Back to Journals » Journal of Pain Research » Volume 9
The effect of intra-articular vanilloid receptor agonists on pain behavior measures in a murine model of acute monoarthritis
Authors Abdullah M, Mahowald M, Frizelle S, Dorman C, Funkenbusch S, Krug H
Received 28 February 2016
Accepted for publication 31 March 2016
Published 17 August 2016 Volume 2016:9 Pages 563—570
DOI https://doi.org/10.2147/JPR.S107385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Mishal Abdullah,1 Maren L Mahowald,2 Sandra P Frizelle,2 Christopher W Dorman,2 Sonia C Funkenbusch,2 Hollis E Krug2,3
1Department of Medicine, Rheumatology Fellowship Training Program, University of Minnesota Medical School, 2Department of Medicine, Minneapolis Veterans’ Affairs Health Care System, 3Department of Medicine, University of Minnesota Medical School, Minneapolis, MN, USA
Abstract: Arthritis is the most common cause of disability in the US, and the primary manifestation of arthritis is joint pain that leads to progressive physical limitation, disability, morbidity, and increased health care utilization. Capsaicin (CAP) is a vanilloid agonist that causes substance P depletion by interacting with vanilloid receptor transient receptor potential V1 on small unmyelinated C fibers. It has been used topically for analgesia in osteoarthritis with variable success. Resiniferatoxin (RTX) is an ultra potent CAP analog. The aim of this study was to measure the analgesic effects of intra-articular (IA) administration of CAP and RTX in experimental acute inflammatory arthritis in mice. Evoked pain score (EPS) and a dynamic weight bearing (DWB) device were used to measure nociceptive behaviors in a murine model of acute inflammatory monoarthritis. A total of 56 C57B16 male mice underwent EPS and DWB testing – 24 nonarthritic controls and 32 mice with carrageenan-induced arthritis. The effects of pretreatment with 0.1% CAP, 0.0003% RTX, or 0.001% RTX were measured. Nociception was reproducibly demonstrated by increased EPS and reduced DWB measures in the affected limb of arthritic mice. Pretreatment with 0.001% RTX resulted in statistically significant improvement in EPS and DWB measures when compared with those observed in carrageenan-induced arthritis animals. Pretreatment with IA 0.0003% RTX and IA 0.01% CAP resulted in improvement in some but not all of these measures. The remaining 24 mice underwent evaluation following treatment with 0.1% CAP, 0.0003% RTX, or 0.001% RTX, and the results obtained were similar to that of naïve, nonarthritic mice.
Keywords: pain, resiniferatoxin, capsaicin, vanilloid, intra-articular
Introduction
Arthritis is the most common cause of disability in the US. It is estimated that >50 million adults (one out of every five people) suffer from some form of arthritis.1 One of the main goals of arthritis management is pain reduction. Currently, available oral systemic analgesics are limited by insufficient joint pain relief, intolerable drug side effects, and adverse drug interactions.2,3
Other agents for intra-articular (IA) administration, such as corticosteroids, hyaluronic acid derivatives, and botulinum toxin A, have variable analgesic effects and duration of action.4–7 Therefore, there is still an unmet need for new therapies for refractory arthritis pain.
Murine models of joint nociception are commonly used in preclinical studies of new analgesic therapies. IA carrageenan (CAR) has been employed to induce a murine model of nociception in acute monoarticular inflammatory arthritis.7,8 Measures of evoked pain thresholds and tenderness have been successfully used to quantify nociception in mouse models of arthritis.8 Analysis of dynamic weight bearing (DWB) measures has been found to be extremely sensitive for quantifying limb nociception severity.9,10
Capsaicin (CAP) and resiniferatoxin (RTX) are vanilloid agonists that have attracted much attention due to their effect on alleviating different kinds of pain.11 CAP is a vanilloid agonist that exerts its analgesic effects by binding to transient receptor potential V1 (TRPV1) channels in sensory nerves.12 These toxins are nonselective cation ionophores involved in integration of afferent noxious signals generated by mediators of inflammation. CAP triggers a calcium influx by binding to TRPV1 channels, followed by generation of an action potential and depletion of substance P making the neuron unresponsive to additional painful stimuli.13 Other potential mechanisms include the effects of sustained high levels of intracellular calcium that may activate calcium-dependent enzyme proteases14 or induce depolymerization of cytoskeletal components such as microtubules.15,16 CAP has been used as a topical analgesic for arthritis pain.17
RTX is a CAP analog derived from latex of Euphorbium resinifera, which is a potent agonist of TRPV1.18 In contrast to CAP, Jeffry et al18 demonstrated that low concentrations of RTX caused a slow and sustained depolarization of membrane potential, thereby preventing the generation of action potentials.
The aim of this study was to determine whether IA administration of CAP or RTX would produce an analgesic effect in experimental acute inflammatory arthritis joint nociception. We compared the evoked and spontaneous nociceptive responses of male C57Bl6 mice before and after IA injection of CAR, which produced severe, acute inflammatory monoarthritis. We then measured the nociceptive pain behaviors in arthritic mice with and without pretreatment with CAP and two doses of RTX.
Materials and methods
Animal subjects
A total of 56 male C57Bl6 mice from the Jackson Laboratory (Bar Harbor, ME, USA) aged 11–12 weeks were studied. Animals were housed in groups of eight in the Animal Care and Research Facility at the Minneapolis Veterans Affairs Medical Center, a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were acclimated to the vivarium for 1 week before experimentation and were housed in standard polycarbonate 183/4×101/4×81/4 in3 cages with water and standard rodent diet ad libitum. Environmental conditions were maintained at 72°F±1°F and 33%±1% humidity, with 12-hour light/dark cycles. All animal procedures and protocols were approved by the Minneapolis Veterans Administration Health Care System (VAHCS) Institutional Animal Care and Use Committee and conformed to the “Guide for the Care and use of Laboratory Animals”.19
Study design
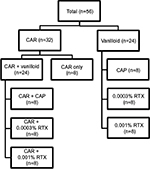
Seven groups of eight animals each (56 animals) were studied. Four groups of eight animals (32 animals) were studied, prior to any IA injections. Eight animals subsequently received IA CAR to produce acute inflammatory arthritis nociception and were examined 3 hours after IA injection of CAR when nociceptive responses peak.
Three vanilloid treatment groups of eight mice each (24 animals) were pretreated with IA vanilloids (0.01% CAP, 0.001% RTX, or 0.0003% RTX) 7 days prior to induction of arthritis and examined 3 hours following IA CAR. Three groups (24 animals) of nonarthritic mice received equivalent doses of IA vanilloids and served as nonarthritic controls for the treatment groups (Figure 1).
Joint injections
Injections were performed into the left knee through the infrapatellar ligament using a 30 G needle with a customized sheath that limited needle penetration to 2.5 mm. The mice were anesthetized with isoflurane in oxygen (induction and maintenance 3%, 1 L/min) for 3±1 minutes during the injections. The left knee was shaved and the skin disinfected with 70% ethanol solution. CAP and RTX groups were injected 7 days prior to examination with 10 mL of either 0.01% CAP (MP Biomedicals, Sigma Aldrich Co., St Louis, MO, USA#202757), 0.0003% RTX (Sigma-Aldrich Co., St Louis, MO, USA, #R8756), or 0.001% RTX diluted in 0.9% sterile saline. The CAR groups were injected 3 hours prior to examination with 10 mL of 2.5% CAR (Sigma-Aldrich Co., #22049-5G-F) diluted in 0.9% sterile saline. Animals recovered under a heat lamp until normal ambulation was observed.
Measures of nociceptive behavior
Both evoked and spontaneous nociceptive pain behaviors were measured. Evoked pain score (EPS) was measured as the total number of fights and vocalizations during 1 minute of firm palpation of the knee at a frequency of 1/s.8 The control right knee was examined before the left knee for comparison. Examinations were performed by a single-blinded examiner trained in consistent manual restraint methods and trained with a Palpometer (Palpometer Systems, Inc., Victoria, BC, Canada) to deliver a series of repeated firm palpations of ~1,100 gf/cm2 or 15.6 psi.
Spontaneous nociceptive behavior was measured as the offloading of body weight (measured as a percentage of total body weight) from the painful limb and reduced time (measured as a percentage of total time in the device) on the painful limb using the DWB device (Bioseb, Vitrolles, France). To increase the sensitivity of detecting spontaneous nociceptive behaviors, each mouse was placed in the DWB device after measurement of EPS. The device is a 4.5×4.5×8.25 in3 plexiglass cube with a floor consisting of 1,936 pressure sensors. The device software calculated the percentage of time spent and percentage of weight placed on each of the four limbs during a 5-minute period. We compared the percentage of time spent and the percentage of weight placed on each of the two hind limbs.
Statistical analysis
EPS and ADWB for the five independent groups (naïve, CAR, CAP, HD RTX, and LD RTX) were compared using a one-way analysis of variance. Dunnett’s post hoc t-test for comparing each of the other four groups with the CAR group was used whenever the overall analysis of variance was significant.
Results
Characterization of nociceptive behaviors produced in the murine model of CAR-induced acute knee arthritis
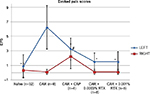
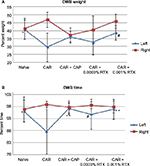
EPSs from the knees of 32 naïve animals were <2 and equivalent bilaterally (SD =1.58 in the left and SD =0.97 in the right). IA CAR-induced arthritis resulted in increased EPSs in the left knee (mean =6.25 fights and/or vocalizations per mouse, SD =3.0; Figure 2) and a difference between left and right knee EPSs was 6.125 (SD =3.18). Spontaneous nociceptive behaviors measured with the DWB device demonstrated equal weight bearing on each hind limb of 40% in naïve nonarthritic animals and a significant decrease to 30% in the left hind limb with CAR-induced arthritis (P<0.05, SD =8.9). The difference between left and right weight bearing increased from <1% to 17% (P<0.0005, SD =11.7). The average time spent on each hind limb was equivalent in naïve nonarthritic mice. Time spent on the left hind limb decreased from 96% to 88% with CAR-induced arthritis in the left knee (P<0.05, SD =9.3) and the difference between the left and right hind limbs increased from <1% in naïve mice to 11% in the arthritic mice (P<0.0001, SD =9.87; Figure 3).
Measuring the analgesic effects of IA vanilloids in CAR-induced acute knee arthritis
Groups of eight mice each underwent pretreatment with 0.01% CAP, 0.0003% RTX, or 0.001% RTX prior to arthritis induction. IA CAP pretreatment reduced the EPS score by 48% from 6.25 to 3.25 (P<0.026; Figure 2). The DWB measures for weight increased from 30% to 36% in the CAP-treated arthritic mice (P=0.16) when compared with the untreated arthritic mice. The time on the arthritic hind limb increased from 88% in arthritic untreated mice to 97% in the CAP-treated mice – the normal amount (P=0.003; Figure 3).
IA 0.001% RTX (high dose) pretreatment significantly reduced the EPS from 6.25 to 1.5 (a near normal score; P<0.0001; Figure 2). DWB measure of percentage of weight on the arthritic limb increased from 30% to 38.5% (P=0.024) and time on rear limbs significantly increased from 88% to 97% (P<0.004; Figure 3).
Pretreatment with the lower dose 0.0003% RTX also reduced the EPS significantly from 6 to 1.5 (P<0.0001; Figure 2). The average weight on hind limbs only increased from 30% to 33% not significant (NS) but time on hind limbs increased from 88% to 95% (P=0.033; Figure 3).
Each vanilloid treatment significantly reduced the right to left knee difference in EPSs (Figure 4).
The left to right difference in percentage of time of weight bearing significantly decreased from 11.4% in arthritic mice to <1%, 3%, and 1% in treated animals (Figure 4).
Left to right differences in percentage of weight bearing decreased from 17.3 to 1.5 with CAP (P=0.004), to 7.9 with low-dose RTX (P=0.142), and to 7.5 with the high-dose RTX (P=0.118; Figure 3). Although the results for RTX were not quite statistically significant, there did seem to be a dose effect seen between low-dose (0.0003%) and high-dose (0.001%) RTX in percentage of weight bearing.
Normal right knee controls
Throughout all stages of this study, the contralateral right knee was a normal, nonarthritic internal control. After induction of arthritis, EPSs in the contralateral knee fell slightly from a baseline mean of 0.34 (SD =0.0.97) to 0.125 (SD =0.35), P = NS. The DWB measures for mean weight and time of the nonarthritic right hind limb were analyzed for each separate group, and these values failed to show any statistically significant difference when compared with the naïve, nonarthritic mice except for an increase in percentage of weight bearing of the right hind limb in the arthritic group (46.89%, SEM 1.68) and the high-dose RTX group (46.0%, SEM 1.56) compared with the naïve group (41.2%, SEM 1.18), P < 0.05 (Figure 3A) and a decrease in right limb weight bearing in the CAP-pretreated arthritic group (37.35%, SEM 1.51) compared with the arthritic group (P<0.001). This appears to indicate compensation by the nonarthritic right hind limb in the face of nociception in the arthritic left hind limb that is reversed when the nociception is reduced in the arthritic limb.
IA vanilloid agonist control
IA vanilloid agonist treatment in naïve, nonarthritic mice did not result in significant change in EPS or DWB measures when compared with data obtained from naïve, nonarthritic mice (Figure 5).
Discussion
This study demonstrated that IA CAR-induced acute inflammatory knee arthritis produced measurable evoked and spontaneous nociceptive behaviors that were used to measure analgesic effects of IA injections of vanilloid agonists.
IA CAR induced an acute inflammatory monoarthritis with peak intensity at 3–4 hours similar to that of previous studies.20,21 EPSs increased 3 hours after IA CAR injections. The spontaneous nociceptive behaviors measured as changes in DWB were related to offloading from the painful limb with compensatory increased loading of the normal limb. Weight bearing then improved with IA analgesia. This study confirms that a shift of body weight from the painful arthritic limb to the contralateral normal limb reflects a weight-bearing deficit and is a reliable measure of spontaneous nociception in models of knee joint arthritis.12,13,22,23 These results were similar to a study of CAR arthritis in rats and our previous results using IA botulinum toxin in mice.8,17
We showed that pretreatment with IA CAP and IA RTX normalized some pain behavior measures. Both vanilloids normalized DWB measures for time spent on the arthritic limb. These improvements were statistically significant. Differential weight bearing deficits tend to improve but the change was statistically significant only in the group pretreated with high-dose RTX compared with the arthritic left hind limbs. Interestingly, even though vanilloid treatment did not normalize weight bearing, in the left hind limbs compared with arthritic left hind limbs, differences between right and left hind limbs disappeared with CAP pretreatment and were reduced with all doses of RTX pretreatment. Quadrupeds may compensate for hind limb pain with fore limb weight bearing. In some cases, this symmetric offloading of the hind limbs seemed to be compensated for by increased loading of the forelimbs, but this was not consistent.
Although there was improvement in pain behaviors with IA vanilloid receptor agonists, this improvement was often incomplete and variable. We controlled for the effect of IA injection so close to the time of examination. IA injection alone had no effect on nociceptive pain measures. Previous studies have demonstrated that IA treatment with saline does not improve EPS in experimental acute inflammatory monoarthritis.8 Therefore, it is not likely that IA injection alone or the vehicle used for the specific treatments had any effect. We were concerned that irritants such as RTX and CAP could produce a nociceptive response in the absence of joint pain, but IA injection of vanilloid alone did not produce a change in nociceptive pain behaviors.
The high-dose IA RTX injection was often more effective suggesting a dose effect. This higher dose is equal to 0.1 mg and is significantly lower than previous locally administered doses.24,25
RTX seemed to provide more reliable analgesia than CAP. RTX is considered to be less of an irritant than CAP and the mechanism of action is different. Although they share a vanillyl group that binds to TRPV1, CAP binds to vanilloid-sensitive peptidergic neurons causing a rapid release of SP, whereas RTX causes a slow and sustained depolarization of the membrane and also binds to serum proteins. This low affinity protein binding may affect the pharmacokinetics of this compound producing a slower onset and more sustained response.18,26,27
The typical time of onset of vanilloid-induced analgesia and the short time of onset of CAP-induced nociception require that the vanilloids should be given prior to the induction of joint pain in this model. It will be important to determine whether IA vanilloid treatment can be effective in chronic arthritis pain when given after the joint pain has been established. Certainly, topical treatments have been shown to be effective,17 but the results presented here support further studies of these two vanilloids as potential IA analgesics in humans as has been previously suggested.28
Our study has several potential limitations. The groups were small so that real differences may not have been detected. The results cannot be extrapolated to other models of experimental arthritis nociception because the experiments were conducted only in mice with acute CAR-induced monoarthritis. In these murine studies, as well as in previous similar studies, the IA procedures were carried out under short duration general anesthesia. This may not be necessary or practical in humans. An alternative would be to administer RTX or CAP mixed with local anesthetic. Time–dose studies with the toxins are needed to optimize toxin dose and dosing intervals. The duration of analgesic effects is not known and would be difficult to establish in this model because the acute arthritis nociception only lasts 3–4 hours. Future studies are planned to examine the effect of IA vanilloids in mouse models of chronic joint pain.
Conclusion
Our experiment showed that the IA administration of the vanilloid agents, CAP and RTX, in mice was safe and produced analgesia in an experimentally induced acute inflammatory arthritis pain model. It may have the potential for use in humans with refractory chronic arthritis pain.
Acknowledgments
Many thanks to Thomas Rector, PhD, who was very helpful in designing and performing the statistical analysis. This work was supported by Merit Review Award # RX000379-05 from the US Department of Veterans Affairs Rehabilitation Research and Development Service. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Disclosure
The authors report no conflicts of interest in this work.
References
Centers for Disease Control and Prevention. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation United States, 2007 – 2009. MMWR Morb Mortal Wkly Rep. 2010;59(39):1261–1265. | ||
Mahowald M. The Role of Nonprescription Analgesics in Treating Mild to Moderate Pain. Clinical and Economic Considerations. Minneapolis: Minneapolis Health Learning Systems; 2000. | ||
Mahowald ML. Chronic pain management. In: Ruddy S, Harris E, Sledge C, Budd R, Sergent J, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: WB Saunders; 2004:7th Edition, p967–995 | ||
Bellamy N, Campbell J, Welch V, Gee TL, Wells GA. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328. | ||
Cheng J, Abdi S. Complications of joint, tendon and muscle injections. Tech Reg Anesth Pain Manag. 2007;11(3):141–147. | ||
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–1711. | ||
Mahowald ML, Krug HE, Singh JA, Dykstra D. Intra-articular botulinum toxin type A: a new approach to treat arthritis joint pain. Toxicon. 2009;54(5):658–667. | ||
Krug HE, Frizelle SP, McGarraugh P, Mahowald ML. Pain behavior measures to quantitate joint pain and response to neurotoxin treatment in murine models of arthritis. Pain Med. 2009;10(7):1218–1228. | ||
Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods. 2002;115(1):107–113. | ||
Tetreault P, Dansereau M-A, Dore-Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav. 2011;104(3):495–502. | ||
Szallasi A, Blumberg PM. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68(2–3):195–208. | ||
Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathology. Annu Rev Neurosci. 2001;24:487–517. | ||
Jessell TM, Iversen LL, Cuello AC. Capsaicin-induced depletion of substance P from primary sensory neurons. Brain Res. 1978;152(1):183–188. | ||
Chard PS, Bleakman D, Savidge JR, Miller RJ. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience. 1995;65(4):1099–1108. | ||
Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007;274(3):760–772. | ||
Han P, McDonald HA, Bianchi BR, et al. Capsaicin causes protein synthesis inhibition and microtubule disassembly throughTRPV1 activities both on the plasma membrane and intracellular membranes. Biochem Pharmacol. 2007;73(10):1635–1645. | ||
Kissin EY, Freitas CF, Kissin I. Effects of intraarticular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005;101(5):1433–1439. | ||
Jeffry JA, Yu S-Q, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4(9):e7021. | ||
The National Academies Press. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 2011. | ||
Min SS, Han JS, Kim YI, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett. 2001;308(2):95–98. | ||
Tonussi CR, Ferreira SH. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain. 1992;48(3):421–427. | ||
Pomonis JD, Boulet JM, Gottshall SL, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114(3):339–346. | ||
Otsuki T, Nakahama H, Niizuma H, Suzuki J. Evaluation of the analgesic effects of capsaicin using a new rat model for tonic pain. Brain Res. 1986;365(2):235–240. | ||
Menendez L, Juarez L, Garcia E, Garcia-Suarez O, Hidalgo A, Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci Lett. 2006;393(1):70–73. | ||
Xu XJ, Farkas-Szallasi T, Lundberg JM, Hokfelt T, Wiesenfeld-Hallin Z, Szallasi A. Effects of the capsaicin analogue resiniferatoxin on spinal nociceptive mechanisms in the rat: behavioral, electrophysiological, and in situ hybridization studies. Brain Res. 1997;752(1–2):52–60. | ||
Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 1990;255(2):923–928. | ||
Holzer P. Capsaicin: cellular targets, mechanism of action and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43(2):143–201. | ||
Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1995;51(2):159–212. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.