Back to Journals » Research and Reports in Urology » Volume 13
The Effect of Intratesticular Injection of Human Adipose-Derived Mesenchymal Cell on Testicular Oxidative Stress and Spermatogenesis Process in the Varicocele Rat Model
Authors Siregar S, Noegroho BS , Adriansjah R , Mustafa A , Bonar A
Received 22 July 2021
Accepted for publication 30 September 2021
Published 11 October 2021 Volume 2021:13 Pages 759—765
DOI https://doi.org/10.2147/RRU.S330634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Safendra Siregar, Bambang Sasongko Noegroho, Ricky Adriansjah, Akhmad Mustafa, Ananta Bonar
Department of Urology, Faculty of Medicine, Universitas Padjadjaran, Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Safendra Siregar
Department of Urology, Faculty of Medicine Universitas Padjadjaran, General Hospital Hasan Sadikin Bandung, Jl. Makmur No. 16, Bandung, Indonesia
Tel +62811227180
Email [email protected]
Introduction: Varicocele is the predominant cause of male infertility. Reactive oxygen species was found in varicocele which induce the lipid peroxidation process in the plasma membrane of spermatozoa and may cause damage to spermatozoa in semen and seminiferous tubules, disrupting spermatogenesis. Human adipose derived stem cells (hADSC) can suppress oxidative stress in some oxidative injury model. This study investigate the effect of intratesticular hADSC injection on malondialdehyde (MDA) level and spermatogenesis process by histopathological examination in the varicocele rat model.
Methods: This is an experimental study. A total sampling of 9 male Wistar rats were divided into three groups. Group I consist of 1 Wistar rats without any treatment or model (sham group), group II consist of 4 Wistar rats with varicocele model without hADSC therapy (control group), and group III consist of 4 Wistar rats with varicocele model and were given injections of 1.0× 106 hADSC cells intratesticularly 30 days after model was made (therapy group). Testicular tissue was harvested for evaluation.
Results: In all varicocele model rats (group II and III), the result of MDA level in therapy group (2.53 mol/liter) was significantly lower than the MDA level in control group (4.43 mol/liter) (p = 0.01). On histopathological examination, the average Johnson’s Score in the therapy and control group was 9.77 and 9.18, respectively. The analysis showed Johnson’s score in the intervention group was significantly higher (p = 0.018).
Conclusion: Intratesticular injection of hADSC can help reduce MDA levels and improve spermatogenesis process, which is damaged by varicoceles.
Keywords: human adipose-derived stem cell, varicocele, spermatogenesis, infertility, malondialdehyde, MDA
Introduction
Infertility is one of the fundamental problems affecting human life.1 According to the Demographic and Health survey in collaboration with WHO which was conducted from 1990 to 2004, it was found that one in every four couples in developing countries was affected by infertility.2
Male infertility is a concern and contributes to 50% of total infertility cases.3,4 Varicocele is the most common cause of male infertility worldwide. Varicocele is found in 19–41% of men with primary infertility and the cause of 45–81% of men with secondary infertility, contributing as the most common cause of male infertility, which can be treated surgically. Studies have shown that varicoceles’ incidence has been associated with increased oxidative stress, which may lead to the overproduction of reactive oxygen species (ROS) such as superoxide anion radicals, hydrogen peroxide, singlet oxygen, and nitrite proxy radicals.5,6
Reactive oxygen species are the oxygen metabolites in the form of a collection of free radicals which react easily. Reactive oxygen species will induce the lipid peroxidation process in the plasma membrane of spermatozoa and may cause damage to spermatozoa in semen and seminiferous tubules, disrupting spermatogenesis. Reactive oxygen species can be assessed by measuring the malondialdehyde (MDA), an organic compound resulting from lipid peroxidation. It can be used to monitor the degree of tissue damage from oxidative stress.1,7–10
Stem cell transplantation to improve organ structure or tissue function has become a new therapeutic strategy. Stem cells are capable of regenerating and differentiate into specific types of cells. Adipose-derived stem cells (ADSC) are one of the mesenchymal stem cells (MSC) types with the most optimal therapeutic cell. These stem cells are mesenchymal stromal cells found in the perivascular adipose tissue. A study showed that ADSC could suppress oxidative stress by increasing superperoxide dismutation that will reduce oxidative stress in azoospermia condition.11
Based on the data above, it can be assumed that the administration of ADSC can suppress oxidative stress in varicoceles and improve spermatogenesis. ADSC is considered to be one of the alternative therapies besides surgical therapy of varicocele. We aim to study the effects of ADSC administration on histopathologically assessed spermatogenesis.
Materials and Methods
This research is a randomized experimental study that used experimental animals (experimental animal study). This research was conducted after obtaining approval and recommendation from the Laboratory Ethics Committee of the Institute of Biosciences, Brawijaya University Malang. All of the participants in this study were from Padjadjaran and the research was done in Malang laboratory due to our laboratory in Padjadjaran was under maintenance so we cannot use our laboratory. All of the ethical approval and animal welfare guidelines are based on Brawijaya University Malang with agreement between both University.12 Ethical approval from Institutional Review Board (IRB) of Brawijaya University Malang and Soetomo Hospital Surabaya was obtained for the use of this tissue. A total of nine male Wistar rats weighing 300–350 grams were used as samples and divided into 3 groups. Group I consisted of one Wistar rat without treatment or model (sham group), Group II consisted of four Wistar rats with varicocele model without hADSC therapy (control group). Group III consisted of four Wistar rats with varicocele model and was given 1.0×106 cells single dose intratesticular hADSC injection in 100µL of PBS solution as deep as the tunica albuginea 30 days after the varicocele model was made (therapy group). All rats were euthanized by Isoflurane gas inhalation, and following euthanasia, left orchidectomy was performed 30 days after ADSC administration in group III (Figure 1). Testicular tissue was harvested for histopathological examination to evaluate the spermatogenesis process using Johnson’s score. Data was collected and analyzed using Shapiro–Wilk test and Mann–Whitney U-test. This study has been accepted by the Ethics Committee with the research number 013-KEP-UB-2020.
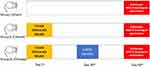 |
Figure 1 Research flow. |
Varicocele Model of Wistar Rat
Adult Wistar rats were put under anesthesia via an intraperitoneal route using 90 mg/kg ketamine and 4 mg/kg xylazine. A midline laparotomy incision was performed to expose the upper left abdominal quadrant. The left kidney and associated blood vessels were then exposed by displacing the intraperitoneal organ to the right. The left kidney, left renal vein, left adrenal vein, and left spermatic vein were then visualized. Through a blunt dissection, the left renal vein was freed from the adhering fat and connective tissue. The left renal vein should be exposed in a medial position from the entry point of the left spermatic vein and left adrenal vein. After the left renal vein was freed from the surrounding tissue, a 4–0 silk suture was used to ligate the vein. Ligation was carried out by inserting a 0.85 mm metal wire in the middle and knotted four times, then the wire was removed, and the left renal vein would slightly expand according to the size of the knot. This would result in consistent partial occlusion of the left renal vein in each model (Figure 2). The intraperitoneal organs were then returned to their original position, and the abdomen was resutured by using 2–0 silk suture.13
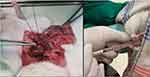 |
Figure 2 Injection of hADSC in Varicocele rats. |
Human Adipose-Derived Stem Cell
Human adipose tissue was obtained from adult male/female fat subjected to other surgeries who had agreed to donate his/her fat for research by signing informed consent. Adipose tissue was obtained with needle biopsy or liposuction aspiration method. Adipose tissue was stored at room temperature for no more than 24 hours before use. Then, the adipose tissue was washed with phosphate-buffered saline (PBS) containing 5% penicillin/streptomycin (P/S). During washing, samples were stored in sterile culture dishes with 0.075% type I collagenase prepared with PBS containing penicillin/streptomycin 2% for tissue digestion. Slice the fat tissue using 2 scalpels, then insert the sample into a 25 or 50 mL pipette up and down several times to facilitate tissue digestion. The samples were then incubated for 30 minutes at 37 degrees Celcius, 5% CO2, then the collagenase activity was neutralized using 5 mL of MEM containing 20% fetal bovine serum (FBS) to the tissue samples. The tissue is then fed into the pipette up and down several times to facilitate tissue disintegration. Centrifuged the sample at 2000 rpm for 5 minutes to obtain SVF containing ASDC. After centrifugation, shake vigorously to mix the pellets and cells. This step completes the separation of the stromal cells from the primary adipocytes. Repeat the centrifugation step. After centrifugation, aspirate all the collagenase solution that was in the top layer without disturbing the cells. Suspense the pellet in 1 mL lysis buffer, incubate for 10 minutes between ice, and then wash with 2% PBS solution containing 2% P/S, then centrifuge at 2000 rpm for 5 minutes. Aspirate the supernatant and re-suspend the pelleted cells in a maximum of 3 mL stromal medium added with 20% FBS, 1% L-Glutamine, and 1% P/S, then the cell suspension was filtered through a 70 mm cell strainer. Wash the cell strainer by adding 2 mL of stromal medium to obtain additional cells. Place the sample containing the cells in a lysine-coated culture dish and incubate at 37 degrees Celsius, 5% CO2.14
Seventy-two hours after placing in culture plates, aspirate all culture medium from the plates. Wash cells with warmed PBS. Spray the pipette over the cells several times to completely clean the cells of tissue fragments and/or blood cells. Add stromal medium to taste. Keep cells in a moist tissue culture incubator at 37 degrees Celsius and 5% CO2. Change medium every 2 days until the cells reach 80–90% confluence. For vital ADSC extraction, add a small volume of warm, sterile PBS and allow to rest. Replace PBS with 0.5% trypsin/EDTA solution. Place in the incubator for 5 minutes and make sure the cells have been removed using a microscope. Make sure more than 90% of the cells have been removed, then add 0.5 mL of stromal medium to neutralize the trypsin reaction. Transfer the medium containing the suspended cells from the culture dish to a 2 mL tube. Centrifugate at 1200 rpm for 5 min. Aspirate the supernatant and suspend the cells in a small volume of the stromal medium. Continue with the cell count by taking a small number of cells diluted in trypan blue (for a 1:2 solution add 12.5 ll of suspended cells to 12.5 ll of trypan blue). Count cells using a hemocytometer. Cells can then be placed back into the plate according to the capacity of the dish in the culture site. The medium was replaced 1 day after manufacture and then every 2 days. Culture and cell multiplication were done by thawing 1 tube, splitting once, and changing the medium once.14
Flow cytometry assay was used to validate the isolated cells using the following markers: CD105 (+), CD45 (-). In the validation application, cell viability was at 86.78%, with 1 cc each containing 1.0×106 cells for use. All tissue harvesting procedures, cell isolation, culture, and characterizations were conducted in the Tissue and Cell Bank of Dr. Soetomo General Hospital Surabaya, sample-numbered with 120.E/MSC/Penelitian/AdiposeTasijo_09/060220 and were declared suitable for the application.
Johnson’s Score
One of the parameters for evaluating spermatogenesis in the seminiferous tubules is by using Johnson’s score. The testicular tissue is dehydrated through a graded series of 70–100% ethanol and embedded in paraffin. One portion with a diameter of 5 μm was obtained to be deparaffinated and stained with Hematoxylin-Eosin (H&E), which was then examined under a light microscope. The histopathological grade of spermatogenesis was assessed between 1 and 10 based on the development of sperm cells found in at least 5 microscopic areas in 100 seminiferous tubules. Johnson’s total score is then determined by dividing the total score by the number of tubules evaluated.
Result
Left Internal Spermatic Vein Width
The success of the varicocele model was assessed based on the left internal spermatic vein width of the male rats 4 weeks after ligation. Comparison of mean internal spermatic vein width in each group is shown in Table 1. If Group II and III were combined into 1 group, namely the group of mice with a varicocele model, and were compared to group I without varicocele model, it was shown that partial ligation of the left renal vein was proven to induce the development of artificial left varicocele in mice. The mean internal spermatic vein width in the group of rats with varicocele model was 0.70 mm. This result was greater compared to group I (sham) without varicocele model, which was 0.23 mm. (p=0.005).
 |
Table 1 Comparison of Left Internal Spermatic Vein Width |
MDA Concentration
There was a significant difference in MDA levels between group II (control) and group III (therapy) as shown in Figure 3 (p = 0.01). The mean MDA levels in the therapy group were lower (2.53 mol/liter) than the group without ADSC (4.43 mol/liter).
 |
Figure 3 MDA concentration between groups. |
Histological Examination (Johnson’s Score)
There is also a significant difference in Johnson’s Score between group II (control) and group III (therapy) (Figures 3 and 4) The average value of Johnson’s Score in group III (therapy) was higher than group II (control). Group III, who received ADSC, had an average Johnson’s score of 9.77, whereas group II without ADSC had an average Johnson’s score of 9.18. Data analysis by using the Kolmogorov Smirnov test showed that the data distribution was not normal (p <0.05). Based on the results of normalization tests that were not normally distributed, data analysis was performed using the Mann–Whitney test. There was a significant difference in the mean value of Johnson’s Score between treatment groups with p = 0.018.
 |
Figure 4 Testicular tissue between groups. (A) Tissue from control group (B) Testicular tissue from therapy group (C) Testicular tissue from group without therapy. |
Discussion
Varicoceles can cause progressive deterioration of the Sertoli cells and trigger the release of spermatogonia cells before their peak maturity. Histopathologic examination in varicocele may show normal and defective spermatogenesis.1,3,4
In a previous study conducted by Qin et.al, it was found that varicocele can cause damage to the endothelial part of the testis and seminiferous tubules, as well as interfering with the spermatogenesis process. They conducted a study by ligating the left renal vein in mice until it was enlarged by two or more times and found a significant reduction in sperm count compared to the control group.8
Several theories hypothesized the relationship between varicocele and infertility, including increased temperature in germ cells sensitive to temperature changes, cadmium accumulation, testicular hypoxia, and metabolite reflux, leading to increased stress oxidative. This oxidative stress causes damage to the function and maturity of spermatogenesis, membrane peroxidation, and hormonal disturbances, which reduce male reproductive potential in the form of decreased sperm count, shape, and motility.15,16
In this study, it was found that there is a significant difference in mean MDA levels between groups (p = 0.01). MDA levels in the therapy group were lower (2.53 mol/liter) than the group without ADSC (4.43 mol/liter), this result was in line with other studies showing that ADSC can self-renew in the vascular stroma and can influence local regulation of angiogenesis and blood vessels remodeling, thereby suppressing oxidative stress by increasing the activity of superoxide dismutase which will cause a decrease in the formation of MDA.17–19
This study showed that the group of mice with varicocele receiving ADSC therapy had a better Johnson’s score (9,77) than the group of mice with varicocele without receiving therapy (9,18)(p = 0.018).
The group model of mice with varicocele that received ADSC therapy had an average Johnson’s Score of 9.77, with the highest score of 9.93 and the lowest score of 9.4. This score showed normal spermatogenesis histopathologically. Normal spermatogenesis is characterized by the presence of seminiferous tubules that are covered by a thin basal membrane and germinal epithelium, indicating normal sperm development from spermatogonia to spermatocytes, including spermatids and spermatozoa. Whereas in the group model of varicocele mice without ADSC therapy, the lowest Johnson’s score was 8.33. The picture in this sample shows the histopathological features of hypospermatogenesis, where the germinal epithelium shows a normal stage of sperm development, but the number is greatly reduced.
We used human ADSC in this study because the number of fat cells from liposuction was abundant, and in the future, we aim to use human ADSC in human patients. Therefore, we started using human ADSC in mice. Human ADSC will not induce xenogenic immunological reactions because it does not express MHC class II.
We performed ADSC intratesticular injection method and found a significant result of spermatogenesis improvement. Intratesticular injection of ADSC gives a better result compared to intravenous injection because there is a blood-testis barrier in the intravenous line that may prevent stem cells from entering testis tissue through the circulation. In addition, it will prevent ADSC from getting trapped in the lungs due to their large size of molecules.20 Another advantage of intratesticular administration of stem cells is that they can significantly protect from testicular damage. Although complications from injection procedure may cause anti-sperm antibody formation that can lead to infertility, long-term follow-up is necessary.21
Sampling was carried out on the 30th day after ADSC administration to the group of rats with varicocele. The 30 days assessment for sampling was used to assess the success of the effect of ADSC on the spermatogenesis cycle of Wistar rats, ranging from 30 to 48 days.21
Stem cell injection may prevent tissue damage caused by oxidative stress. After administration of stem cells, stem cells were integrated into interstitial area and seminiferous tubules and into the vascular walls of the testis, secreting various growth factors, such as Basic Fibroblast Growth Factor and Stem Cell Factor, which would reduce the level of oxidative stress, regulating hormonal levels and promoting regeneration of Leydig cells and germ cells, which would improve the process of spermatogenesis and increase Johnson’s Score in the testis.20 Intratesticular injection of ADSC also has the effect of increasing and repairing testosterone levels. It suggests that ADSC may improve the endocrine function of the testis.21
Conclusion
Based on the results and discussion of this study, it can be concluded that the administration of ADSC may improve the spermatogenesis process damaged by varicoceles. This shows the benefits of ADSC as a stem cell therapy which may be further investigated as one of the therapeutic options in improving the spermatogenesis process for patients with varicocele.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Noegroho BS. Oxidative stress assessment of artificial left varicocele in rabbit (Oryctalagus cuniculus) model treated with or without antioxidant vitamin C. Bandung: Universitas Padjadjaran; 2010
2. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):1–12. doi:10.1371/journal.pmed.1001356
3. Agarwal A, Mulgund A, Hamada Chyatte MRA. Unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;37:1–9. doi:10.1186/s12958-015-0032-1
4. Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77(3):491–498. doi:10.1016/S0015-0282(01)03229-0
5. Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl. 2016;18(2):163. doi:10.4103/1008-682X.172638
6. Khatri DK, Juvekar A. Preliminary phytochemical and antioxidant evaluation of a polyherbal formulation. Int J Phytopharm. 2013;4(5):322–328.
7. Saleh RA, Esfandiari N, Sharma RK. Diagnostic and prognostic value of measurement of reactive oxygen species in neat semen. Am Soc Reprod Med. 2001;76(3). doi:10.1016/S0015-0282(01)02042-8
8. Qin Q, Liu J, Ma Y, et al. Aberrant expressions of stem cell factor/c-KIT in rat testis with varicocele. J Formos Med Assoc. 2016;2:116.
9. Ge Y, Zhang Q, Jiao Z, Li H, Bai G, Wang H. Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 2018;1(214):62–69. doi:10.1016/j.lfs.2018.10.054
10. Asadi N, Kheradmand A, Gholami M, Saidi SH, Mirhadi SA. Effect of royal jelly on testicular antioxidant enzymes activity, MDA level and spermatogenesis in rat experimental Varicocele model. Tissue Cell. 2019;57:70–77. doi:10.1016/j.tice.2019.02.005
11. Sun G. Effects of adipose-derived stem cells on antioxidant enzymes and malondialdehyde in radiation damaged rat. Radiat Prot Bull. 2012;32(4):1–5.
12. Universities Federation for Animal Welfare. The UFAW Handbook on the Care & Management of Laboratory Animal. United Kingdom: Bath Press, Avon; 1987.
13. Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod. 2006;21(4):986–993. doi:10.1093/humrep/dei429
14. Su JS, Farber NJ, Vij SC. Pathophysiology and treatment options of varicocele: an overview. Andrologia. 2021;53(1):e13576. doi:10.1111/and.13576
15. Dohle GR, Elzanaty S, van Casteren NJ. Testicular biopsy: clinical practice and interpretation. Asian J Androl. 2012;14(1):88–93. doi:10.1038/aja.2011.57
16. Katz MJ, Najari BB, Li PS, Goldstein M. The role of animal models in the study of varicocele. Transl Androl Urol. 2014;Mar(1):593.
17. Cakici C, Buyrukcu B, Duruksu G, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013;2013:529589. doi:10.1155/2013/529589
18. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049. doi:10.1155/2016/1245049
19. Hikim AP, Maiti BR, Ghosh A. Spermatogenesis in the bandicoot rat. I. Duration of the cycle of the seminiferous epithelium. Arch Androl. 1985;14(2–3):151–154.
20. Meligy FY, Abo Elgheed AT, Alghareeb SM. Therapeutic effect of adipose-derived mesenchymal stem cells on Cisplatin induced testicular damage in adult male albino rat. Ultrastruct Pathol. 2019;43(1):28–55. doi:10.1080/01913123.2019.1572256
21. Hamada A, Esteves SC, Agarwal A. Varicocele and male infertility: current concepts, controversies and consensus [Internet]. SpringerBriefs in Reproductive Biology. Springer International Publishing; 2016 [
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
