U, Roos D, Loos BG. Plasma levels of mannan-binding lectin in relation to periodontitis and smoking. J Periodontol. 2005;76(11):1881–1889. doi:10.1902/jop.2005.76.11.1881
23. Ridao-Sacie C, Segura-Egea JJ, Fernández-Palacín A, Bullón-Fernández P, Ríos-Santos JV. Radiological assessment of periapical status using the periapical index: comparison of periapical radiography and digital panoramic radiography. Int Endod J. 2007;40(6):433–440. doi:10.1111/iej.2007.40.issue-6
24. Sopińska K, Bołtacz-Rzepkowska E. The influence of tobacco smoking on dental periapical condition in a sample of an adult population of the Łódź region, Poland. Int J Occup Med Environ Health. 2019;1–13.
25. Orstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2(1):20–34. doi:10.1111/j.1600-9657.1986.tb00119.x
26. Estrela C, Leles CR, Hollanda ACB, Moura MS, Prevalence PJ. Risk factors of apical periodontitis in endodontically treated teeth in a population of Brazilian adults. Braz Dent J. 2008;19(1):34–39. doi:10.1590/S0103-64402008000100006
27. Alafif H. Impact of the quality of coronal restoration and root canal filling on the periapical health in adult syrian subpopulation. Indian J Dent. 2014;5(2):75–80. doi:10.4103/0975-962X.135265
28. Gunsolley JC, Quinn SM, Tew J, Gooss CM, Brooks CN, Schenkein HA. The effect of smoking on individuals with minimal periodontal destruction. J Periodontol. 1998;69:165–170. doi:10.1902/jop.1998.69.2.165
29. Marini GM, Greeghi Sebastie AL, Passanezi E, Santana Adriana PC. Gingival recession: prevalence, extension and severity in adults. J Appl Oral Sci. 2004;12(3):250–255. doi:10.1590/S1678-77572004000300017
30. Miller JRPD. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8–13.
31. Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi:10.1111/j.1600-051X.1975.tb01734.x
32. Nyman S, Lindhe J, Lundgren D. The role of occlusion for the stability of fixed bridges in patients with reduced periodontal tissue support. J Clin Periodontol. 1975;2(2):53–66. doi:10.1111/cpe.1975.2.issue-2
33. Ali BJ, Ibrahim LM, Majid AY. Periodontal health status of heavy and light smokers and its correlation with salivary superoxide dismutase enzyme (A comparative study). J Bagh College Dentistry. 2013;25(3):97–102. doi:10.12816/0015004
34. Correia-Sousaa J, Madureira AR, Carvalhoc MF, Teles AM, Pina-Vazc I. Apical periodontitis and related risk factors: cross-sectional study. Rev Port Estomatol Med Dent Cir Maxilofac. 2015;56(4):226–232.
35. Peršić Bukmir R, Jurčević Grgić M, Brumini G, Spalj S, Pezelj-Ribaric S, Brekalo P. Influence of tobacco smoking on dental periapical condition in a sample of croatian adults. Wien Klin Wochenschr. 2016;128(7–8):260–265. doi:10.1007/s00508-015-0910-8
36. Bahammam LA. Tobacco smoking and dental periapical condition in a sample of Saudi Arabian sub-population. JKAU: Med Sci. 2012;19(1):35–41.
37. Balto HA, Alabdulaaly L, Bahammam S, Al-Ekrish AA. Comparative analysis of prevalence of apical periodontitis in smokers and non-smokers using cone-beam computed tomography. Saudi Dent J. 2019;31(1):52–57. doi:10.1016/j.sdentj.2018.09.006
38. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28(1):12–18. doi:10.1111/iej.1995.28.issue-1
39. Hommez GMG, Coppens CRM, DeMoor RJG. Periapical health related to the quality of coronal restorations and root fillings. Int Endod J. 2002;35(8):680–689. doi:10.1046/j.1365-2591.2002.00546.x
40. Ahmed HMA, Neelakantan P, Dummer PMH. A new system for classifying accessory canal morphology. Int Endod J. 2018;51(2):164–176. doi:10.1111/iej.2018.51.issue-2
41. Manakil J. Periodontology and Dental Implantology.
42. Rocas IN, Alves FR, Santos AL, Rosado AS, Siqueira JF
43. Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP. Antigenic activity of bacterial endodontic contents from primary root canal infection with periapical lesions against macrophage in the release of interleukin-1beta and tumor necrosis factor alpha. J Endod. 2010;36(9):1467–1474. doi:10.1016/j.joen.2010.06.012
44. Rodriguez FR, Taner B, Weiger R, Walter C. Is smoking a predictor of apical periodontitis? Clin Oral Investig. 2013;17(8):1947–1955. doi:10.1007/s00784-012-0893-z
45. Kweon HH, Lee JH, Youk TM, Lee BA, Kim YT. Panoramic radiography can be an effective diagnostic tool adjunctive to oral examinations in the national health checkup program. J Periodontal Implant Sci. 2018;48(5):317–332. doi:10.5051/jpis.2018.48.5.317
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
