Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
The effect of oral clindamycin and rifampicin combination therapy in patients with hidradenitis suppurativa in Singapore
Authors Ochi H, Tan LC, Oon HH
Received 11 March 2017
Accepted for publication 3 August 2017
Published 18 January 2018 Volume 2018:11 Pages 37—39
DOI https://doi.org/10.2147/CCID.S136730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Harumi Ochi, Lixian Chris Tan, Hazel H Oon
Department of Dermatology, National Skin Centre, Singapore
Abstract: Hidradenitis suppurativa (HS) is a chronic inflammatory disease of follicular occlusion characterized by abscesses, draining sinuses, and scarring. The efficacy and tolerability of combination treatment with oral clindamycin and rifampicin have previously been assessed in 4 studies including groups of Caucasian patients. Overall results are promising with reported improvement rates between 71.4% and 85.7%. In this study, we propose that combination therapy is safe and efficacious in the treatment of HS, not only among Caucasians, but also in a group of Asian patients in Singapore.
Keywords: hidradenitis suppurativa, combination therapy, clindamycin, rifampicin
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of follicular occlusion. Although HS is not primarily an infectious disease, Staphylococcus aureus and Staphylococcus epidermidis are pathogens most frequently isolated as secondary colonizers.1 In this study, we propose that combination therapy with oral clindamycin and rifampicin is efficacious in the treatment of HS in a group of Asian patients in Singapore.
Methodology
This retrospective study assessed the efficacy of a 10-week course of oral clindamycin 300 mg twice daily and oral rifampicin 300 mg twice daily in the treatment of HS. Patients who received this combination therapy between 1 December 2012 and 31 July 2013 in a tertiary dermatological center in Singapore were included.
This study was approved as an audit by the Head of Acne Clinic of National Skin Centre (NSC), Singapore. As this was performed retrospectively, permission to access the medical records of the patients was granted by the Director of NSC. Patient consent was waived by the Head of Acne Clinic as data were de-identified and retrospective.
Results
Eleven patients (9 males) had a mean age of 24.5±8.8 years. There were 6 Chinese (54.5%), 4 Malays (36.3%) and 1 Indian (9.1%). Five were smokers (45.5%), 6 were obese (54.5%) and 1 had a family history of HS (9.1%). The duration of HS prior to commencement of oral clindamycin and rifampicin ranged from 2 to 20 years. Eight patients (72.7%) had previous treatments, including retinoids and antibiotics, with limited effect and persistent disease. At the end of 10 weeks of treatment, 7 of the 11 patients (63.6%) reported clinical improvement. Four patients had digital photography documenting response before and after treatment, and 2 blinded assessors evaluated the improvement using the HS Physician Global Assessment (PGA) score. Three patients achieved clear, minimal or mild scoring from all sites after completion of therapy, and 2 patients reported a 2-grade improvement relative to baseline from at least 1 site. There was only 1 patient (9.1%) who reported side effects of nausea and vomiting and 1 patient (9.1%) who defaulted follow-up (Table 1).
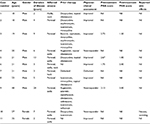  | Table 1 Demographics of patients, previous treatments, response and side effects of combination therapy Abbreviation: PGA, Hidradenitis Suppurativa Physician Global Assessment. |
Discussion
The efficacy and tolerability of this combination treatment had previously been assessed in 4 studies. Overall results are promising with reported improvement rates between 71.4% and 85.7%.1–4 Statistically significant improvements in all quality-of-life dimensions of the Skindex-France questionnaire were also described in 1 study.2
It is hypothesized that both the antibacterial and anti-inflammatory properties of clindamycin and rifampicin are responsible for the beneficial effects in treating HS. Clindamycin is a lincosamide antibiotic that is active against Gram-positive cocci and anaerobic bacteria. It mediates inflammation by suppressing complement-derived chemotaxis of polymorphonuclear leukocytes. Rifampicin is a lipid-soluble, broad-spectrum antibiotic highly effective against S. aureus. Additionally, it modifies cell-mediated hypersensitivity by suppressing antigen-induced transformation of sensitized lymphocytes. Rapid emergence of bacterial resistance may result with rifampicin monotherapy.5 Hence, combination therapy is synergistic with reduced resistance rates and increased anti-inflammatory properties. Although a longer duration of treatment appears warranted in chronic diseases like HS, no large differences in outcome between patients treated for 10 weeks or more and those treated for a shorter period have been reported.4
Other studies have similarly described good tolerability with low rates of side effects between 13.0% and 38.2% (Table 2). Gastrointestinal complaints were most commonly reported, but there were no cases of clindamycin-associated Clostridium difficile colitis.1–4
  | Table 2 Summarized data of the available studies on rifampicin–clindamycin in HS Abbreviation: HS, hidradenitis suppurativa. |
In a recent systematic review of HS treatment, only combination clindamycin–rifampicin regimen, infliximab, Nd:YAG laser and surgical excision were considered effective treatments. However, some of these modalities have limitations. Infliximab has resulted in adverse events including severe allergic reactions, multifocal motor neuropathy and drug-induced lupus reactions. Recurrence rates of up to 42.8% after surgical excision have also been described.6
Conclusion
Oral clindamycin and oral rifampicin combination therapy is safe and efficacious in the treatment of HS in groups of Caucasian and Asian patients in Singapore.
Acknowledgment
The authors thank Dr Heng Yee Kiat for assisting with the PGA scoring.
Disclosure
Dr Hazel H Oon has received research grants from Pfizer and Novartis and acted as a speaker for Novartis, Galderma, and AbbVie. The authors report no other conflicts of interest in this work.
References
Bettoli V, Zauli S, Borghi A, et al. Oral clindamycin and rifampicin in the treatment of hidradenitis suppurativa-acne inversa: a prospective study on 23 patients. J Eur Acad Dermatol Venereol. 2014;28(1):125–126. | ||
Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219(2):148–154. | ||
Mendonça CO, Griffiths CE. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154(5):977–978. | ||
van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219(2):143–147. | ||
Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24(4):275–291. | ||
Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148(4):439–446. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
