Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
The Effect of Rooibos (Aspalathus linearis), Honeybush (Cyclopia intermedia) and Sutherlandia (Lessertia frutescens) on Testicular Insulin Signalling in Streptozotocin-Induced Diabetes in Wistar Rats
Authors Omolaoye TS , Windvogel SL, Du Plessis SS
Received 5 October 2020
Accepted for publication 10 February 2021
Published 19 March 2021 Volume 2021:14 Pages 1267—1280
DOI https://doi.org/10.2147/DMSO.S285025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Temidayo S Omolaoye,1,2 Shantal Lynn Windvogel,1,3 Stefan S Du Plessis1,2
1Division of Medical Physiology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa; 2Department of Basic Sciences, College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates; 3Centre for Cardio-Metabolic Research in Africa, Stellenbosch University, Cape Town, South Africa
Correspondence: Stefan S Du Plessis
Department of Basic Sciences, College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, P.O. Box 505055, Dubai, 505055, United Arab Emirates
Email [email protected]
Background: Testicular insulin signalling is altered in diabetic (DM) males. While unravelling the mechanism through which DM exert these detrimental effects, studies have shown the importance of insulin regulation in glucose homeostasis, and how a lack in insulin secretion indirectly led to reduced male fertility. The current study aimed to investigate the role of rooibos, honeybush and Sutherlandia on insulin signalling in the testicular tissue of type I diabetic rats.
Methods: Animals (n=60) were randomly divided into six groups. The groups include a control group, a vehicle group, and diabetes was induced in the remainder of animals via a single intraperitoneal injection of STZ at 45mg/kg. The remaining four groups included a diabetic control (DC), diabetic + rooibos (DRF), diabetic + honeybush (DHB) and diabetic + Sutherlandia group (DSL). Animals were sacrificed after seven weeks of treatment, and blood and testes were collected.
Results: All diabetic groups (DC, DRF, DHB, DSL) presented with a significant increase in blood glucose levels after diabetes induction compared to the control and vehicle (p< 0.001). The DC animals presented with decreased testicular protein expression of IRS-1, PkB/Akt and GLUT4 compared to controls. DRF and DHB animals displayed an acute upregulation in IRS-1, while the DSL group showed improvement in IRS-2 compared to DC. Although, DRF animals presented with a decrease in PkB/Akt, DHB and DSL animals displayed upregulation (22.3%, 48%) compared to controls, respectively.
Conclusion: The results taken together, it can be suggested that these infusions may enhance insulin signalling through diverse pathways.
Keywords: testis, rooibos, honeybush, Sutherlandia, diabetes, insulin signalling
Introduction
Diabetes mellitus (DM), a disease that occurs because of either a lack in the synthesis and secretion of insulin (type I) or the insensitivity of the tissue to the effect of insulin (type II), is a non-communicable disease that brings about complications in various systems, including the male reproductive system. Both experimental and human studies have highlighted the diverse adverse effects of DM (both type I and type II) on male fertility.1–6 In experimental type I diabetic animals, studies have reported altered spermatogenesis, diminished epididymal sperm reserve,7 abnormal Sertoli-Sertoli cell junction complexes, reduction in the testicular weight, sperm concentration, testicular morphology index and decreased fertility potential.8 Lopez-Alvarenga et al reported that the suppression of both endogenous pulsatile and exogenous gonadotropin-releasing hormone (GnRH) stimulated the secretion of luteinizing hormone (LH) in men with type I DM, which resulted in hypogonadotropism.4 Studies have also shown that there are reduced levels of testosterone, LH and follicle-stimulating hormone (FSH) in the sera of diabetic men.6,9,10 Also reported is an increase in spermatozoa with nuclear and mitochondrial DNA fragmentation,11,12 reduced sperm motility13 and decreased sperm with normal morphology.14 In the course of unravelling the mechanism(s) through which DM exert these detrimental effects, studies have shown the importance of insulin regulation in glucose homeostasis, insulin signalling and how a lack in insulin secretion indirectly led to reduced male fertility.15–18
Insulin receptors, such as insulin-like growth factor 1-receptor (IGF1-R) and insulin receptor related-receptor (IRR), are tetrameric proteins having four functional subunits (2α and 2β subunits). These receptors can form functional complexes with insulin, such that the inhibitory mutation in one receptor subunit can inhibit the activity of the others.19 Interestingly, studies have shown the presence of these receptors in the Sertoli cells, Leydig cells, spermatogonia and spermatocytes.20,21 This suggests that they are involved in glucose metabolism, insulin signalling, and are important in the development of the testis.22,23
Gomez et al reported a decrease in both systemic and testicular insulin concentration in type I diabetic rats with resultant disrupted spermatogenesis. It was suggested that, although, the testis produces insulin, systemic hypoinsulinemia due to type I DM adversely affect male fertility.17 Despite findings showing that Sertoli cells secrete their own insulin, a lack in pancreatic β-cell insulin production still affected male reproduction adversely. Schoeller et al investigated the role of both systemic and testicular insulin signalling in male fertility using the Akita mouse diabetic model. They validated the presence of insulin in the testes and further reported that insulin affects male fertility through its indirect regulatory effect on the hypothalamic-pituitary-gonadal axis (HPGA).16 It was hypothesized that in DM, due to hypoinsulinemia/hyperglycaemia, insulin signalling is altered and the function of HPGA is disrupted, which cumulatively resulted in altered spermatogenesis and reduced sperm function. This was evidenced by the decrease in the levels of serum GnRH, LH, FSH and testosterone as well as testicular LH, FSH and testosterone in the diabetic animals. However, when insulin was administered, the levels of these hormones were increased, and normal spermatogenesis was restored. They concluded that insulin promoted fertility by restoring function to the HPGA, thus normalizing the hormone levels of LH and testosterone.16 However, other studies have further shown the importance of normal insulin signalling in normal male reproduction.24,25
Since the importance of maintaining normal insulin signalling has been highlighted, it is essential to explore natural and artificial agents that have health benefits, and that can possibly enhance this process. Rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) and Sutherlandia (Lessertia frutescens) are plants native to Southern Africa.26,27 Studies have highlighted some of their health benefits,28–32 including anti-diabetic properties.33,34 Kawano et al reported that aspalathin, a component of rooibos tea, improved impaired glucose tolerance in db/db mice at 30, 60, 90 and 120 minutes.35 They further added that rooibos, in a dose-dependent manner, enhanced glucose uptake in cultured L6 myotubes and also increased the secretion of insulin by RIN-5F cells. The results of Kawano et al are supported by several other authors36–41 with the inclusion that aspalathin promoted activated protein kinase (AMPK) phosphorylation, enhanced GLUT4 translocation and also improved protein kinase B activation. This suggests that rooibos may improve glucose metabolism by enhancing glucose uptake and restoring normal insulin signalling. Studies have also highlighted the anti-diabetic potential of honeybush42–46 and Sutherlandia47–49 in both in vitro and in vivo experiments. All these studies showcasing the hypoglycaemic effects of rooibos, honeybush and Sutherlandia were carried out in models of cardiovascular and other systemic diseases. There are a few studies that have investigated these infusions (apart from rooibos) in the context of DM-related male reproductive disorder. Hence, this study was designed to investigate the role of rooibos, honeybush and Sutherlandia on testicular insulin signalling in type I diabetic rats.
Materials and Methods
Infusion Preparation
Rooibos (Aspalathus linearis, 2% fermented), was obtained from Carmien SA PTY LTD, South Africa, while honeybush (Cyclopia intermedia, 4% fermented) and Sutherlandia (Lessertia frutescens, 0.2% unfermented) were obtained from Afrinaturals, South Africa. They were prepared according to previously established laboratory protocols. Rooibos was prepared according to the method described by Marnewick et al,50,51 honeybush, by Du Toit and Joubert52 and Sutherlandia by Tobwala et al.53 In summary, 2% fermented rooibos was prepared by adding 20g of dried rooibos in 1 litre of boiling water and allowed to steep for 30 minutes. The mixture was filtered using a cheesecloth, followed by a number 4 and then a number 1 filter paper (WhatmanTM, Buckinghamshire, UK). Filtered teas/infusions were transferred into dark plastic containers and stored at 4°C. Infusions were stored in dark plastics, and also prepared freshly every other day (48 hours), to prevent the degradation of polyphenols that have a short half-life and are light sensitive.54 Fermented honeybush (4%; 40g in 1 litre) and unfermented Sutherlandia (0.2%) were prepared following the same procedures. The herbal teas served as the only drinking fluid for these infusion groups and fluid intake of the animals was measured thrice weekly.
Determination of the Phenolic Content Present in Rooibos, Honeybush and Sutherlandia
All analyses were carried out using the infusion concentrations that the animals were treated with, ie, 2% fermented rooibos, 4% fermented honeybush and 0.2% unfermented Sutherlandia.
Soluble Solid Content
The soluble solid content of the infusions (rooibos, honeybush and Sutherlandia) was determined gravimetrically (6 repetitions) and examined in triplicate at each time point. Briefly, the glass beakers used were firstly washed, placed in the oven overnight at 70°C and cooled in a desiccator for another 24 hours. For the assay, dried beakers were weighed prior to adding 1mL of the respective infusions. The aliquots were dried in an oven for 24 hours at 70°C, whereafter, they were placed in a desiccator for another 24 hours. Glass beakers containing the dried content were weighed again. The soluble solid content in each herbal infusion was determined by subtracting the initial weight from the final weight.
Total Polyphenol Content
The total polyphenol content was measured as described by Arthur et al, using the Folin–Ciocalteau method.55 In Brief, an initial 20µL of blank (deionized water), standard (10–100mg/L gallic acid) and the respective infusions were loaded into a 96-well plate. Thereafter, 100µL of Folin-Ciocalteau reagent and 80µL of 7.5% (m/v) Na2CO3 were added, respectively. The plate was gently vortexed and allow to stand for 2 hours at room temperature in the dark. The absorbance was measured at 765 nm and expressed as mg gallic acid equivalents per mg soluble solids.
Animal Care and Study Design
Fourteen-week-old adult male Wistar rats weighing 250–300g were housed in the Animal Unit of the Faculty of Medicine and Health Sciences, Stellenbosch University (18–23°C, 12:12 light/dark cycle). Animals were caged individually, and had free access to food and water/infusions and were treated according to the recommendations of the Laboratory Animal Care of the National Society of Medical Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.56 Ethics approval was obtained from the Stellenbosch University Animal Ethics Committee (SU-ACUD17-00016).
Type I diabetes was induced with streptozotocin (STZ, S0130-IG, Sigma, South Africa) and a stock solution (30mg/mL) was prepared by dissolving it in freshly prepared 0.1M sodium citrate buffer (pH 4.5). From the stock solution, rats were administered a single intraperitoneal injection of STZ (45mg/kg body weight). The successful induction of diabetes was confirmed after one week, if animals showed a blood glucose level of ≥14mmol/L, using a Glucoplus™ glucometer. Animals in the vehicle group were injected citrate buffer intraperitoneally.
Sixty rats were randomly assigned to six groups (n=10) to avoid bias. The groups include a control group (water), vehicle group (0.1M citrate buffer), a diabetic control group (DC, STZ45mg/kg + water), a diabetic + rooibos (DRF, STZ45mg/kg + 2% fermented rooibos), a diabetic + honeybush (DHB, STZ45mg/kg + 4% fermented honeybush) and a diabetic + Sutherlandia group (DSL, STZ45mg/kg + 0.2% unfermented Sutherlandia). Food intake, fluid intake and body weights were measured three times a week, and blood glucose levels were measured once weekly. Animals were sacrificed after 7 weeks of DM induction. Blood samples for hormonal analysis were collected immediately after sacrifice, and the testes were harvested and weighed. Plasma was used for hormonal assays, while the testis was used for Western blotting. The relative testicular weight was expressed as a percentage of body weight. The reported average blood glucose levels were the mean of blood glucose measured over seven weeks, and the fasting blood glucose levels were recorded immediately before sacrifice.
Intraperitoneal Glucose Tolerance Test
The intraperitoneal glucose tolerance test (IPGTT) was performed 2–3 days before sacrifice. Animals were fasted overnight but allowed free access to water/infusion as appropriate. After 14–16 hours of fasting, animals were injected intraperitoneally with a 20% glucose solution at a dose of 2g/kg body weight (10µL/g). Blood glucose levels were then measured at 12 different time points (0, 3, 5, 10, 15, 20, 25, 30, 45, 60, 90 and 120 minutes).
Western Blot Analysis
Tissue homogenates and protein determination were obtained as previously described.57,58 Tissue lysates were prepared by diluting samples in Laemmli sample buffer and lysis buffer, boiled for 5 minutes and 50ug protein/µL was separated by electrophoresis on either a 12% SDS-PAGE mini-PROTEAN gel or a 4–20% stain-free precast gel (Criterion TGX, Biorad, USA). The running protocol consisted of an initial 10 minutes electrophoresis at 100V and 200mA followed by 30–40 minutes at 200V and 200mA for the mini-PROTEAN gels, while the running protocol for the precast gel consisted of an initial 10 minutes electrophoresis at 100V and 200mA followed by 25–30 minutes at 140V and 140mA. Gels were activated using ChemiDoc (BioRad), and thereafter, proteins were transferred onto a Millipore Immobilon-P transfer membrane (0.45µm) (Immobilon®-P, Merck Millipore Ltd, Germany). Non-specific sites were blocked with 5% fat-free milk in TBS-tween. Primary antibodies were diluted in TBS-Tween in a 1:1000 ratio while the secondary antibody was diluted in TBS-Tween in a 1:4000 ratio. All data points are from independent biological repeats (n=4–5). The measured biomarkers were GLUT 4, tPkB/Akt, p/tERK1/2 and p/tIRS 1/2. All primary antibodies were obtained from Cell Signalling Technology. A goat anti-mouse/rabbit-horseradish peroxidase-conjugated antibody (Sigma-Aldrich) was used as the secondary antibody.
Hormonal Assays
The plasma concentration of testosterone (E-EL-0072), estradiol (E-EL-0065) and insulin (E-EL-R2466) was measured using a commercially available ELISA kit (Elabscience Biotechnology, Hubei) as per the manufacturer’s instructions.
Statistical Analysis
GraphPad Prism™ software (GraphPad™ Software, Version 8.2, San Diego, CA, USA) was used for the statistical analysis. Normal data distribution was measured using the Shapiro–Wilk normality test. When data passed normality test, a one-way ANOVA of variance with a Tukey’s Post-hoc Test was performed. Where data were not evenly distributed, a Kruskal–Wallis test and a Dunn’s Post-hoc Test were carried out. To investigate the plausible mixed effect of the phosphorylated to total protein ratios, a mixed effect or ordinary two-tailed, two-way ANOVA was performed. Where appropriate, a Pearson’s correlation matrix was carried out. Significance was accepted at p< 0.05. Results are expressed as mean ± SD.
Results
Biometric Data
Diabetic control (DC) animals consumed more food and drank more water compared to the control (p<0.001) and vehicle (p<0.001) groups. Diabetic animals receiving rooibos (DRF), honeybush (DHB) and Sutherlandia (DSL) followed the same trend as DC when compared to the control (p<0.001) and vehicle (p<0.001). However, the diabetic animals treated with the infusions (DRF, DHB, and DSL) displayed a significant decrease in food intake when compared to the DC animals (35.38±4.936g, 32.22±5.440g, 34.92±4.144g vs 40.43±2.872g, p<0.001).
DC animals presented with elevated fasting blood glucose levels when compared to the control (19.62±7.49mmol/L vs 6.150±0.7246mmol/L, p<0.001) and vehicle (19.62±7.49 mmol/L vs 5.660±1.02mmol/L, p<0.001) groups. Although the diabetic infusion treatment groups (DRF, DHB and DSL) presented with a significant increase in fasting blood glucose levels when compared to the control and vehicle groups (p<0.001), their (DRF, DHB and DSL) respective fasting blood glucose levels mildly decreased when compared to the DC group, (18.91±9.148, 14.98±9.436, 16.60±8.925 vs 19.62±7.492; p>0.05) (Table 1). DC animals showed a significant reduction in body weight when compared to control (p<0.05). The respective infusion treatment groups (DRF, DHB and DSL) also presented with a decrease in body weight when compared to the control (p<0.001) and vehicle (p<0.001) (Table 2).
 |
Table 1 Biometric Data |
 |
Table 2 Body, Organ, and Tissue Weights |
Total Polyphenols
There was a significant difference in the amount of soluble solid contents (SS) between the infusions (P<0.0001). The SS content of fermented RF was significantly lower than that of HB (P<0.001) and SL (P<0.001).
The phenolic content of fermented RF was significantly higher than HB (P<0.0001) and SL (P<0.0001), while that of HB was higher than that of SL (P<0.001) (Table 3). Regarding the daily phenolic consumption relative to 100g body weight, the diabetic animals treated with the infusions had a significantly increased intake in the total amount of total polyphenols (Table 3).
 |
Table 3 Total Polyphenolic Contents |
IPGTT
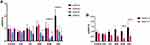
There was a significant difference in the measured glucose levels between 0 and 120 minutes, in the DC, DRF, DHB and DSL groups (p=0.001), as indicated with glucose tolerance. There was, however, a non-significant improvement in the glucose tolerance of DHB animals (Figure 1A). Additionally, there was a significant positive correlation (r=0.7631) between fasting blood glucose levels and fluid intake per day (p<0.0001) (Figure 1B).
Western Blot
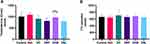
All diabetic animals (DC, DRF, DHB and DSL) presented with increased phosphorylated ERK1/2 (pERK1/2) when compared to the vehicle (0.988±0.252, 1.226±0.368, 1.028±0.369, 1.408±0.682 vs 0.766±0.138, p<0.05). These groups (DC, DRF, DHB and DSL, respectively) also displayed a reduction in the total ERK1/2 (tERK1/2) when compared to the control and vehicle (0.6438±0.5, 0.51±0.398, 0.316±0.323, 0.56±0.537 vs 0.847±0.281 and 0.7407±0.094, p<0.001) (Figure 2A–C). Furthermore, DHB animals presented with a significant difference in the ratio of phosphorylated to total ERK1/2 proteins compared to controls (p=0.02). This ratio was however increased in the DSL compared to the vehicle (p=0.02).
Diabetic control animals presented with a decrease in tIRS-1 (−48% and −32%) when compared to the control and vehicle groups, respectively. The diabetic infusion treatment groups (DRF, DHB, DSL) however displayed a non-significant decrease in trend in tIRS-1 when compared to the control and vehicle groups. DRF and DHB were however acutely upregulated when compared to DC (0.515±0.56, 0.5±0.453 vs 0.48±0.246) (Figure 3). Despite the reduced tIRS-1 displayed by DSL animals when compared to the DC, they showed a nearly 50% upregulation in tIRS-2 when compared to the DC (Figure 3A and B).
DC animals presented with a non-significant decrease (−41.7%, −34%) in the expression of PkB/Akt when compared to the control and vehicle groups, respectively. The diabetic infusion treatment groups (DRF, DHB, DSL) also displayed a percentage decrease in PkB/Akt when compared to the control (−42%, −28%, −13%) and vehicle (−35%, −19%, −2%) groups, however, DRF animals presented with a further decrease in the total PkB/Akt compared to DC group. While, the DHB and DSL animals displayed an upregulation in total PkB/Akt (22.3%, 48%) when compared to DC, respectively (Figure 4A and B).
DC animals showed a reduction in tGLUT4 (−30%, −4%) when compared to the control and vehicle, respectively. DRF and DHB groups also displayed a non-significant decrease in GLUT4 expression when compared to the DC and control groups. In contrast, DSL animals presented with an increase in GLUT4 (70.9%) when compared to the DC group (p=0.08) (Figure 5A and B).
Hormonal Assays
Although, not significant, DC animals presented with a mild decrease in the level of plasma testosterone concentration (−16.5%) when compared to the vehicle. DRF and DSL groups displayed a significant decrease compared to vehicle (811.2±126.5nmol/L vs 1085±95.66nmol/L, p=0.01) (793.1±192.9nmol/L vs 1085±95.66nmol/L, p=0.02) respectively. The testosterone concentration in DHB animals was mildly increased, when compared to DC (Figure 6A). Furthermore, there were no significant differences in the level of plasma 17β-estradiol concentrations between the groups (Figure 6B).
Discussion
The potential hypoglycaemic effects of rooibos, honeybush and Sutherlandia extracts have been widely investigated in both in vivo35,42,47 and in vitro studies.49,59,60 The mechanisms through which this occur have been mostly determined in in vitro studies using cell lines, such as adipocytes, cardiomyocytes, fibroblast, myotubes/myoblast, and etcetera. However, remarkably few to no studies have reported the effects of rooibos, honeybush and Sutherlandia on testicular insulin signalling. Hence, the current study was designed.
As expected, the diabetic control animals of the current study displayed polyphagia, polydipsia, excessive weight loss, sustained increased blood glucose and impaired glucose tolerance. These are features typical of type I DM. This is in agreement with several studies reporting the symptomatic characteristics of DM.61 However, the diabetic animals treated with rooibos (DRF), honeybush (DHB) or Sutherlandia (DSL) had significantly decreased food intake, and polydipsia was suppressed, as evidenced by the percentage reduction in fluid intake and by the significant positive correlation seen between fluid intake and the fasting blood glucose (FBG) (r=0.7631, p<0.0001). This was accompanied with a reduction of 23.6% and 15% in the FBG levels of DHB and DSL animals, respectively. This supports the theory that increased FBG can lead to osmotic diuresis thereby stimulating osmoreceptors to increase fluid intake. Although, the glucose tolerance of DRF and DHB animals improved, it did not reach significance and the glucose tolerance of DSL animals remained unchanged. The slight/mild decrease in FBG levels observed in the DRF animals of the current study is partly supported by a study that showed a significant reduction in the FBG levels of obese diabetic mice after treatment with aspalathin (a component of rooibos)62 and was accompanied with improved glucose tolerance. This is also in agreement with the findings of Muller et al, who showed that rooibos has a glucose-lowering effect in diabetic rats by improving the glucose tolerance.41 The decrease in the FBG of up 23.6% observed in the DHB animals of the current study concurs with Muller et al, who reported reduced FBG levels in obese insulin-resistant rats after administration of honeybush extract.42 Additionally, the decrease in FBG levels of DSL animals in the current study is supported by Chadwick et al who showed diabetic rats receiving Sutherlandia displayed lower blood glucose as evidenced by normoinsulinaemic levels and increased glucose uptake.47 The SS content (mg/mL) of rooibos was lower than that of honeybush and Sutherlandia (13.4±0.003; 16.26±0.012 vs 5.18±0.003), while the TPC of rooibos was higher than honeybush and Sutherlandia. Awoniyi et al in the same vein, reported that the SS content of 2% fermented rooibos used in their study was 5±1.2mg/mL.28 The SS content of honeybush differed from the report of North et al.63 The difference in the SS content of honeybush, and the TPC of rooibos, honeybush and Sutherlandia may in part be due to the difference in harvest times, production process and the part of the plant material used, amongst others.
In normal insulin signalling, IRS 1/2 are the most important insulin receptor substrate for glucose homeostasis,64 as they act as binding sites for Src-homology-2 domain containing molecules such as PI3K. Binding of these molecules to IRS1/2 activates series of cascade signalling which eventually activates PkB. Activation of PkB/Akt in turn stimulates the translocation of GLUT4 from the intracellular pools to the plasma membrane.
The absence of GLUT4 in the testis has been reported by several authors,65–67 however, Verma and Haldar (2016) reported the presence of GLUT4 in the testis of adult golden hamster.68 Gomez et al showed that GLUT8 is the dominant glucose transporter in the testis and that both insulin depletion and hyperglycaemia do not regulate its expression.17 A year after this report, Lampioa and du Plessis reported that insulin stimulates GLUT8 expression in human spermatozoa and they further speculated that GLUT8 translocation is aided through the PkB/Akt pathway.69 In the current study, GLUT4 was measured in the testis and all groups expressed GLUT4. Furthermore, both the control and vehicle groups of this study expressed the proteins IRS1/2, PkB/Akt, ERK1/2 and GLUT4 in the testis. These results suggest the involvement of GLUT4 in testicular insulin signalling, in addition to the already known stimulation of GLUT8 during testicular glucose metabolism.
Additionally, the testicular tissue of diabetic control animals of the current study had reduced IRS1/2, PkB/Akt and GLUT4 protein expression levels. This is in agreement with another study in which the authors reported impaired insulin signalling in C3A liver cells induced with insulin resistance, as these cells displayed decreased PtdIns (3, 4, 5)P2 and subsequent reduction in PkB.60 In addition to the impaired insulin synthesis and secretion observed in diabetes, studies have also shown that this disorder in part develops because of disruption in IRS ½.64,70,71 Araki et al reported impaired glucose tolerance, decreased insulin/insulin-like growth factor-1 (IGF-1) stimulated glucose uptake, and reduced intrauterine growth, in targeted gene mutated IRS-1 deficient mice.70 Withers et al additionally showed that disruption of IRS-2 impaired both pancreatic β cell function and peripheral insulin signalling in IRS-2 deficient mice. They further reported that these mice displayed dysfunctional glucose homeostasis, and hence concluded that disruption of IRS-2 might contribute to the pathophysiology of human type II DM.71 Pertaining to male reproduction, Griffeth et al reported that deletion of IRS-2 in mice resulted in a 45% reduction in testicular weight with consequent decreases in Sertoli cells, spermatogonia, spermatocytes, spermatids and the epididymal sperm reserve. However, there was a normal cellular association, that is, the structural architecture of the seminiferous tubules was intact.24 Yagci and Zik also reported that IGF-1 plays a significant role in testicular function and germ cell development. As the receptor for IGF-1 was distributed in the pachytene primary spermatocyte and Leydig cells of aged rats.25 This suggests the importance of maintaining normal testicular insulin signalling, as disruption in signalling molecules, especially, IRS 1/2, PkB/Akt and GLUT may impair testicular function. Additionally, the diabetic control animals of this study had lower plasma testosterone levels. This is in agreement with several studies that reported reduced serum testosterone following diabetes induction.15,72
Regarding the effects of rooibos on testicular insulin signalling, the diabetic animals receiving rooibos (DRF) presented with an increase in testicular protein expression of IRS proteins 1 and 2 Interestingly, there was also a reduction in the expression of PkB/Akt and GLUT4. The decrease in PkB/Akt and GLUT4 observed in the testicular tissue of DRF rats of the current study is in contrast with the findings of Mazibuko et al, which showed the reversal of insulin resistance in 3T3-L1 adipocytes treated with rooibos through the suppression of phosphorylated IRS-1, nuclear factor kappa beta (Nf-kB), and AMPK and increased GLUT4 expression.39 In another manuscript, the same group of authors reported the ameliorative effects of rooibos on insulin-resistant C2C12 muscle cells. This was evidenced by the enhanced glucose uptake, mitochondrial activity, and ATP production after treating the muscle cells with rooibos. Mechanistically, it was shown that rooibos increased the activation of Akt, AMPK and GLUT4, suggesting that rooibos can exert its ameliorative effects through the PkB/Akt/GLUT4 pathway in these cells.38 The difference between the results of the current study and the reports of Mazibuko et al may be due to (i) different models of investigation (in vivo/in vitro), (ii) different tissues (testis/adipocytes and muscle cells) and (iii) the plausibility that rooibos does not enhance testicular insulin signalling through the PkB/Akt/GLUT4 pathway.
Studies have shown that insulin can also activate the mitogen-activated protein kinases (MAPK), especially the ERKs through the binding of growth factor receptor-bound protein-2 (GRB-2) and Son-of-sevenless proteins (SOS) to IRS1/2. GRB-2 and SOS can bind IRS1/2 because they contain the Src-homology-2 domain. Binding of these proteins to IRS1/2 activates membrane bound Ras. The phosphorylation of Ras stimulates a stepwise activation of kinase signalling of Raf, MEK and ERK. ERK is then translocated into the nucleus where it stimulate the phosphorylation of transcription factors to initiate gene expression needed for cell proliferation and differentiation.73 Interestingly, the DRF animals of the current study presented with an upregulation (13.2%) in phosphorylated ERK 1/2. Although, the upstream signalling molecules (GRB-2 and SOS) were not measured, it may be suggested that rooibos may rather exert its effect on diabetic testicular insulin signalling through the IRS/ERK pathway. Regarding testosterone levels, diabetic animals receiving rooibos displayed a non-significant decrease in plasma testosterone levels. This partly concur with Opuwari and Monsees who reported that both fermented and unfermented rooibos reduces testosterone production in TM3 Leydig cells.74 This suggests that although rooibos may play a role in insulin signalling, it does not influence testosterone levels.
While studies have shown the hypoglycaemic effects of honeybush,75,76 little to nothing is known about the mechanism/pathway at which these effects are exerted. In the current study, diabetic animals receiving honeybush (DHB) displayed a mild increase in testis protein expression of IRS 1/2 and PkB/Akt while GLUT4 remained unchanged. Since GLUT8 was not measured in the current study, little can be said about it. However, extrapolating from previous reports, it can be speculated that honeybush may enhance insulin signalling through the stimulation of IRS1/2, PkB/GLUT8 pathway. Additionally, the DHB animals presented with a mild increase (7%) in the level of plasma testosterone. Since, this is the first study demonstrating this property, it can be suggested that honeybush may have a positive effect on testosterone production, although the mechanism through this occur still needs further investigation.
Diabetic animals receiving Sutherlandia presented with increased tIRS-2, tPkB and tGLUT4. The increase in GLUT4 observed in the current study concur with Dang et al who reported that pinitol (a Sutherlandia component) enhanced GLUT4 translocation from the cytosol (intracellular vesicles) to the plasma membrane in skeletal muscle of C57BL/6 mice.77 Bates et al also showed that pinitol exerts a mild but sustained hypoglycaemic insulin-like effect in the murine model of type I DM.33 Additionally, Williams et al reported that Sutherlandia prevents changes in DM-related gene expression in insulin-resistant cell model and that it upregulates GLUT4 translocation to the cell surface.49 Hence, it can be suggested that the decrease in the blood glucose levels of diabetic animals receiving Sutherlandia observed in the current study may be through the stimulation of the IRS1/2, PkB/Akt, GLUT4 pathway.
In conclusion, the result of the present study has shown the presence of GLUT4 in the testis and its plausible role in insulin signalling as seen in a typical cell. We have also demonstrated the role of rooibos, honeybush and Sutherlandia in insulin signalling. Therefore, it can be suggested that these infusions may enhance insulin signalling through diverse pathways, but further investigations are required to elucidate the specific upstream and downstream proteins that are plausibly activated during this process.
Acknowledgments
The authors would like to thank Dr Michelle Smit-van Schalkwyk for the generous donation of tissue samples.
Disclosure
A portion of this study was made possible by a research contribution from the National Research Foundation and Stellenbosch University. Rooibos used during the conduct of this study was a gift from Carmién Tea PTY LTD. The authors report no other conflicts of interest in this work.
References
1. Hassan AA, Hassouna MM, Taketo T, Gagnon C, Elhilali MM. The effect of diabetes on sexual behavior and reproductive tract function in male rats. J Urol. 1993;149(1):148–154. doi:10.1016/S0022-5347(17)36028-7
2. Sanguinetti RE, Ogawa K, Kurohmaru M, Hayashi Y. Ultrastructural changes in mouse leydig cells after streptozotocin administration. Exp Anim. 1995;44(1):71–73. doi:10.1538/expanim.44.71
3. Bondarenko LB, Shayakhmetova GM, Byshovets TF, Kovalenko VM. Pyrazinamide-mediated changes in rat type I collagen and spermatogenesis indices. Acta Pol Pharm Drug Res. 2011;68(2):285–290.
4. López-Alvarenga JC, Zariñán T, Olivares A, González-Barranco J, Veldhuis JD, Ulloa-Aguirre A. Poorly controlled type I diabetes mellitus in young men selectively suppresses luteinizing hormone secretory burst mass. J Clin Endocrinol Metab. 2002;87(12):5507–5515. doi:10.1210/jc.2002-020803
5. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(11):5462–5468. doi:10.1210/jc.2004-0804
6. Maneesh M, Jayalakshmi H, Singh TA, Chakrabarti A. Impaired hypothalamic-pituitary-gonadal axis function in men with diabetes mellitus. Indian J Clin Biochem. 2006;21(1):165–168. doi:10.1007/BF02913088
7. Amaral S, Mota PC, Lacerda B, et al. Testicular mitochondrial alterations in untreated streptozotocin-induced diabetic rats. Mitochondrion. 2009;9(1):41–50. doi:10.1016/j.mito.2008.11.005
8. Cameron DF, Rountree J, Schultz RE, Repetta D, Murray FT. Sustained hyperglycemia results in testicular dysfunction and reduced fertility potential in BBWOR diabetic rats. Am J Physiol Endocrinol Metab. 1990;259(6):622–626. doi:10.1152/ajpendo.1990.259.6.e881
9. Hussein Z, Al-Qaisi J. Effect of diabetes mellitus type 2 on pituitary gland hormones (FSH, LH) in men and women in Iraq. J Al-Nahrain Univ Sci. 2012;15(3):75–79. doi:10.22401/jnus.15.3.11
10. Rezvani MR, Saadatjoo SA, Sorouri S, Fard MH. Comparison of serum free testosterone, luteinizing hormone and follicle stimulating hormone levels in diabetics and non-diabetics men- a case-control study. J Res Health Sci. 2012;12(2):98–100. doi:10.9790/0853-13536571
11. Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–1877. doi:10.1093/humrep/dem077
12. Agbaje IM, McVicar CM, Schock BC, et al. Increased concentrations of the oxidative DNA adduct 7,8-dihydro-8-oxo-2-deoxyguanosine in the germ-line of men with type 1 diabetes. Reprod Biomed Online. 2008;16(3):401–409. doi:10.1016/S1472-6483(10)60602-5
13. La Vignera S, Vicari E, Calogero AE, Condorelli R, Lanzafame F. Diabetes, oxidative stress and its impact on male fertility. J Andrological Sci. 2009;16(1):42–46.
14. Delfino M, Imbrogno N, Elia J, Capogreco F, Mazzilli F. Prevalence of diabetes mellitus in male partners of infertile couples. Minerva Urol e Nefrol. 2007;59(2):131–135.
15. Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25(5):706–719. doi:10.1002/j.1939-4640.2004.tb02845.x
16. Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary- gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012;61(7):1869–1878. doi:10.2337/db11-1527
17. Gómez O, Ballester B, Romero A, et al. Expression and regulation of insulin and the glucose transporter GLUT8 in the testes of diabetic rats. Horm Metab Res. 2009;41(5):343–349. doi:10.1055/s-0028-1128146
18. Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update. 2018;24(1):86–105. doi:10.1093/humupd/dmx033
19. Butler AA, LeRoith D. Tissue-specific versus generalized gene targeting of the IGF1 and IGF1R genes and their roles in insulin-like growth factor physiology. Endocrinology. 2001;142(5):1685–1688. doi:10.1210/endo.142.5.8148
20. MacLean JA, Hu Z, Welborn JP, et al. The RHOX homeodomain proteins regulate the expression of insulin and other metabolic regulators in the testis. J Biol Chem. 2013;288(48):34809–34825. doi:10.1074/jbc.M113.486340
21. Griffeth RJ, Bianda V, Nef S. The emerging role of insulin-like growth factors in testis development and function. Basic Clin Androl. 2014;24(1):1–10. doi:10.1186/2051-4190-24-12
22. Nakayama Y, Yamamoto T, Abé SI. IGF-I, IGF-II and insulin promote differentiation of spermatogonia to primary spermatocytes in organ culture of newt testes. Int J Dev Biol. 1999;43(4):342–347. doi:10.1387/ijdb.10470651
23. Pitetti JL, Calvel P, Zimmermann C, et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol. 2013;27(5):814–827. doi:10.1210/me.2012-1258
24. Griffeth RJ, Carretero J, Burks DJ. Insulin receptor substrate 2 is required for testicular development. PLoS One. 2013;8(5):1–11. doi:10.1371/journal.pone.0062103
25. Yagci A, Zik B. Immunohistochemical localization of insulin-like growth factor-I receptor (IGF-IR) in the developing and mature rat testes. J Vet Med Ser C Anat Histol Embryol. 2006;35(5):305–309. doi:10.1111/j.1439-0264.2006.00689.x
26. Morton JF. Rooibos tea, aspalathus linearis, a caffeineless, low-tannin beverage. Econ Bot. 1983;37(2):164–173. doi:10.1007/BF02858780
27. van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J Ethnopharmacol. 2008;119(3):620–629. doi:10.1016/j.jep.2008.08.003
28. Awoniyi DO, Aboua YG, Marnewick J, Brooks N. The effects of rooibos (Aspalathus linearis), green te (Camellia sinensis) and commercial rooibos and green te supplements on epididymal sperm in oxidative stress-induced rats. Phytother Res. 2012;26(8):1231–1239. doi:10.1002/ptr.3717
29. Hong IS, Lee HY, Kim HP. Anti-oxidative effects of Rooibos tea (Aspalathus linearis) on immobilization-induced oxidative stress in rat brain. PLoS One. 2014;9(1):1–9. doi:10.1371/journal.pone.0087061
30. Sanderson M, Mazibuko SE, Joubert E, et al. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine. 2014;21(2):109–117. doi:10.1016/j.phymed.2013.08.011
31. Marnewick JL, Joubert E, Swart P, Van Der Westhuizen F, Gelderblom WC. Modulation of hepatic drug metabolizing enzymes and oxidative status by rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), green and black (Camellia sinensis) teas in rats. J Agric Food Chem. 2003;51(27):8113–8119. doi:10.1021/jf0344643
32. Marnewick JL, van der Westhuizen FH, Joubert E, Swanevelder S, Swart P, Gelderblom WCA. Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem Toxicol. 2009;47(1):220–229. doi:10.1016/j.fct.2008.11.004
33. Bates SH, Jones RB, Bailey CJ. Insulin-like effect of pinitol. Br J Pharmacol. 2000;130(8):1944–1948. doi:10.1038/sj.bjp.0703523
34. Ojewole JAO. Analgesic, antiinflammatory and hypoglycemic effects of Sutherlandia frutescens R. BR. (variety incana E. MEY.) [Fabaceae] shoot aqueous extract. Methods Find Exp Clin Pharmacol. 2004;26(6):409–416.
35. Kawano A, Nakamura H, Hata S-I, Minakawa M, Miura Y, Yagasaki K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine. 2009;16(5):437–443. doi:10.1016/j.phymed.2008.11.009
36. Johnson R, Beer D, Dludla PV, Ferreira D, Muller CJF, Joubert E. Aspalathin from rooibos (Aspalathus linearis): a bioactive C -glucosyl dihydrochalcone with potential to target the metabolic syndrome. Planta Med. 2018;84(9–10):568–583. doi:10.1055/s-0044-100622
37. Kamakura R, Son MJ, de beer D, Joubert E, Miura Y, Yagasaki K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-Ay mice. Cytotechnology. 2015;67(4):699–710. doi:10.1007/s10616-014-9816-y
38. Mazibuko SE, Muller CJF, Joubert E, et al. Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis). Phytomedicine. 2013;20(10):813–819. doi:10.1016/j.phymed.2013.03.018
39. Mazibuko SE, Joubert E, Johnson R, Louw J, Opoku AR, Muller CJF. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol Nutr Food Res. 2015;59(11):2199–2208. doi:10.1002/mnfr.201500258
40. Mikami N, Tsujimura J, Sato A, et al. Green rooibos extract from Aspalathus linearis, and its component, aspalathin, suppress elevation of blood glucose levels in mice and inhibit α-amylase and α-glucosidase activities in vitro. Food Sci Technol Res. 2015;21(2):231–240. doi:10.3136/fstr.21.231
41. Muller CJF, Joubert E, De Beer D, et al. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine. 2012;20(1):32–39. doi:10.1016/j.phymed.2012.09.010
42. Muller CJ, Joubert E, Gabuza K, De Beer D, Fey SJ, Louw J. Assessment of the antidiabetic potential of an aqueous extract of honeybush (Cyclopia intermedia) in streptozotocin and obese insulin resistant wistar rats. In: Phytochemicals - Bioactivities and Impact on Health; 2011:313–331. doi:10.5772/28574
43. Wang HL, Li CY, Zhang B, et al. Mangiferin facilitates islet regeneration and β-cell proliferation through upregulation of cell cycle and β-cell regeneration regulators. Int J Mol Sci. 2014;15(5):9016–9035. doi:10.3390/ijms15059016
44. Saleh S, El-Maraghy N, Reda E, Barakat W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-α. An Acad Bras Cienc. 2014;86(4):1935–1947. doi:10.1590/0001-3765201420140212
45. Jung UJ, Lee M-K, Jeong K-S, Choi M-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004;134(10):2499–2503. doi:10.1093/jn/134.10.2499
46. Akiyama S, Katsumata SI, Suzuki K, Ishimi Y, Wu J, Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2009;46(1):87–92. doi:10.3164/jcbn.09-82
47. Chadwick WA, Roux S, van de Venter M, Louw J, Oelofsen W. Anti-diabetic effects of Sutherlandia frutescens in wistar rats fed a diabetogenic diet. J Ethnopharmacol. 2007;109(1):121–127. doi:10.1016/j.jep.2006.07.012
48. M J, C. K T, R S, Vdv M, Dealtry G, Effect of Sutherlandia frutescens on the lipid metabolism in an insulin resistant rat model and 3T3-L1 adipocytes. Phytother Res. 2012;26(12):1830–1837. doi:10.1002/ptr.4653
49. Williams S, Roux S, Koekemoer T, Van De Venter M, Dealtry G. Sutherlandia frutescens prevents changes in diabetes-related gene expression in a fructose-induced insulin resistant cell model. J Ethnopharmacol. 2013;146(2):482–489. doi:10.1016/j.jep.2013.01.008
50. Marnewick JL, Rautenbach F, Venter I, et al. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J Ethnopharmacol. 2011;133(1):46–52. doi:10.1016/j.jep.2010.08.061
51. Omolaoye T, Windvogel S, Du Plessis S. Testicular oxidative stress and apoptosis status in streptozotocin-induced diabetic rats after treatment with rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia), and Sutherlandia (Lessertia frutescens) infusions. Asian Pacific J Reprod. 2021;10(1):11–20. doi:10.4103/2305-0500.306432
52. Du Toit J, Joubert E. Optimization of the fermentation parameters of honeybush tea (Cyclopia). J Food Qual. 1999;22(3):241–256. doi:10.1111/j.1745-4557.1999.tb00555.x
53. Tobwala S, Fan W, Hines CJ, Folk WR, Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement Altern Med. 2014;14(1):1–11. doi:10.1186/1472-6882-14-271
54. Santos JS, Deolindo CTP, Esmerino LA, et al. Effects of time and extraction temperature on phenolic composition and functional properties of red rooibos (Aspalathus linearis). Food Res Int. 2016;89:476–487. doi:10.1016/j.foodres.2016.08.041
55. Arthur H, Joubert E, De Beer D, Malherbe CJ, Witthuhn RC. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011;127(2):581–588. doi:10.1016/j.foodchem.2011.01.044
56. National Research Council. Guide for the Care and Use of Laboratory Animals.
57. Bradford MM. A rapid and sensitive microgram quantities of protein utilizing the principle of protein dye biding. Anal Biochem. 1976;72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
58. Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA, Lochner A. Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J Mol Cell Cardiol. 2001;33(4):769–778. doi:10.1006/jmcc.2001.1347
59. Chellan N, Joubert E, Strijdom H, Roux C, Louw J, Muller CJF. Aqueous extract of unfermented honeybush (cyclopia maculata) attenuates stz-induced diabetes and β-cell cytotoxicity. Planta Med. 2014;80(8–9):622–629. doi:10.1055/s-0034-1368457
60. Mazibuko-Mbeje SE, Dludla PV, Roux C, et al. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int J Mol Sci. 2019;20(3):1–16. doi:10.3390/ijms20030633
61. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22(4):359–370. doi:10.1111/j.1464-5491.2005.01499.x
62. Son MJ, Minakawa M, Miura Y, Yagasaki K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur J Nutr. 2013;52(6):1607–1619. doi:10.1007/s00394-012-0466-6
63. North MS, Joubert E, de Beer D, de Kock K, Joubert ME. Effect of harvest date on growth, production and quality of honeybush (Cyclopia genistoides and C. subternata). South African J Bot. 2017;110:132–137. doi:10.1016/j.sajb.2016.08.002
64. Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling. Nat Genet. 1999;23(1):32–40. doi:10.1038/12631
65. Kokk K, Veräjänkorva E, Wu XK, Tapfer H, Põldoja E, Pöllänen P. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina (Kaunas). 2004;40(2):156–160.
66. Schürmann A, Axer H, Scheepers A, Doege H, Joost HG. The glucose transport facilitator GLUT8 is predominantly associated with the acrosomal region of mature spermatozoa. Cell Tissue Res. 2002;307(2):237–242. doi:10.1007/s00441-001-0499-2
67. Burant CF, Davidson NO. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol Regul Integr Comp Physiol. 1994;267(6):1488–1495. doi:10.1152/ajpregu.1994.267.6.r1488
68. Verma R, Haldar C. Photoperiodic modulation of thyroid hormone receptor (TR-α), deiodinase-2 (Dio-2) and glucose transporters (GLUT 1 and GLUT 4) expression in testis of adult golden hamster, Mesocricetus auratus. J Photochem Photobiol B Biol. 2016;165:351–358. doi:10.1016/j.jphotobiol.2016.10.036
69. Lampiao F. Insulin stimulates glut8 expression in human spermatozoa. J Biosci Tech. 2010;1(2):90–93.
70. Araki E, Lipes MA, Patti ME, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372(6502):186–190. doi:10.1038/372186a0
71. Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–904. doi:10.1038/36116
72. Vikram A, Tripathi DN, Ramarao P, Jena GB. Intervention of d-glucose ameliorates the toxicity of streptozotocin in accessory sex organs of rat. Toxicol Appl Pharmacol. 2008;226(1):84–93. doi:10.1016/j.taap.2007.09.006
73. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism.. Insight Rev Artic. 2001;414(December):799–806.
74. Opuwari CS, Monsees TK. Reduced testosterone production in TM3 Leydig cells treated with Aspalathus linearis (Rooibos) or Camellia sinensis (tea). Andrologia. 2015;47(1):52–58. doi:10.1111/and.12221
75. Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176(3):165–173. doi:10.1016/S0300-483X(02)00069-0
76. Ichiki H, Miura T, Kubo M, et al. New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull. 1998;21(12):1389–1390. doi:10.1248/bpb.21.1389
77. Dang NT, Mukai R, Yoshida KI, Ashida H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci Biotechnol Biochem. 2010;74(5):1062–1067. doi:10.1271/bbb.90963
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.