Back to Journals » Pragmatic and Observational Research » Volume 10
The effect of lercanidipine or lercanidipine/enalapril combination on blood pressure in treatment-naïve patients with stage 1 or 2 systolic hypertension
Authors Rayner B
Received 1 September 2018
Accepted for publication 24 December 2018
Published 22 January 2019 Volume 2019:10 Pages 9—14
DOI https://doi.org/10.2147/POR.S186070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Brian Rayner
Kidney and Hypertension Research Unit, University of Cape Town, Groote Schuur Hospital, Observatory, Cape Town 7925, South Africa
Purpose: To describe the efficacy of a stratified approach on automatic office blood pressure (BP), 24-hour ambulatory BP, and BP variability (BPV) in treatment-naïve patients with systolic hypertension using lercanidipine for stage 1 and lercanidipine/enalapril for stage 2.
Patients and methods: This was an open-label, prospective interventional study conducted in 22 general practices in South Africa. Treatment-naïve patients with stage 1 hypertension received lercanidipine 10 mg and patients with stage 2 received lercanidipine 10 mg/enalapril 10 mg. After 6 weeks, patients not reaching target (<140/90 mmHg) were up-titrated to lercanidipine 10 mg/enalapril 10 mg or lercanidipine 10 mg/enalapril 20 mg, respectively, for a further 6 weeks. Office BP was determined at each visit, and 24-hour ambulatory BP monitor (ABPM) at baseline and 12 weeks. The primary end point was changes in office BP, and secondary end points were changes in 24-hour ABPM and BPV.
Results: Of the 198 patients, 48% had stage 1 and 52% stage 2 hypertension. The mean age was 55 years, body mass index was 29.2 kg/m2, 48.5% were female, and 15.1% were diabetic. The mean (SD) office SBP and DBP at baseline, 6 weeks, and 12 weeks was 158.2 (13.8), 141.6 (11.1), and 138.7 (16.7) mmHg (P<0.00001), and 92.2 (10.6), 84.6 (11.1), and 82 (13.3) mmHg (P<0.00001), respectively. The mean (SD) systolic and diastolic daytime ABPM at baseline and 12 weeks was 157 (16.63) and 142 (14.41) mmHg (P<0.0001) and 88 (12.34) and 81 (10.79) mmHg (P<0.0001), and the nighttime ABPM was 146 (15.68) and 133 (13.94) mmHg (P<0.0001) and 79.5 (11.64) and 72.5 (10.05) mmHg (P<0.009), respectively. There were few adverse events.
Conclusion: Lercanidipine and lercanidipine/enalapril for stage 1 or 2 hypertension highly improves office SBP and DBP, overall 24-hour BP, daytime BP, and nighttime BP, also reducing BPV with few adverse effects.
Keywords: European hypertension guidelines, primary therapy for treatment-naïve patients, stage 1 hypertension, stage 2 hypertension, efficacy
Introduction
Elevated blood pressure (BP) is a major risk factor for cardiovascular (CV) events, and subsequently, it is also a leading contributor to the global disease burden. Overwhelming evidence suggests that prompt BP control leads to reduction in CV events. To address this issue, the European Hypertension Guidelines issued by the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) recommend initiation of antihypertensive therapy stratified according to stage of hypertension.1 Patients with stage 1 hypertension received monotherapy, while patients with stage 2 a combination therapy, preferably in a single pill. Despite these recommendations, this strategy has not been tested widely in clinical trials.
In the ACCELERATE study, the strategy of initial combination of aliskiren and amlodipine was tested for superiority to each monotherapy in early control of BP without excess of adverse events.2 Patients with SBP between 150 and 180 mmHg were randomized to one of the three groups. Initial combination therapy had a 6.5 mmHg greater reduction in mean SBP than the monotherapy groups without increase in adverse events. However, in this study, patients were not stratified according to stages of hypertension as recommended by the guidelines.
Hypertension guidelines also propose the out-of-office BP measurement in the diagnosis and management of hypertension.1,3,4 Twenty-four-hour ambulatory BP monitoring (ABPM) provides a greater number of readings and minimization of the white coat effect, observer bias, and possible measurement errors. This contributes to better diagnostic accuracy and prediction of target-organ damage and adverse CV outcome compared with office BP measurement.5,6 In addition to elevated mean levels of BP, also short-term daytime or 24-hour BP variability (BPV) has been shown to carry an independent prognostic value in hypertensive patients,7,8 and is directly related to target-organ damage.9,10
Automated office BP (AOBP) is currently gaining recognition as a preferred method of measuring BP, as it correlated closely with 24-hour daytime ambulatory BP, and is a better predictor of target-organ damage than traditional office measurement.4,10
With the growing acknowledgment of the importance of these factors, this Phase IV study was designed to gather more knowledge on the efficacy of a stratified guideline approach on AOBP, 24-hour ambulatory BP, and BPV in treatment-naïve patients with systolic hypertension using lercanidipine for stage 1 and lercanidipine/enalapril for stage 2.
Patients and methods
Participants
This was an open-label, prospective interventional study conducted in 22 general practices in South Africa. The trial was approved by Pharma Ethics, South Africa, on February 2015 (reference number 141110708), and all patients provided written informed consent prior to treatment start, but the study was not registered as a clinical trial. Adult (>35 years) treatment-naïve patients or those without antihypertensive treatment for >4 weeks with stage 1 or stage 2 hypertension based primarily on office SBP were assigned to lercanidipine 10 mg (if in stage 1) or to a single pill combination of lercanidipine 10 mg/enalapril 10 mg (if in stage 2). The choice of antihypertensive therapy was based on the ESH/ESC Hypertension Guidelines where a calcium channel blocker (CCB) or a combination of a CCB and an angiotensin-converting-enzyme (ACE) inhibitor can be initiated as first-line therapy.1
The major exclusion criteria were: body mass index (BMI) >35 kg/m2; pregnancy or planned pregnancy; lactation; previous diagnosis of stage 3 hypertension (BP ≥180/110 mmHg); use of any other agent registered for the use in hypertension; hypersensitivity to any dihydropyridine CCB or any ACE inhibitor; estimated glomerular filtration rate (eGFR) <60 mL/min (based on the Modification of Diet in Renal Disease study equation); and history of angioedema and strong inhibitors of CYP3A4.
Procedures
AOBP was performed according to the South African Hypertension guidelines using a (Welch Allyn 6100) monitor.11 We used a mean of three readings 1 minute apart after 5 minutes rest in the seated position with the arm supported at heart level. Stage 1 was defined as 140 ≤ SBP ≤ 159 and 90 ≤ DBP ≤ 99; stage 2 as 160 ≤ SBP ≤ 179 and 100 ≤ DBP ≤ 109.
The trial flow diagram is outlined in Figure 1 and procedures in Table 1. After informed consent, we did an initial screening visit, drew safety bloods for potassium and creatinine, and performed an electrocardiogram (ECG). Eligibility was established at this visit based on the mean of the AOBP, and patients underwent a 24-hour ABPM using a (Welch Allyn 6100) monitor prior to baseline visit. At baseline, patients were assigned to monotherapy or combination therapy according to stage of hypertension. At 6 weeks, office BP, potassium, and creatinine were repeated. Patients not at target (BP >140/90 mmHg) were up-titrated – lercanidipine to lercanidipine 10 mg/enalapril 10 mg and lercanidipine 10 mg/enalapril 10 mg to lercanidipine 10 mg/enalapril 20 mg. After 12 weeks (end of study), patients underwent repeat 24-hour ABPM, office BP, ECG, potassium, and creatinine.
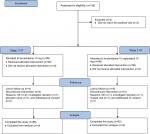  | Figure 1 Study flow diagram. Abbreviations: HT, hypertension; AE, adverse event. |
The primary end point was changes in AOBP from baseline, at week 6 and week 12, and the percentage of patients reaching target. The secondary end points were: changes in 24-hour ABPM and BPV. The BPV was calculated from the SD of both the daytime and nighttime SBP and DBP during the 24-hour ABPM. Only patients with a successful ABPM at both baseline and week 12 were considered for analysis. Discontinuation and adverse events were recorded.
Statistical analysis
All data were captured on the electronic data management system. The sample size was calculated to be a total of 196 patients – 98 patients in stage 1 and 98 patients in stage 2 hypertension. This assumed that the clinical effect of interest is a decrease in SBP of 10 mmHg with a SD of 25 mmHg with an 80% power to detect a difference with a significance level of 5%. The patient’s demographic and clinical outcomes were presented as mean (± SD) or median (with interquartile range) for normally distributed and non-normally distributed data, respectively. Categorical variables were analyzed by chi-squared or Fisher’s exact test, while comparison of continuous variables between groups was analyzed using Student’s t-test or Wilcoxin rank-sum test using StatisticaTM version 13.3.
Results
Of the 198 patients included in the study, 48% had stage 1 and 52% had stage 2 hypertension. The mean age was 55 years and BMI was 29.2 kg/m2. The demographic breakdown was as follows: 48.5% were female, 51.5% male, 15.1% diabetic, 24.2% white, 38.4% black, 18.7% mixed ancestry, and 18.7% Asian.
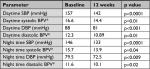
The mean (SD) office SBP and DBP and pulse rate are presented in Figure 2. During treatment, 35.7% of stage 1 patients and 32% of stage 2 patients reaching week 6 were up-titrated. At the final visit (week 12), 73.6% of patients achieved control of DBP and 58.6% of SBP (Table 2). For group 1 it was 79.5% for diastolic and 59% for systolic, and for group 2 it was 68% for diastolic and 58% for systolic BP.
BP response according to ethnic group is shown in Table 3. All ethnic groups showed highly significant improvements in SBP and DBP.
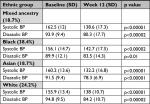  | Table 3 BP response in mmHg according to ethnic group Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure |
Twenty-hour ABPM was performed in 85 patients, but only 66 had successful ABPM readings at both baseline and 12 weeks. Seventy percent of patients were non-dippers based on SBP. The mean (SD) systolic and diastolic daytime ABPM and the nighttime ABPM at 12 weeks showed a significant reduction vs the baseline measurements (Table 2). BPV was also significantly reduced as determined by the SD of 24-hour ABPM readings.
There were 26 discontinuations from the study – nine due to adverse events, ten were lost to follow-up, three withdrew consent, two due to investigator decision, and two for various reasons.
Adverse events of interest are shown in Table 4. The majority of adverse events recovered. There were four serious adverse events unrelated to study drug namely diverticulitis, inguinal hernia, asthma, and severe upper respiratory tract infection.
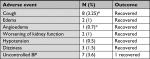  | Table 4 Adverse events of interest Note: *Percentage based on 135 patients exposed to enalapril. Abbreviation: BP, blood pressure. |
Safety bloods at screening, week 6, and week 12 showed no significant change in serum K+, creatinine, and eGFR at weeks 6 and 12.
Discussion
BP control remains suboptimal in many countries for complex reasons. However, a combination of physician inertia and patient non-adherence are important contributory factors. The ESH Guidelines has recommended the wider use of single pill combinations to improve adherence and initiation of two drugs in combination for patients with more severe hypertension or high CV risk to counter physician inertia.1 However, few studies have tested this approach in clinical practice.
In this study, we tested this approach by using monotherapy with a CCB (lercanidipine) for stage 1 hypertension and lercanidipine/enalapril single pill combination for stage 2 over a 12-week period with up-titration at 6 weeks. (Table 1, Figure 1). This was done in a diverse patient population with 48.5% being female, 15.1% diabetic, 24.2% white, 38.4% black, 18.9% mixed ancestry, and 18.9% Asian with approximately equal numbers of patients with stage 1 and 2 hypertension. During treatment, 35.7% of stage 1 patients and 32% of stage 2 patients were up-titrated. At 12 weeks, 73.6% of patients achieved control of DBP and 58.6% of SBP. There were also highly significant reductions in both SBP and DBP (Figure 2). BP response was observed across all ethnic groups including blacks, mixed ancestry, whites, and Asians. There was a slight but significant rise in mean pulse rate of 3.2 beats per min at week 6, which was well described after instituting a dihydropyridine CCB but was not clinically relevant.12
Twenty-four-hour ABPM was obtained in 68 patients at both baseline and 12 weeks. It is important to note that non-dipping status for SBP was present in 70.5% of patients undergoing 24-hour ABPM. There were highly significant reductions in overall 24-hour, daytime, and nighttime systolic and diastolic BP as well as reductions in BPV (Table 2).
These reductions in BP and BPV are likely to translate into significant reduction in CV and stroke events. However, to improve control rates, it is suggested that single pill combination therapy should be considered in patients at a level of SBP 150 and not 160 mmHg, especially in patients at higher CV risk.
The approach to treatment in this study was associated with very few adverse events related to study drug. In particular, there was only one documented case of hypotension and three cases of dizziness that were possibly related to low BP. There was no significant change in kidney function or serum K+. In relation to the individual antihypertensive drugs used in this study, remarkably only 1% of patients were documented to have ankle edema, which is often an important reason for discontinuation of dihydropyridine CCBs. This is probably related to the intrinsic properties of lercanidipine and the reduction of edema due to combination with renin-angiotensin blocker.13,14 In regard to enalapril, there was one case of mild angioedema and a low incidence of dry cough (3.25%). Other published data suggest that the combination of a dihydropyridine CCB reduces the incidence of ACE-inhibitor cough.15
Limitations of the study were that the participants were not randomized, adherence was not analyzed, and the study was of a relatively short duration. In addition, not all patients underwent 24-hour ABPM and the sample size was small.
Conclusion
A guideline approach using lercanidipine and lercanidipine/enalapril for stage 1 and 2 hypertension, respectively, resulted in highly significant improvements in office SBP and DBP, overall 24-hour BP, daytime BP, and nighttime BP, plus a significant reduction in BPV with few adverse effects. This is also likely to result in better adherence and reduced side effects, and a reduction in CV events.
Acknowledgments
The author would like to thank the principal investigators for participating in the study: E Kotze, N Fourie, MS Tayob, R Fortuin-De Smidt, MI Phayane, NJ Ngcakani, M Duki, GB Madisakwane, E van der Walt, AE Smook, WF Steynberg, C Kahanovitz, YM Haffajee, GH Zipp, PJDV Odendaal, O Banderker, K Lewis, JE Rosenthal, MCE McDonald, MA Fulat, DM De Jong, E Hellstrom, and V Govender. He would also like to thank participants and study nurses; OnQ Research, South Africa, for assistance in writing the protocol, conducting the study, collecting the data, and analyzing the study; and editorial assistance for the preparation of this manuscript was provided by Laura C Collada Ali. Litha Pharma (South Africa) – study sponsor – provided appropriate finance to the author for conducting the study and writing of this manuscript, and editorial assistance for the preparation of this manuscript was provided by Laura C Collada Ali (medical writer) and financed by Recordati S.p.A.
Disclosure
The author has received speaker honoraria from Litha Pharma and – not necessarily on talks related to this research – from several companies like Cipla, AstraZeneca, Novartis, Boehringer Ingelheim, and Servier. The author reports no other conflicts of interest in this work.
References
ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 practice guidelines for the management of arterial hypertension of the European Society of hypertension (ESH) and the European Society of cardiology (ESC): ESH/ESC Task Force for the management of arterial hypertension. J Hypertens. 2013;31(10):1925–1938. | ||
Brown MJ, Mcinnes GT, Papst CC, Zhang J, Macdonald TM. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (accelerate): a randomised, parallel-group trial. Lancet. 2011;377(9762):312–320. | ||
Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice Guidelines. Hypertension. 2018;71(6):1269–1324. | ||
Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557–576. | ||
Banegas JR, Ruilope LM, de La Sierra A, et al. Clinic versus daytime ambulatory blood pressure difference in hypertensive patients: the impact of age and clinic blood pressure. Hypertension. 2017;69(2):211–219. | ||
Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156–161. | ||
Tatasciore A, Zimarino M, Tommasi R, et al. Increased short-term blood pressure variability is associated with early left ventricular systolic dysfunction in newly diagnosed untreated hypertensive patients. J Hypertens. 2013;31(8):1653–1661. | ||
Parati G. Blood pressure variability: its measurement and significance in hypertension. J Hypertens Suppl. 2005;23(1):S19–S25. | ||
Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50(2):325–332. | ||
Myers MG, Kaczorowski J, Dolovich L, Tu K, Paterson JM. Cardiovascular risk in hypertension in relation to achieved blood pressure using automated office blood pressure measurement. Hypertension. 2016;68(4):866–872. | ||
Hypertension guideline working group, Seedat YK, Rayner BL, Veriava Y. South African hypertension practice guideline 2014. Cardiovasc J Afr. 2014;25(6):288–294. | ||
Toal CB, Meredith PA, Elliott HL. Long-acting dihydropyridine calcium-channel blockers and sympathetic nervous system activity in hypertension: a literature review comparing amlodipine and nifedipine GITS. Blood Press. 2012;21(Suppl 1):3–10. | ||
Philipp T, Smith TR, Glazer R, et al. Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther. 2007;29(4):563–580. | ||
Burnier M, Pruijm M, Wuerzner G. Treatment of essential hypertension with calcium channel blockers: what is the place of lercanidipine? Expert Opin Drug Metab Toxicol. 2009;5(8):981–987. | ||
Bahl VK, Jadhav UM, Thacker HP. Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the strong prospective, observational, multicenter study. Am J Cardiovasc Drugs. 2009;9(3):135–142. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.