Back to Journals » Drug Design, Development and Therapy » Volume 13
The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and non-cooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study
Authors Kawai M, Kurata S , Sanuki T , Mishima G , Kiriishi K , Watanabe T, Ozaki-Honda Y , Yoshida M, Okayasu I, Ayuse T, Tanoue N, Ayuse T
Received 13 December 2018
Accepted for publication 19 March 2019
Published 17 May 2019 Volume 2019:13 Pages 1729—1737
DOI https://doi.org/10.2147/DDDT.S198123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Mari Kawai,1 Shinji Kurata,1 Takuro Sanuki,2 Gaku Mishima,1 Kensuke Kiriishi,1 Toshihiro Watanabe,1 Yu Ozaki-Honda,1 Mizuki Yoshida,1 Ichiro Okayasu,1 Terumi Ayuse,3 Naomi Tanoue,3 Takao Ayuse1–3
1Department of Dental Anesthesia, Nagasaki University Hospital, Nagasaki, Japan; 2Nagasaki University Institute of Biomedical Sciences, Course of Medical and Dental Sciences, Dental Anesthesiology, Nagasaki, Japan; 3Department of Special Care Dentistry, Nagasaki University Hospital, Nagasaki, Japan
Background: Sevoflurane is generally the preferred anesthetic agent for general anesthesia in pediatric patients, due to its rapid induction and recovery characteristics. However, it has been recognized that a major complication is emergence agitation when awakening from general anesthesia. The aim of this study was to evaluate the occurrence rate of emergence agitation in the operating room and postoperative recovery area following intraoperative administration of midazolam to pediatric patients under general anesthesia.
Patients and methods: One hundred and twenty pediatric patients undergoing dental treatment under sevoflurane anesthesia were enrolled in this study. The patients were divided into three groups (n=40 each in the 0.1 mg/kg midazolam, 0.05 mg/kg midazolam, and control with saline groups). Midazolam or saline was injected intravenously approximately 30 minutes before the end of the dental treatment. We used the Richmond Agitation and Sedation Scale (RASS) to assess the level of sedation and drowsiness at emergence phase in the operating room. We also used the Pediatric Anesthesia Emergence Delirium Scale (PAED) to assess the level of agitation and delirium at the full recovery phase from anesthesia in the recovery area.
Results: At the emergence phase, the incidence of emergence agitation in the 0.1 mg/kg midazolam group was significantly lower than in the other groups (p=0.0010). At the recovery phase, there was no significant difference among the three groups. The odds ratio between PAED score and RASS score was 4.0 using logistic regression analysis. The odds ratio between PAED score and Disability was 2.5.
Conclusion: Administration of a single dose of 0.1 mg/kg midazolam dose significantly decreases the incidence of severe emergence agitation at the emergence after sevoflurane anesthesia, but not at the recovery phase. Furthermore, the evaluation of sedative and agitation condition using RASS score at emergence from anesthesia is useful to predict occurrence of agitation in the recovery phase.
Keywords: emergence agitation, sevoflurane anesthesia, pediatric patients, extreme non-cooperation against dental treatment
Introduction
Sevoflurane is generally the preferred anesthetic agent for induction and maintenance of general anesthesia in pediatric patients, including patients with extreme non-cooperation against dental treatment and procedure, due to its rapid induction and recovery characteristics. However, it has been recognized that one of the major complications after sevoflurane anesthesia in pediatric patients is emergence agitation when awakening from general anesthesia, with the reported incidence ranging between 10% and 80%. Emergence agitation is recognized as a major risk factor for significant complications, such as anxiety, eating and sleeping disorders, and enuresis, along with persistent secondary alteration of emotional and cognitive development.1 Furthermore, the prolonged postoperative severe agitation might be another major complication for safety management of these patients. These have negative implications from a hospital management point of view, and also cause a decline in patient satisfaction and that of their families due to the potential for self-injury and other injurious behaviors, and are a burden on nursing staff.
Several different kinds of drugs are used to prevent emergence agitation from sevoflurane anesthesia in pediatric patients in the operating room, such as non-opioid analgesics, opioids,2 benzodiazepines,3 intravenous anesthetics,4 α2 agonists,5 and use of short acting midazolam. Kulka et al6 previously described the efficacy of a single dose of midazolam (0.1 mg/kg intravenously) given at the end of the procedure for reducing mild emergence agitation in pediatric patients aged 2–7 years undergoing minor ambulatory surgery, but stated that it was ineffective in cases with severe emergence agitation. Previously, Kim et al7 also revealed that a smaller dose of midazolam (0.05 mg/kg) administered just before the end of surgery had no effect on the incidence of severe emergence agitation requiring pharmacologic treatment. On the other hand, most recently, Cho et al8 found that a lower dose (0.03 mg/kg) of midazolam could suppress emergence agitation with minimal prolongation of the emergence time. However, this dose also had no effect on severe agitation at emergence from general anesthesia.
In the management of general anesthesia for dental treatment in pediatric patients, who represent an extremely uncooperative population due to extreme dental fear and the excessive need for treatment,9,10 we frequently observe a high incidence of severe agitation leading to a potentially disruptive and dangerous situation, such as self-harm and delirium in both operating room and recovery area. Therefore, it is important to prevent severe emergence agitation in pediatric patients with extreme non-cooperation against dental treatment as part of safe anesthesia management in both operating room and recovery area during perioperative phase.
We hypothesized that a single higher dose of 0.1 mg/kg midazolam 30 minutes before the completion of treatment would be needed to reduce severe agitation at both the emergence phase from general anesthesia and at the recovery phase in award. The aim of this study is to evaluate the occurrence rate of emergence agitation and the level of sedation or agitation at two different phases after sevoflurane anesthesia following intraoperative administration of midazolam to pediatric patients who underwent dental treatment under general anesthesia.
Materials and methods
This study was designed as a randomized, double-blind study to examine our hypothesis, and was performed with the approval of the Ethics Committee of Nagasaki University Hospital. We have confirmed that this study was conducted in accordance with the Declaration of Helsinki. After institutional review board approval and written informed consent from the patients’ parents, 120 patients aged less than 12 years, American Society of Anesthesiologists physical status I or II, and scheduled to undergo dental treatment under general anesthesia were enrolled. Additionally, to investigate the effect of the midazolam dose on emergence agitation, patients were randomly divided into three groups. Pediatric patients, who represent an extremely uncooperative population due to extreme dental fear and the excessive need for treatment, were included in this study. Several attempts had been made to treat these patients in ordinary circumstances awake, but they failed to accept any procedure. Exclusion criteria included cerebral palsy and neurological disorders.
The children were made to fast for 8 hours, except a small amount of clear fluid 2 hours before anesthesia. No sedative premedication was administered prior to anesthesia induction because they refuse to have any additional oral medication. After application of an electrocardiogram, pulse oximetry and noninvasive arterial blood pressure monitors, inhalational anesthesia induction was performed with sevoflurane. After achieving an adequate depth of anesthesia, an intravenous line was inserted on the dorsum of the hand. Then, 0.6 mg/kg rocuronium was administered to facilitate tracheal intubation and controlled ventilation during surgery. Anesthesia was maintained with 2% sevoflurane in 40% oxygen and a remifentanil infusion at the rate of 0.2 μg/kg/hour. Standard monitoring included electrocardiogram, blood pressure, pulse oximetry, capnography, temperature, and end-tidal anesthetic gas measurements. Ventilation was controlled to maintain end-tidal CO2 at 35±4 mmHg. As required for pain after tooth extraction cases or root canal treatment in 90 patients (75%), an adequate doses of suppository acetaminophen (10 mg/kg) was used during surgery to prevent postoperative pain. Approximately 30 minutes before the completion of treatment, according to the estimation of completion time, patients in the midazolam group received 0.1 mg/kg or 0.05 mg/kg midazolam intravenously, depending on the group they were allotted to, while patients in the saline group received the same volume of 2 ml of saline. At the completion of treatment, sevoflurane was discontinued and the endotracheal tube was removed when the child resumed adequate spontaneous breathing, associated with a normal range of minutes volume and End-tidal CO2. Then, when the patients were hemodynamically stable and free of pain and vomiting, they were discharged from the operating room and moved into the recovery area within 15–20 minutes. We compared the incidence and severity of emergence agitation, as well as emergence time from discontinuation of sevoflurane to extubation and discharge times from the operating room, in the two groups of children receiving a single dose of midazolam 30 minutes before the completion of treatment versus the third group of children receiving saline.
Assessment of agitation and sedation level
Of the many scales that have been proposed to evaluate the incidence and severity of emergence agitation or sedation, we used the two different purpose of evaluating scale, PAED4,11 and RASS,12,13 which classifies agitation, sedation and delirium following general anesthesia.
At the emergence phase just after an extubation after sevoflurane aesthesia in the operating room, RASS was used for evaluation of level of agitation and sedation because the patients might still be drowsy due to the influence of sevoflurane anesthesia (Table 1). The RASS is a single tool that is intuitive, easy to use, and includes both agitation and sedation. It is a valid scale for assessing the responsiveness level of critically ill children. The patients were divided into two categories based on agitation and sedation levels: non-agitated condition (RASS score: -5–0) and agitated condition (RASS score: 1–4). At the time of emergence from anesthesia, agitation was assessed immediately after removal of the endotracheal tube by nurses who were unaware of the child’s group assignment.
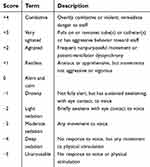 | Table 1 The description of Richmond Agitation-Sedation Scales (RASS) |
At the full recovery phase in the recovery area within 15 minutes, the Pediatric Anesthesia Emergence Delirium Scale (PAED) was used for evaluation of agitation and delirium in recovery phase because the patients might be clear enough to be assessed using criteria by a nurse (Table 2). PAED was the most reliable evaluating scale for agitation and delirium in pediatric patients after recovery from anesthesia, as found by Sikich and Lerman11.
 | Table 2 The description of Pediatric Anesthesia Emergence Delirium Scale (PAED) |
For safe anesthetic practice, the anesthesiologists who administered midazolam were aware of the dose, but they did not share this information with the investigator who evaluated the agitation level using the RASS and PAED score.
Analysis
Statistical analysis
This study was powered on the basis of preliminary results showing 50% incidence of emergence agitation in the control group. A sample size of 39 in each group was calculated as being required to detect a decrease in the incidence of agitation to 15% with α=0.05 and β=0.2. All statistical analyses were performed using Prism version 5.0 (Graphpad Software, Inc., San Diego, CA, USA). Demographic data, such as age, weight, and height, and surgical duration were compared using the Kruskal–Wallis test or one-way analysis of variance among the three groups. The occurrence rate of emergence agitation and sedation was based on the score level 1<RASS<4, defined as “agitated condition”, and the sedative symptom based on the score level -5<RASS<0, defined as “non-agitated condition”. The occurrence rate of emergence agitation and delirium was based on the score level 1<PAED<9, defined as “lower level of agitation”, and the severe level of agitation and delirium based on the score level 10<RASS<20, defined as “severe agitated condition”. According to the indication by Sikich and Lerman11 that a PAED with greater than 10 should be regarded as the cut-off criteria for emergence agitation from anesthesia, we categorized 10<RASS<20 as “severe agitated condition”.
Categorical variables, such as the incidence of emergence agitation, are reported as numbers and percentages; these variables were compared among groups using the chi-square test and logistic regression analysis, as appropriate. The multivariable logistic regression analysis for occurrence rate of agitation assessed by PAED at recovery phase with Multiple imputations was performed using JAP Pro14 software. In order to estimate the odds ratio between PAED score and RASS score, the existence of disability, age, gender, weight, and anesthesia time were analyzed. A p-value<0.05 was considered to be significant.
Results
One hundred and twenty children were enrolled in the study (n=40 each in the 0.1 mg/kg midazolam, 0.05 mg/kg midazolam, and control groups). The demographic and perioperative clinical data of patients in the three groups are shown in Table 3. The mean age of the children in the three groups was comparable. There were no significant differences among the three groups in terms of perioperative clinical data. The characteristics of subjects in each group divided according to the suspected and/or the definitive diagnosis of disability are shown in Table 4.
At the emergence phase just after an extubation after sevoflurane aesthesia in the operating room, emergence agitation occurred in 21 cases (52.5%) in the 0.05 mg/kg midazolam group, 15 cases (37.5%) in the 0.1 mg/kg midazolam group, and 31 cases (77.5%) in the control group. The incidence of emergence agitation in the 0.1 mg/kg group was significantly lower than in the other groups (P=0.0010). In the 0.1 mg/kg midazolam group, the estimated emergence time for successful extubation was significantly longer than in the control group (P=0.020). However, there was no significant difference between emergence times in the 0.1 and 0.05 mg/kg groups.
At the full recovery phase in the recovery area within 15 minutess, postoperative agitation and delirium were assessed by PAED in 10 cases in the 0.05 mg/kg midazolam group, 15 cases in the 0.1 mg/kg midazolam group, and 14 cases in the control group. There was no significant difference among the three groups (Table 5). Three patients claimed slight postoperative pain in spite of an injection of acetaminophen, and two patients expressed slight pain without having acetaminophen. There was no case of sustained severe agitation that should be treated with additional sedative drug in eithier emergence phase or recovery phase.
The odds ratio between PAED score and RASS score was 4.0 (p=0.0020) using logistic regression analysis (Table 6). The odds ratio between PAED score and Disability was 2.5 (p=0.0290). The odds ratio between PAED score and sex was 1.3 (p=0.49). The odds ratio between PAED score and postoperative pain was 1.9 (p=0.55). The ROC analysis to test predictability of the occurrence rate of agitation at emergence from anesthesia (1<RASS<4) and the occurrence rate of agitation at recovery phase in award (10<PAED<20) was 0.77 sensitivity and 0.54 specificity.
 | Table 6 Multivariable logistic regression analysis for PAED at recovery phase with multiple imputations |
Discussion
The data in our study indicate that administration of a single dose of 0.1 mg/kg midazolam 30 minutes before the completion of treatment in pediatric patients with extreme non-cooperation significantly decreases the incidence of severe emergence agitation just after anesthesia, but not in the recovery phase. Furthermore, the odds ratio of 4.0 between PAED score and RASS score and the odds ratio of 2.5 between PAED score and Disability may indicate that the evaluation of sedative condition using RASS score of Disability pediatric patients at emergence from anesthesia might be useful methods to predict an occurrence of agitation in the recovery phase.
Effects of midazolam and other drugs on agitation
Several investigators have reported that intravenous injection of benzodiazepines (0.05–0.1 mg/kg midazolam) as anxiolytic drugs is effective for the drug treatment of emergence agitation.7,8,14 Cohen et al14 suggested that 0.1 mg/kg of midazolam given intravenously at the induction of anesthesia did not alter the incidence of emergence agitation, but it caused a delay in emergence time and recovery in pediatric patients undergoing adenotonsillectomy. Kim et al7 revealed that intravenous administration of 0.05 mg/kg midazolam just before the end of surgery is effective in reducing emergence agitation, although it delays emergence time from sevoflurane anesthesia. Most recently, Cho et al8 suggested that a lower dose (0.03 mg/kg) of midazolam just before the end of surgery could suppress emergence agitation with minimal disturbance of the emergence time. However, this dose of midazolam also had no effect on severe agitation at the emergence from general anesthesia. Furthermore, they excluded patients with excessively uncooperative behaviors, developmental delay, and neurological illness. In the present study, we observed significant prolongation (4.5 minutes longer than the control group) of emergence time, including the time required to perform extubation. Therefore, we speculate that a 0.1 mg/kg midazolam dose injected intravenously just before the end of surgery might be adequate for reducing severe emergence agitation in pediatric patients after sevoflurane anesthesia, although this dose may cause delayed emergence compared to the control group, which is, however, a negligible level of prolongation in clinical situations.
It should be noted that a single dose of 0.1 mg/kg midazolam could prevent severe emergence agitation immediately after sevoflurane anesthesia. However, this dose of midazolam could not prevent prolonged agitation in the recovery area. We speculate that this might indicate either the feature of a short acting effect of midazolam or the occurrence of side-effects of midazolam, such as the paradoxical response to midazolam, including symptoms of agitation, confusion, delirium, and inconsolable hysteria. Midazolam has a short elimination half-life, with approximately 0.4 hours Tmax as compared to other benzodiazepines, due to its rapid metabolism.15 The response varies from individual to individual, depending on the patient’s age, susceptibility to midazolam, general condition, and drug interactions, and hence its effect may be prolonged. However, we believe that by setting the timing of administration at 30 minutes before the end of treatment, these variables were not likely to have affected the study results. It should also be noted that there may be an influence of the paradoxical response to midazolam. As has been previously described, the paradoxical response to midazolam includes symptoms such as violent anger, aggression, paroxysmal excitement, and assault, and may occur in very young pediatric patients.16–18 Since the anxiolytic effect of midazolam depends on the dosage and the patient’s background characteristics, it is necessary to consider that the administration of midazolam may conversely cause excitation. Taken together, we speculate that the strategy of use of midazolam for reduction of emergence agitation depends on the preoperative prediction of emergence agitation based on the severity of uncooperativeness. Furthermore, we believe that other long-lasting sedative drugs with no major influence on upper airway patency, such as dexmedetomidine, should also be considered to prevent severe agitation both in the operating room and recovery area, as previously suggested.5 Recently, Keles et al19,20 suggested that administration of oral dexmedetomidine premedication may reduce postoperative agitation and delirium in patients undergoing dental procedures. Tsiotou et al21 also clearly revealed that dexmedetomidine 1 mcg/kg reduces the incidence and severity of emergence agitation after 20 and 30 minutes in post-anesthesia care unit in tonsillectomy with Propofol anesthesia. Therefore, further studies are needed to test effective and safe methods to reduce agitation during the perioperative period.
It is known that, in pediatric patients receiving sevoflurane for induction and maintenance of anesthesia, administration of a single dose of propofol 30 minutes before the completion of treatment decreases the incidence of emergence agitation without delaying emergence from anesthesia or discharge from the operating room.4 Compared with propofol administration, benzodiazepines have a longer effect time. A previous report showed that premedication with benzodiazepines is effective for sedation before surgery, but not for emergence agitation at arousal from sevoflurane anesthesia. Kulka et al6 published a study in German describing a single dose technique for children aged 2–7 years undergoing minor ambulatory surgery, involving administration of 0.1 mg/kg midazolam intravenously at the end of the procedure. They reported this technique as being effective in the reduction of mild emergence agitation, but ineffective in severe cases. According to Cho et al,8 intravenous administration of 0.03 mg/kg of midazolam just before the end of surgery reduces emergence agitation without delaying emergence in children undergoing strabismus surgery under sevoflurane anesthesia. Since previous studies have been performed to test emergence agitation in healthy children, which have reported different outcomes compared to the results of the present study due to the severity of agitation, we conclude that higher doses of midazolam 0.1 mg/kg might be effective in preventing severe emergence agitation after sevoflurane anesthesia in pediatric patients with extreme non-cooperation, but not in the recovery phase.
Another method of minimizing emergence agitation following general anesthesia is use of propofol instead of sevoflurane as the maintenance agent. It is well recognized that use of propofol for anesthesia maintenance after induction with sevoflurane is a valuable anesthetic management strategy to prevent emergence agitation in pediatric patients.22 Recently, Kocaturk and Keles23 recommended performance of pediatric dental procedures under total intravenous anesthesia with propofol plus remifentanil because of the resultant comfortable postoperative period due to less emergence delirium. Therefore, combination of sevoflurane induction with total intravenous anesthesia maintenance might be an alternative method to reduce emergence agitation in dentally disabled patients who refuse intravenous access.
Prediction of agitation in perioperative phase
In this study, we used the RASS to evaluate the level of agitation and sedation at emergence from anesthesia12,13,24 and used PAED to evaluate agitation and delirium at the full recovery phase from anesthesia in the recovery area. Previous studies to test the effect of midazolam on emergence agitation have used a delirium scale of PAED,7,8 which is appropriate for evaluation of postoperative delirium in pediatric patients in the ward and recovery area.7,25 The reason why we selected the RASS as an evaluation method is that this scale can assess overall levels of responsiveness under remaining influence of sevoflurane anesthesia, which facilitates the reduction of symptoms of severe emergence agitation. The RASS is a well validated and highly reliable 10-point scale, with scores ranging from +1 to +4 assigned for levels of agitation up to combativeness, and from −5 to −1 assigned for levels of sedation.13
We observed that there was a significant positive correlation between PAED score and RASS score with confounding factor of existence of disability. This observation might indicate that the evaluation of a wide range of sedative and agitated condition using RASS score at emergence from anesthesia is useful to predict the occurrence rate of severe agitation in the recovery phase. If we observe predicted risk for occurrence of severe agitation in the recovery phase, continuous higher attention should be needed for these patients during the recovery phase until discharge from hospital.
Clinical implication
Numerous clinical studies have shown that the emergence agitation after sevoflurane anesthesia is a common phenomenon in pediatric patients. The typical manifestations of severe emergence agitation are reportedly self-injury, including tilting their bodies from the operation table, extending their necks to avoid any medical equipment, and typical kicking behavior while being emotionally inconsolable.26 It was also revealed that the possible risk factors associated with emergence agitation include younger age,27sleep disturbances in the preoperative period due to the hostile environment, intolerance against perceived postoperative pain, preoperative fear and anxiety, use of short acting volatile anesthetic agents such as sevoflurane or desflurane,28 and procedures in the oral-maxillofacial region.29
It is well known that rapid emergence from general anesthesia with dependable return of airway reflexes and cognitive abilities are important minimum requirements following general anesthesia. The use of sevoflurane as the sole anesthetic agent can speed awakening in pediatric patients undergoing dental treatment. Unfortunately, this rapid awakening is accompanied by a high incidence of postanesthetic emergence agitation.30
Most recently, we have reported that there was a significant disturbance of sleep cycle after general anesthesia in a disability patient who underwent dental treatment, due to possible severe agitation after anesthesia.31 Therefore, we should carefully manage the behavioral condition of these pediatric patients during perioperative periods estimated with useful score.
In conclusion, administration of a single dose of 0.1 mg/kg midazolam 30 minutes before the completion of treatment in extremely uncooperative pediatric patients undergoing dental treatment under sevoflurane anesthesia significantly decreases the incidence of severe emergence agitation at the emergence after sevoflurane anesthesia, but not at the recovery phase. Furthermore, the evaluation of sedative and agitation condition using RASS score at emergence from anesthesia is a useful method to predict the occurrence of severe agitation in the recovery phase.
Acknowledgments
This study was approved by Nagasaki University Hospital ethical committee (Nagasaki University Hospital 2016.1.5. IRB number:15022321-2). This study was funded by departmental resources.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stipic SS, Carev M, Kardum G, Roje Z, Litre DM, Elezovic N. Are postoperative behavioural changes after adenotonsillectomy in children influenced by the type of anaesthesia?: a randomised clinical study. Eur J Anaesthesiol. 2015;32(5):311–319. doi:10.1097/EJA.0000000000000104
2. Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth. 2013;110(2):274–280. doi:10.1093/bja/aes382
3. Arai YC, Fukunaga K, Hirota S. Comparison of a combination of midazolam and diazepam and midazolam alone as oral premedication on preanesthetic and emergence condition in children. Acta Anaesthesiol Scand. 2005;49(5):698–701. doi:10.1111/j.1399-6576.2005.00700.x
4. Aouad MT, Yazbeck-Karam VG, Nasr VG, El-Khatib MF, Kanazi GE, Bleik JH. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107(5):733–738. doi:10.1097/01.anes.0000287009.46896.a7
5. Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98(1):60–63. table of contents.
6. Kulka PJ, Bressem M, Wiebalck A, Tryba M. Prevention of “post-sevoflurane delirium” with midazolam. Anaesthesist. 2001;50(6):401–405.
7. Kim YH, Yoon SZ, Lim HJ, Yoon SM. Prophylactic use of midazolam or propofol at the end of surgery may reduce the incidence of emergence agitation after sevoflurane anaesthesia. Anaesth Intensive Care. 2011;39(5):904–908. doi:10.1177/0310057X1103900516
8. Cho EJ, Yoon SZ, Cho JE, Lee HW. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology. 2014;120(6):1354–1361. doi:10.1097/ALN.0000000000000181
9. Savanheimo N, Sundberg SA, Virtanen JI, Vehkalahti MM. Dental care and treatments provided under general anaesthesia in the Helsinki Public Dental Service. BMC Oral Health. 2012;12:45. doi:10.1186/1472-6831-12-45
10. Savanheimo N, Vehkalahti MM. Five-year follow-up of children receiving comprehensive dental care under general anesthesia. BMC Oral Health. 2014;14:154. doi:10.1186/1472-6831-14-90
11. Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–1145.
12. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi:10.1164/rccm.2107138
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi:10.1001/jama.291.14.1753
14. Cohen IT, Drewsen S, Hannallah RS. Propofol or midazolam do not reduce the incidence of emergence agitation associated with desflurane anaesthesia in children undergoing adenotonsillectomy. Paediatr Anaesth. 2002;12(7):604–609.
15.
16. Shin YH, Kim MH, Lee JJ, et al. The effect of midazolam dose and age on the paradoxical midazolam reaction in Korean pediatric patients. Korean J Anesthesiol. 2013;65(1):9–13. doi:10.4097/kjae.2013.65.1.9
17. Jackson BF, Beck LA, Losek JD. Successful flumazenil reversal of paradoxical reaction to midazolam in a child. J Emerg Med. 2015;48(3):e67–e72. doi:10.1016/j.jemermed.2014.09.062
18. Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004;24(9):1177–1185.
19. Keles S, Kocaturk O. The effect of oral dexmedetomidine premedication on preoperative cooperation and emergence delirium in children undergoing dental procedures. Biomed Res Int. 2017;2017:6742183. doi:10.1155/2017/6742183
20. Keles S, Kocaturk O. Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anesthesia: a retrospective study. Drug Des Devel Ther. 2018;12:647–653. doi:10.2147/DDDT.S163828
21. Tsiotou AG, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth. 2018;28(7):632–638. doi:10.1111/pan.13397
22. Chandler JR, Myers D, Mehta D, et al. Emergence delirium in children: a randomized trial to compare total intravenous anesthesia with propofol and remifentanil to inhalational sevoflurane anesthesia. Paediatr Anaesth. 2013;23(4):309–315. doi:10.1111/pan.12090
23. Kocaturk O, Keles S. Recovery characteristics of total intravenous anesthesia with propofol versus sevoflurane anesthesia: a prospective randomized clinical trial. J Pain Res. 2018;11:1289–1295. doi:10.2147/JPR.S164106
24. Jo JY, Jung KW, Kim HJ, et al. Effect of total intravenous anesthesia vs volatile induction with maintenance anesthesia on emergence agitation after nasal surgery: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. Epub 2018 Nov 29.
25. Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth. 2010;20(8):704–711. doi:10.1111/j.1460-9592.2010.03328.x
26. Malarbi S, Stargatt R, Howard K, Davidson A. Characterizing the behavior of children emerging with delirium from general anesthesia. Paediatr Anaesth. 2011;21(9):942–950. doi:10.1111/j.1460-9592.2011.03646.x
27. Cole JW, Murray DJ, McAllister JD, Hirshberg GE. Emergence behaviour in children: defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth. 2002;12(5):442–447.
28. Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. 2000;10(4):419–424.
29. Silva LM, Braz LG, Modolo NS. Emergence agitation in pediatric anesthesia: current features. J Pediatr (Rio J). 2008;84(2):107–113. doi:10.2223/JPED.1763
30. Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth. 1999;9(4):299–304.
31. Ayuse T, Kurata S, Sanuki T, et al. Effects of general anesthesia on postoperative sleep cycles in dentally disabled patients. Spec Care Dentist. 2018;39:3–9.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



