Back to Journals » Cancer Management and Research » Volume 12
The Effect of Hormone Therapy on the Expression of Prostate Cancer and Multi-Epigenetic Marker Genes in a Population of Iranian Patients
Authors Mohammadi M, Irani S , Salahshourifar I, Hosseini J, Moradi A , Pouresmaeili F
Received 25 February 2020
Accepted for publication 30 April 2020
Published 19 May 2020 Volume 2020:12 Pages 3691—3697
DOI https://doi.org/10.2147/CMAR.S251297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Mahan Mohammadi,1 Shiva Irani,1 Iman Salahshourifar,1 Jalil Hosseini,2 Afshin Moradi,3 Farkhondeh Pouresmaeili2,4
1Department of Molecular Genetics, Faculty of Science, Science and Research Branch, Islamic Azad University, Tehran, Iran; 2Men’s Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Department of Pathology, Shahid Beheshti University of Medical Sciences, Shohadaye Tajrish Hospital, Tehran, Iran; 4Medical Genetics Department, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Correspondence: Farkhondeh Pouresmaeili
Men’s Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Tel/ Fax +982 123872572
Email [email protected]
Background and Aim: Many recent studies have shown a direct relationship between the decrease in the expression of GSTP1 and RASSF1 with the incidence and progression of prostate cancer. Moreover, the expression level of these genes is greatly affected by epigenetic factors and their methylation pattern. Given the prevalence of prostate cancer and the importance of choosing the best method to inhibit the progression of the disease and provide specific treatment, it is important to evaluate the effect of hormone therapy on the expression of effective prostate cancer genes and epigenetic markers.
Patients and Methods: In this case–control study, 35 prostate cancer samples were examined before and after hormone therapy. Following the blood sampling, RNA extraction, and cDNA synthesis, the expression of GSTP1, RASSF1, HDAC, DNMT3A, and DNMT3B was assessed by real-time PCR.
Results: The results analysis showed that the expression of GSTP1, RASSF1, and DNMT3B was significantly increased, DNMT3A was significantly decreased (P value< 0.05) and HDAC expression did not change significantly (P value=0.19) after hormone therapy.
Discussion: Significant changes in the expression of GSTP1, RASSF1, DNMT3B and DNMT3A in the studied samples indicate that these genes are susceptible targets for cancer hormone therapy in Iranian men like in the other populations. Evaluation of gene activity in a larger population of patients may support these findings.
Keywords: GSTP1, RASSF1, HDAC, DNMT3A, DNMT3B, prostate cancer, hormone therapy, real-time PCR
Introduction
Prostate cancer (PCa) is one of the most mortal malignancies in American men with an approximate rate of one in nine with a higher incidence in African origin people.1 Approximately 192,000 individuals were diagnosed with prostate cancer in the United States in 2009, while 27,000 were expected to die of the disease.2 The disease is rarely diagnosed in men under the age of 50 (less than 0.1% of all patients). The maximum incidence of the cancer is between 70 and 74 years of age, and 85% of cases are diagnosed after the age of 65 years.3
GSTs are a group of isozymes that perform detoxification by binding glutathione to electrophilic compounds. Of this family, the role of GSTP1 has been identified more prominently in cancers, especially in prostate cancer.4 The Ras-association domain family 1 (RASSF1) gene is an important RAS signaling pathway effector which is located in the 3p21.3 chromosomal region and plays an important role in the tumorigenesis of cancers.5 Previous studies have demonstrated GSTP1 and RASSF1 important role in prostate malignancies and introduced them as candidate biomarkers for diagnosis of the disease through their expression changes.6,7 Moreover, epigenetic markers such as HDAC and DNMTs are among the factors that influence the methylation and consequent expression of these genes.8
Researchers have shown that in the prostate cancer tissue, testosterone levels are higher than in normal non-cancerous tissue and sex hormones are of high importance in the pathogenesis of prostate cancer, which is less prevalent in infertile men.9 It has long been recognized that prostate cancer is dependent on androgens for growth and proliferation. Therefore, androgen deprivation has been suggested as an effective treatment and is commonly used in clinical practice for androgen-dependent patients.10,11
Numerous studies have confirmed the accumulation of testosterone and dihydrotestosterone in the enlarged prostate stroma and its relation to the prostate mass. Reducing male sex hormones by hormone therapy can decrease the prostate mass,12 and affect the expression of many genes and microRNAs associated with the cancer development.13,14
Given the inhibitory effects of hormone therapy and the multifactorial nature of prostate cancer and its significant prevalence in Iran, this study could provide an effective guide to the treatment of the cancer in Iranian men.
In this cross-sectional study, we aimed to investigate the effect of hormone therapy on the expression of several candidate biomarker genes such as GSTP1 and RASSF1 and some epigenetic markers that have been shown to be effective on the incidence and progression of prostate cancer in a group of patients nominated for advanced treatment. Therefore, we evaluated the expression of GSTP1, RASSF1, HDAC, DNMT3A and DNMT3B in understudy patients at diagnosis time and after Bicalutamide therapy for a period of 3 months.11
Patients and Methods
Patients
Seventy blood samples from prostate cancer patients were collected before and after treatment in EDTA-containing tubes and stored at 20°C. The diagnosis has been reported by a specialist in the urology department following biopsy and pathology confirmation for prostate cancer (Grades 1–5). Appropriate blood samples were obtained from Shohadaye Tajrish and Modares hospitals (Tehran, Iran) for gene expression analysis. Clinical and pathological data were collected from patients after PCa diagnosis due to high prostate-specific antigen (PSA) levels and the presence of cancer cells in histological analysis following ultrasound rectal biopsy. Sampling was performed following a three-month hormone therapy with the Bicalutamide family drugs. By default, all stages of this research were covered by the Hospital Ethics Committees and SBMU. Written informed consent was obtained from the patients.
RNA Extraction
Five milliliters of each blood sample was lysed by RiboEx TMLS, centrifuged at 13,000 × g for 12 min at 4°C. RNA was extracted according to the manufacturer’s instructions (Cat.No.315–150). A spectrophotometer (NanoDropTM 2000/2000c, USA) was used to quantify RNA concentration. Agarose gel electrophoresis (1%) was used to evaluate the quality of the extracted RNA.
cDNA Synthesis and Quantitative RT‑PCR
HyperScript First-Strand Synthesis Kit GeneAll (Cat No: 605-005) was applied to synthesize cDNA from the extracted total RNA following the manufacturer’s guidelines. Primer3 and BLAST web sites were used to design gene-specific primers. Qiagen Rotor-Gene Q was used for the cDNA denaturation at 95°C for 15 mins, then amplification at 95°C for 10 s and annealing for 35 s at an appropriate amplicon temperature for 45 cycles (Table 1). Negative controls were used to confirm that no genomic DNA contamination existed. Beta-2- macroglobulin (B2M) was selected as a housekeeping gene for normalization and 2% agarose gel was used for verification of amplification products accuracy.
 |
Table 1 Details on Primers Used for qRT-PCR Analysis. |
Statistical Analysis
In this study, 2−ΔΔCT was used to analyze the relative gene expression changes. REST 2009 Software was used for gene expression analysis and normalization using Real-Time PCR data. Significant differences between samples’ gene expression before and after therapy were determined by two-tailed Student’s t-test using GraphPad Prism 8.0.2 (GraphPad prism Software, Inc. San Diego CA, USA). To determine the efficiency of the examined genes as diagnostic PCA biomarkers, receiver operating characteristic (ROC) curve analyses was performed by using MedCalc-version19.1.2. In statistical analysis, a P value of <0.05 was considered significant.
Results
Patient Dataset
Blood samples were obtained from 35 PCa patients, 52 to 85 years old with a mean of 71 years, before and after hormone therapy. A summarized clinicopathological feature of understudy patients is shown in (Table 2).
 |
Table 2 Clinicopathological Characteristics of Patients with Prostate Cancer |
Expression Variation of the Examined Genes
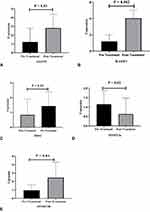
The expression of prostate cancer genes GSTP1, RASSF1, HDAC; and DNMT3A, and DNMT3B epigenetic markers were evaluated by qRT-PCR at the time of diagnosis and after 3 months of hormone therapy by Bicalutamide. The mRNA expression level of GSTP1, RASSF1, and DNMT3B in PCa patients who responded to hormone therapy was significantly upregulated (p<0.05), HDAC gene expression did not show any significant changes (P=0.19) and DNMT3A gene expression was significantly decreased after the treatment (P=0.02) (Figure 1). The qRT-PCR results were used for assessment of the relative expression of the examined genes in two evaluated groups.
ROC Curve Analysis of Examined Genes
The receiver operating characteristic curve (ROC) was plotted to find the value of evaluating the expression of the under study genes with the drug response of patients after hormone therapy. ROC analysis showed area under the ROC curve of A) 0.78 for GSTP1 (Sensitivity 80%, Specificity75% and P value =0.001), B) 0.73 for RASSF1 (Sensitivity 77.5%, Specificity 70% and P value =0.0004), C) 0.77 for DNMT3A (Sensitivity 72.5%, Specificity 82.5% and P value<0.0001), D) 0.75 for DNMT3B (Sensitivity 85.37%, Specificity 62% and P value<0.0001) and E) 0.89 for HDAC (Sensitivity 81%, Specificity 76% and P value <0.0001) (Figure 2).
Discussion
Since the clinical significance of GSTP1, RASSF1, HDAC, DNMT3A and DNMT3B genes expression in PCa patients has not been thoroughly studied in Iran, we aimed to investigate the effect of hormone therapy on their expression to introduce a possible combination of genetic and epigenetic biomarkers that can be effective in the disease treatment and to evaluate the drug efficacy and follow-up the patients during hormone therapy.
Over the past decades, researchers have focused on identifying the genetic background of prostate cancer. A number of genetic alterations in association with epigenetics and gene expression have made a better prediction of the disease.15 GSTP1 and RASSF1 are two genes whose expression in healthy and cancerous groups has been discussed in various articles on the incidence and progression of prostate cancer.16 It is shown that the expression of these genes is affected by changes in the methylation pattern through which their expression is reduced by hypermethylation.17 Therefore, they are among the candidate genes as biomarkers for early detection of prostate cancer in Kashmir, Vietnam, and a large number of other populations.16,18,19 Inês Graça (2016) stated that potential therapeutic agents for managing prostate cancer are epigenetic changes of the disease which highlights the important role of epigenetic modulators in pre-trial and clinical trials.20 In addition, it is well understood that HDAC, DNMT3A, and DNMT3B are major epigenetic factors regulating methylation patterns of GSTP1 and RASSF1 genes which alter the expression of the genes in prostate cancer.7,21,22
Christopher J. Luskeb in 2002 discussed the association of GSTP1 glutathione S-transferase genotypes with the response to androgen deprivation therapy in patients with advanced prostate cancer.23 Our results showed a significant increase in GSTP1 and RASSF1 expression in post-treatment specimens (P <0.05). These results confirm the specificity of these two genes for an earlier and faster detection of cancer and their use as possible biomarkers tools.
Giovanni Luca Gravina in 2011 showed that a significant increase in the expression of DNMT including DNMT3A and DNMT3B in the advanced stages of the disease was a great achievement to apply hormone therapy to the development of the hormone resistance phenotype in cancer patients.24 Tarek K. Motavi in 2018 highlighted the important role of HDAC in the progression of prostate cancer and suggested that a combination of HDAC inhibitors and hormone therapy could be ideal for the treatment of advanced prostate cancer.25 Due to the effect of methyltransferases on the methylation of GSTP1 and RASSF1 genes, and their divergent expression in PCa patients in comparison to BPH group, an elevation in DNMT3A and DNMT3B expression level could be expected. Our study had some interesting results, when DNMT3A expression decreased significantly (P = 0.02) and DNMT3B expression was enhanced after treatment (P = 0.04). HDAC also did not show significant expression changes (P = 0.19). This contradiction in the predicted results could be attributed to drug resistance in patients or sample size.
Our results indicated a decrease in the expression of GSTP1 and RASSF1 as candidate markers for the prognostic profile of PCa patients in Iran. GSTP1 and RASSF1 may act as risk factors in the diagnosis of the patients with poor prognosis and can also be known as therapeutic targets for killing cells that are specifically running and causing the recurrence. Increased expression of both GSTP1 and RASSF1 genes after treatment may indicate a favorable patient response to hormone therapy and remission.
Conclusion
In conclusion, the analysis of our data suggests that reduced levels of GSTP1 and RASSF1 expression in patients with prostate cancer before hormonal treatment in comparison to the post-treatment stage indicate that these genes are potential cancer markers in Iranian population as was reported in the previously approved data. Therefore, evaluating the patient at different stages of the disease can determine the severity and/or progression as well as the response to appropriate treatment.
Ethics and Consent Statement
This work was approved by the Ethics and Clinical Studies Committee of Shahid Beheshti University of Medical Sciences and the ethics committee of Men’s Health and Reproductive Health Research Center (Tehran, Iran) and Islamic Azad University, Science and Research Branch, Tehran, Iran, Review board. Each patient signed a written consent according to the Declaration of Helsinki.
Acknowledgments
The authors would like to express their deep gratitude to the doctors and nursing staff of the hospitals for their co-operation and sampling; and wish health for patients who agreed to participate in the study.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63. doi:10.14740/wjon1191
2. Plata Bello A, Concepcion Masip T. Prostate cancer epidemiology. Arch Esp Urol. 2014;67(5):373–382.
3. Castillejos-Molina RA, Gabilondo-Navarro FB. Prostate cancer. Salud Publica Mex. 2016;58(2):279–284. doi:10.21149/spm.v58i2.7797
4. Singal R, van Wert J, Bashambu M. Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. Cancer Res. 2001;61(12):4820–4826.
5. Dammann R, Schagdarsurengin U, Strunnikova M, et al. Epigenetic inactivation of the Ras-association domain family 1 (RASSF1A) gene and its function in human carcinogenesis. Histol Histopathol. 2003;18(2):665–677. doi:10.14670/HH-18.665
6. Patel PG, Wessel T, Kawashima A, et al. A three-gene DNA methylation biomarker accurately classifies early stage prostate cancer. Prostate. 2019;79(14):1705–1714. doi:10.1002/pros.23895
7. Gurioli G, Martignano F, Salvi S, Costantini M, Gunelli R, Casadio V. GSTP1 methylation in cancer: a liquid biopsy biomarker? Clin Chem Lab Med. 2018;56(5):702–717. doi:10.1515/cclm-2017-0703
8. Nowacka-Zawisza M, Wisnik E. DNA methylation and histone modifications as epigenetic regulation in prostate cancer (review). Oncol Rep. 2017;38(5):2587–2596. doi:10.3892/or.2017.5972
9. Grozescu T, Popa F. Prostate cancer between prognosis and adequate/proper therapy. J Med Life. 2017;10(1):5–12.
10. Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi:10.1146/annurev-med-051517-011947
11. Brower V. Bicalutamide with radiotherapy for prostate cancer. Lancet Oncol. 2017;18(3):e141. doi:10.1016/S1470-2045(17)30097-9
12. Pejcic T, Tosti T, Tesic Z, et al. Testosterone and dihydrotestosterone levels in the transition zone correlate with prostate volume. Prostate. 2017;77(10):1082–1092. doi:10.1002/pros.23365
13. Lehmusvaara S, Erkkila T, Urbanucci A, et al. Goserelin and bicalutamide treatments alter the expression of microRNAs in the prostate. Prostate. 2013;73(1):101–112. doi:10.1002/pros.22545
14. Auchus RJ, Sharifi N. Sex hormones and prostate cancer. Annu Rev Med. 2020;71(1):33–45. doi:10.1146/annurev-med-051418-060357
15. Sanchez BE, Aguayo A, Martinez B, et al. Using genetic and epigenetic markers to improve differential diagnosis of prostate cancer and benign prostatic hyperplasia by noninvasive methods in mexican patients. Clin Genitourin Cancer. 2018;16(4):e867–e877. doi:10.1016/j.clgc.2018.02.004
16. Haluskova J, Lachvac L, Nagy V. The investigation of GSTP1, APC and RASSF1 gene promoter hypermethylation in urine DNA of prostate-diseased patients. Bratisl Lek Listy. 2015;116(2):79–82. doi:10.4149/bll_2015_014
17. Kaminska K, Bialkowska A, Kowalewski J, Huang S, Lewandowska MA. Differential gene methylation patterns in cancerous and noncancerous cells. Oncol Rep. 2019;42(1):43–54. doi:10.3892/or.2019.7159
18. Syeed N, Syed Sameer A, Hamid A, et al. Promoter methylation profile of GSTP1 and RASSF1A in benign hyperplasia and metastatic prostate cancer patients in a Kashmiri population. Mol Med Rep. 2010;3(5):883–887. doi:10.3892/mmr.2010.348
19. Vo TT, Ta BT, Ta VT, Vuong DL, Nguyen QU. Promoter methylation profile of GSTP1 and RASSF1A in prostate cancer and benign hyperplasia in Vietnamese men. Turkish J Med Sci. 2016;46(1):228–235. doi:10.3906/sag-1410-65
20. Graca I, Pereira-Silva E, Henrique R, Packham G, Crabb SJ, Jeronimo C. Epigenetic modulators as therapeutic targets in prostate cancer. Clin Epigenet. 2016;8:98.
21. Tzelepi V, Logotheti S, Efstathiou E, et al. Epigenetics and prostate cancer: defining the timing of DNA methyltransferase deregulation during prostate cancer progression. Pathology. 2019.
22. Makarevic J, Rutz J, Juengel E, et al. Influence of the HDAC inhibitor valproic acid on the growth and proliferation of temsirolimus-resistant prostate cancer cells in vitro. Cancers. 2019;11:4. doi:10.3390/cancers11040566
23. Luscombe CJ, French ME, Liu S, et al. Glutathione S-transferase GSTP1 genotypes are associated with response to androgen ablation therapy in advanced prostate cancer. Cancer Detect Prev. 2002;26(5):376–380. doi:10.1016/S0361-090X(02)00089-2
24. Gravina GL, Marampon F, Piccolella M, et al. Hormonal therapy promotes hormone-resistant phenotype by increasing DNMT activity and expression in prostate cancer models. Endocrinology. 2011;152(12):4550–4561. doi:10.1210/en.2011-1056
25. Motawi TK, Darwish HA, Diab I, Helmy MW, Noureldin MH. Combinatorial strategy of epigenetic and hormonal therapies: A novel promising approach for treating advanced prostate cancer. Life Sci. 2018;198:71–78. doi:10.1016/j.lfs.2018.02.019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.