Back to Journals » Journal of Blood Medicine » Volume 11
The Effect of Serum Ferritin Level on Gonadal, Prolactin, Thyroid Hormones, and Thyroid Stimulating Hormone in Adult Males with Sickle Cell Anemia
Authors Mostafa GG , Zahran FE, Omer SA , Ibrahim A , Elhakeem H
Received 4 October 2019
Accepted for publication 15 January 2020
Published 28 January 2020 Volume 2020:11 Pages 27—32
DOI https://doi.org/10.2147/JBM.S232562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Gamal G Mostafa,1 Fawkia E Zahran,2,3 Sawsan A Omer,3,4 Ahmed Ibrahim,5 Heba Elhakeem6
1Department of Clinical Adult Hematology, Faculty of Medicine, Aswan University, Aswan, Egypt; Haematology, Hereditary Blood Disease Centre- Hofuf, Hofuf, Kingdom of Saudi Arabia; 2Department of Internal Medicine, Faculty of Medicine for Girls, Al- Azhar University, Cairo, Egypt; 3King Fahad Hospital, Hofuf, Al Hofuf, Kingdom of Saudi Arabia; 4Department of Internal Medicine, Faculty of Medicine, University of Gezira, Wad Madani, Sudan; 5Department of Community Medicine, Faculty of Medicine, University of Western Kordofan, Al-Foula, Sudan; Prince Sultan Cardiac Center, Al Hassa, Al Hasa, Kingdom of Saudi Arabia; 6Clinical Pathology Department, Faculty of Medicine for Girls, Alazhar University, Cairo, Egypt
Correspondence: Sawsan A Omer
King Fahad Hospital, Hofuf, Eastern Region, Kingdom of Saudi Arabia
Tel +966 553210343
Email [email protected]
Background: Sickle cell anaemia (SCA) is an inherited hemoglobinopathy resulting in sickling of erythrocytes that cause micro-vascular obstruction leading to acute complications and chronic organ damage. Adults with SCA have endocrine complications and metabolic alterations. The aim of this study was to assess the association between gonadal and thyroid hormones with iron indices and to explore the potential association between serum ferritin levels and sex hormones in adult males with sickle cell disease in New Prince Saud Bin Jalawy Hospital (NPSBJH) in Hofuf city (Eastern region of Saudi Arabia) where there are many patients with SCA.
Methods: A cross-sectional analytical study was carried out in the Haematology Clinic at NPSBJH in 2018. A total of eighty (80) male patients with sickle cell anaemia were included in this study and were divided into two groups according to serum ferritin level. Group I (G-I): Included 40 male patients with high serum ferritin level and group II (G-II): included 40 male patients with normal serum ferritin level.
Results: There was a significant difference in height/cm between GI and Gil, P value= 0.006. Serum ferritin was significantly higher in GI (P value= 0.000), and serum TIBC was significantly higher in G-II. (P value= 0.022). Testosterone level was significantly higher in G-II (P value= 0.018). Luteinizing hormone (LH) was significantly higher in group I (P-value 0.019). There was a significant relation between serum ferritin level in G-I and the following: serum iron, TIBC, serum testosterone, LH, prolactin, free T3 and free T4.
Conclusion: Adult males with SCD with high serum ferritin level were shorter than adult males with SCD who had normal serum ferritin level and had a significant lower level of serum testosterone and significant high level of LH and this was most likely due to endocrine dysfunction secondary to high ferritin level and iron overload.
Keywords: sickle cell disease, ferritin, testosterone, prolactin
Introduction
Sickle cell anaemia (SCA) results from an inherited abnormality of human adult haemoglobin structure. The resultant abnormal haemoglobin is called Sickle haemoglobin (Hb SS) which has a glutamic acid to valine substitution at position six of the beta-globulin chain. Hb S is normally carrying oxygen however begins to produce intracellular polymers at low oxygen tension. This polymerization is the key event of SCA pathophysiology.1,2 The SCA is characterized by haemolytic anaemia, intermittent episodes of vaso-occlusion in microcirculation and organ dysfunction.3 Although blood transfusion can prevent a number of SCA complications, it leads to iron overload in the body, besides the excess iron absorption due to anaemia.4 Another factor for increased iron in SCA patients is haemolysis with subsequent recycling and accumulation of iron. In steady-state sickle cell anaemia, there are several studies reported an increase in serum ferritin level.4,5 Ferritin is a protein found inside the cells of the body and only a small amount is secreted into the serum. It is well known in the literature that some factors, like liver disease or chronic inflammation, may increase serum ferritin level. The primary role of ferritin is the storage of iron and there is a positive correlation between the ferritin level and the total amount of body iron. Hence, serum ferritin is used as a reliable indicator of the total amount of iron in the body.6 So, it is one of the most important tools in the measurement of the state of iron balance in steady-state sickle cell disease.5 The damaging effect of SCA on endocrine organs is indirect, it occurs through chronic anaemia, tissue hypoxia, and iron overload.7 The endocrine system dysfunction is reported as the most common and earliest complication of SCA.8 Infertility appears to be a big problem among males than females with SCA. Delayed puberty, gonadal insufficiency and thyroidal disorders were frequently reported in patients with SCA.8 It is well known that iron overload leads to gonadal atrophy. Therefore, this study aimed to assess the association between Thyroid hormones, luteinizing hormone (LH), Follicle-stimulating hormone (FSH), Testosterone hormone, Prolactin hormone, and Thyroid-Stimulating Hormone (TSH) with serum ferritin level and other iron indices in males with SCA.
Patients and Method
A cross-sectional analytical study was carried out in the Hematology Clinic Department at the New Prince Saud Bin Jalawy Hospital (NPSBJH) between January and August 2018. A total of eighty (80) male patients with Sickle Cell Anemia (Hb SS) were included in our study and distributed into two groups according to the serum ferritin level. The normal range of serum ferritin level is 20 to 270 ng/mL in adult male, generally, the serum ferritin level of more than 300 ng/mL in male is considered as elevated.
Group I: Including 40 male patients had a serum ferritin level >300 ng/mL with their age ranged between 18 and 45 years.
Group II: Including 40 male patients had a serum ferritin level >20 < 270 ng/mL with their age ranged between 19 and 43 years.
Inclusion criteria:
- An adult male of age from 18 to 45 years.
- A patient diagnosed with SCA (Hb SS).
- Patients are on regular follow-up
Exclusion criteria:
- Heart failure
- Severe liver disease
- Renal failure.
- Blood transfusion within 4weeks prior to study entry
- Vaso-occlusive crisis within the last 4 weeks.
- Patient admitted with fever and infection or sepsis
- Patient on hydroxyurea
- Patient on chronic oral narcotics
Ethical Considerations
Approval for the study was obtained from the research and ethics committee at the hospital. The study was conducted in accordance with the Declaration of Helsinki, and informed written consent was obtained from all participants in the study.
All patients were subjected to the following:
Complete medical history was taken and complete clinical examination was done and blood tests were taken for complete blood count (CBC), iron profile (serum iron, serum ferritin, and total iron-binding capacity) and hormonal assay (serum testosterone, prolactin, LH, FSH, TSH-free T3 and T4 levels) as well as liver function test (ALT-AST- INR-PT) and renal function test (serum creatinine-blood urea).
Electrocardiogram.
Specimen Collection and Handling
Five mL of venous blood was withdrawn in a plastic syringe under complete aseptic condition using routine precautions observed in venipunctures and divided into two parts.
- Two mL were added to ethylenediaminetetraacetic acid (EDTA) vacutainers for complete blood count (CBC) including differential white blood cells count which was done on a Leishman stained peripheral blood smear with evaluation using a fully automated cell counter (Sysmex XN, Germany).
- Three mL were taken in plain vacutainers and left to clot for 30 mins, then centrifugations were done at 1500 xg for 10 mins. Serum was separated for assessment of ferritin, FSH, LH, prolactin, testosterone, T3, T4 and TSH using chemical auto analyzer (Cobas 401), Germany and kits supplied by Roche Diagnostic Kits according to manufacturer instructions.
Complete blood count:
- White blood cell count (WBC) (N0. Range in adult 3.000–10.500 million cells/microliter) (mcL)
- Red blood cell count (RBCs) (N0. Range in adult 4.32–5.72 million cells/microliter) (mcL).
- Haematocrit (Hct)
- Haemoglobin (Hbg)
- Mean corpuscular volume (MCV) (N0. Range in adult male 80–100 femtoliter/red cell)
- Mean Corpuscular Hemoglobin (MCH) (N0. Range in adult male 27 – 31pg/red cell)
- Mean corpuscular Hemoglobin concentration (MCHC) (N0. Range in adult male 32–36 g/dl).
Iron profile:
- Serum Iron (No. range in adult male 35–15 µg/dL)
- TIBC (No. range in adult male 252–479 µg/dL)
- Serum Ferritin (No. in adult male 20 to 270 ng/mL)
- Liver function test (ALT-AST- INR-PT)
- Renal function test (serum creatinine-blood urea)
Hormonal assay:
- Thyroid-Stimulating Hormone (TSH) (N0. Range 0.4 mL/L- 4.0 µlU/mL in adult male).
- Free Thyroxin (free T4) (No. 9.00–26.00 pmol/L in the adult male).
- Free triiodothyronine (free T3) (No. 2.80–7.10 pmol/L in the adult male).
- Serum Prolactin hormone (No. <15 ng/mL in adult male).
- Luteinizing hormone (LH) (No. 1–10 mIU/mL in the adult male).
- Follicle-stimulating hormone (FSH) (No. 1.5–12.4 IU/L in the adult male).
- Testosterone hormone (No. range 2.70–10.0 ng/mL in male with 20 to 39 years) (No. range 3.50–8.90 ng/mL in male with 40 to 59 years).
Statistical Analysis
Data were analysed using SPSS version 20. Descriptive statistics was provided. Continuous data were reported in the form of mean, median, range, standard deviation. The t-test was used to assess the significant difference between continuous variables. A significant difference between categorical variables was assessed by using the Chi-squared test. The significance level was set at 95% and the P value was considered significant if <0.05%.
Results
The Characteristics of Data Between Groups
Table 1 shows the comparative study of the clinical and laboratory characteristics data between the study groups. The current study enrolled 80 male SCA (Hb SS) patients who were divided into; 40 patients with serum ferritin level above 300 ng/mL as Group I and 40 patients with normal serum ferritin level as Group II. There was no statistically significant difference between G-I and G-II with respect to the age/year (G-I: 31.09 ± 8.64 vs G-II: 31.89 ± 11.07, P-value = 0.682) and weight/Kg (G-I: 59.58 ± 13.84 vs G-II: 73.26 ± 10.33, P-value = 0.371). But there was a significant difference of height/cm between G-I (152.15 ± 4.94) vs G-II (154.79 ± 4.74), P value= 0.006.
 |
Table 1 Comparison Between the Two Groups of SCA Male Patients |
Comparison of Groups Based on the Complete Blood Count
It was found that, there was a statistically significant difference between G-I and G-II as regarding the RBCs (G-I 3.73 ± 0.89 vs G-II 4.27 ± 0.84, P value = 0.002) and Hb g/dl (G-I: 9.25 ± 1.50 vs G-II: 10.12 ± 1.30, P-value = 0.002) as shown in Table 1.
While there was no statistically significant difference between G-I and G-II as regarding MCV/femtoliter (G-I: 73.26 ± 10.33 vs GII: 74.48 ± 9.71, P value= 531), MCH pg/red cell (G-I: 25.48 ± 4.01 vs G-II: 25.49 ± 3.96, P value=0.994), and MCHC g/dL (G-I: 34.00 ± 0.99 vs G-II: 33.80 ± 2.16, P value=0.553). Also, there was no significant difference between the median of platelet count 109/L in G-I 211 (175–400) and G-II (287 (181–410), P value=0.604, as shown in Table 1.
Comparison of Groups Based on Iron Profile
In Table 1 the serum iron/µg/L was a non-significant difference between G-I (12.81 ± 5.08) and G-II (11.44 ± 4.89) (P value= 0.159). The median of serum Ferritin/ng/mL was significantly higher in G-I: 346.5 (312.1–590) than the median in G-II 113.6 (48.9–225.8) (P value= 0.000) and serum TIBC/µg/dL was significantly higher in G-II (61.24 ± 16.39) than G-I (54.25 ± 14.54) (P value= 0.022).
Comparison of Groups Based on Hormonal Assay
In Table 1 the testosterone ng/mL level was significantly higher in G-II (5.18 ± 2.85) than G-I (4.11 ± 1.61) (P value= 0.018). While the median (IQR) of LH mIU/mL was significantly higher in G-I: 8.3 (2.57–10.50) than G-II: 4.14 (3.50–4.99) (P value= 0. 019). The serum level of FSH/IU/L was the statistically non-significant difference when compared between G-I (5.15 ± 2.49) and G-II (6.09 ± 3.37) (P value= 0.105). Also, there was a non-significant difference of serum prolactin ng/mL level when compared between G-I (8.02 ± 2.96) and G-II (8.65 ± 5.63) (P value= 0.475).
Moreover, there were non-significant differences in free T3 pmol/L, free T4 pmol/L and TSH µlU/mL levels in G-I (5.53 ± 1.72, 13.47 ± 2.65, and 2.03 ± 1.59), respectively, when compared with G-II (5.38 ± 1.07, 12.39 ± 3.10, and 1.95 ± 0.98), respectively (P value=0.606, P value= 0.057, and P value= 0.734), respectively, as shown in Table 1.
The Correlation Study
Pearson’s correlation showed a non-significantly association between serum ferritin level and serum Testosterone/ng/mL level (r= 0.181, P= 0.194), serum FSH/IU/L level (r= 0.124, P= 0.375), prolactin ng/mL level (r= 0.020, P= 0.889) and Free T4 pmol/L level (r= −0.206, P= 0.140) in G-II, as shown in Table 2. Also, there was a non-significantly association between serum ferritin level and serum FSH/IU/L level (r= 0.175, P= 0.211) in G-I, but there was significant relation between serum ferritin level in G-I and the followings: serum iron (r= 0.27, P=0.04), TIBC (r=0.3, P=0.01), serum testosterone (r= 0.32, P= 0.01), LH (r=−0.30, P=0.02), prolactin (r= 0.56, P=0.00), free T3 (r= 0.30, P=0.02), and free T4 (r=−0.37, P=0.006) as shown in Table 2.
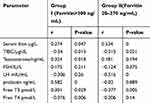 |
Table 2 Correlation Between Serum Ferritin Level and Other Parameters Within Group I and Group II |
Discussion
This cross-sectional analytical study which was carried out in the Hematology Clinic Department at the New Prince Saud Bin Jalawy Hospital (NPSBJH) consisted of eighty (80) male patients with Sickle Cell Anaemia (Hb SS) and were divided into two groups according to serum ferritin level, group I (G I) with high serum ferritin level and group II (GII) with normal serum ferritin level. Each group consisted of 40 patients. It showed that the height of patients who had serum level of ferritin above 300ng/mL were significantly shorter than the control who had serum ferritin below 270 ng/mL, this shorter height could be due to abnormal endocrine function in these patients either secondary to iron overload which was reflected by high serum ferritin level or it could be repeated vasoocclusive crises in microcirculation leading to endocrine glands dysfunction. Also, it was reported that both red cell count and Hb level were significantly lower in the same group with shorter height. This was in keeping with several studies that showed decreased height in men with SCA, Friedman et al in 1974, Olambiwonnu et al in 1975, Platt et al study in 1984 and Zemel et al in 2007where they found that 38% of patients with SCA were below the 5th percentile for height.9 Ferritin was significantly higher in G-I compared to G-II (P value= 0.000), and serum TIBC was significantly higher in G-II. (P value= 0.022). This high serum ferritin level could be explained by increased iron absorption due to anaemia or it could be due to increased haemolysis in these SCD patients with subsequent recycling and accumulation of iron or it could be due to repeated blood transfusion in these patients, anyway this could be assumptive rather than conclusive. This was similar to the study carried out by Hagag et al in Egypt in females with SCA.10 Testosterone level was significantly higher in G-II than G-I (P value= 0.018), while Luteinizing hormone (LH) was significantly higher in group I (P-value 0.019), the lower level of testosterone in GI (SCA with high ferritin level) could be attributed to gonadal hypo-function in patients with SCA secondary to iron overload or it could be due to intra-vascular sickling vasoocclusive occlusion infraction or hypoxia associated with chronic anaemia causing testicular failure. This was in keeping with the result of the study carried out by Garadah et al in Bahrin in adult patients with SCA where they found low serum testosterone level,11 and also similar to the result of the study done by Ezeiruaku F.C in Nigeria where he found significant low level of testosterone in patients with SCA compared to control.12 It was also similar to the results of the study done by Martins et al in 2015, were they found low testosterone level in males with SCA.9 The levels of follicle-stimulating hormone (FSH), prolactin hormone, free T3, free T4 and thyroid-stimulating hormone (TSH) were statistically non-significant between the two groups. Pearson’s correlation showed a significant relation between serum ferritin level in GI and the followings: serum iron (r= 0.27, P=0.04), TIBC (r=0.3, P=0.01), serum testosterone (r= 0.32, P= 0.01), LH (r=−0.30, P=0.02), prolactin (r= 0.56, P=0.00), free T3 (r= 0.30, P=0.02), and free T4 (r=−0.37, P=0.006), this was similar to the result of the study done by Garadah et al in Bahrin in adult patients with SCA where they found, low serum T4 level.11 This was also similar to the study done by Ozen et al in 2013 among males with SCA, where they found a low free T4 level with normal TSH.9 There was a non-significant association between serum level of ferritin and serum levels of Testosterone/ng/mL l FSH/IU/L, prolactin and Free T4 in G-II. Also, there was a non-significantly association between serum ferritin level and serum FSH/IU/L level in GI.
Conclusion
From this study, we can conclude that adult males with SCD Who had high serum ferritin level were shorter than adult males with SCD who had normal serum ferritin level and they had also significant lower level of serum testosterone and significant high level of LH and this was most likely due to endocrine dysfunction secondary to high ferritin level and iron overload, anyway, these results were not conclusive and ferritin was an only indicator of iron overload and other factors like vaso-occlusive occlusion, infraction or hypoxia associated with chronic anaemia could be responsible for endocrine dysfunction in patients with SCD. There was a significant relation between serum ferritin level in patients with SCA and high ferritin level and the following: serum iron, TIBC, serum testosterone, LH, prolactin, free T3 and free T4. These results could have serious impact on the fertility of male with SCD.
Recommendations
Male patients with SCA with high ferritin level may have gonadal hormone deficiency, so they should be subjected to regular medical follow-up with good monitoring to iron profile studies and receiving chelating agents for iron to prevent gonadal damage. More researches are needed to address if disease severity affects hormonal levels in patients with SCA.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sergent GR. Nomenclature and genetics of sickle cell disease. In: Textbook of Haematology on Sickle Cell Disease.
2. Silva M, Vargas S, Coelho A, et al. Hemorheological alterations in sickle cell anemia and their clinical consequences - The role of genetic modulators. Clin Hemorheol Microcirc. 2016;64(4):859–866. doi:10.3233/CH-168048
3. Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood. 2013;122(24):3892–3898. doi:10.1182/blood-2013-05-498311
4. Makulo JR, Itokua KE, Lepira RK, et al. Magnitude of elevated iron stores and risk associated in steady-state sickle cell anemia Congolese children: a cross-sectional study. BMC Hematol. 2019;19(3). doi:10.1186/s12878-019-0134-7.
5. Akinbami AA, Dosunmu AO, Adediran AA, et al. Serum ferritin levels in adults with sickle cell disease in Lagos, Nigeria. J Blood Med. 2013;4:59–63. doi:10.2147/JBM.S42212
6. Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm (Vienna). 2011;118(3):
7. Wood JC, Noetzl L, Hyderi A, Joukar M, Coates T, Mittelman S. Predicting pituitary iron and endocrine dysfunction. Ann NY Acad Sci. 2010;1202:123–128. doi:10.1111/j.1749-6632.2010.05545.x
8. Özen S, Ünal *, Erçetin N, Taşdelen B. Frequency and risk factors of endocrine complications in turkish children and adolescents with sickle cell anemia. Turk J Haematol. 2013;30(1):25–31. doi:10.4274/tjh
9. Huang AW, Muneyyiric-Delale O. Reproductive endocrine issues in men with sickle cell anaemia. Andrology. 2017;5(4):679–690.
10. Hagag AA, El-Farargy MS, Elrefaey S, Amany M, Elenien A. Study of gonadal hormones in Egyptian female children with sickle anaemia in correlation with iron overload; Single centre study. Hematol Oncol Stem Cell Ther. 2016;9:1–7. doi:10.1016/j.hemonc.2015.11.005
11. Garadah TS, Jaradat AA, Alalawi ME, Hassan AB. Hormonal and echocardiographic abnormalities in adult patients with sickle cell anaemia in Bahrin. J Blood Med. 2016;7:283–289. doi:10.2147/JBM.S124426
12. Ezeiruaku FC. Sex hormones and prolactin ranges in sickle cell disease subjects in Southern Nigeria. J Nat Sci Res. ISSN 2224-3186(Paper) ISSN 2225-0921(Online). 2016;6(18).
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
