Back to Journals » Local and Regional Anesthesia » Volume 13
The Effect of Different Doses of Intravenous Dexmedetomidine on the Properties of Subarachnoid Blockade: A Systematic Review and Meta-Analysis
Authors Al Nobani MK , Ayasa MA, Tageldin TA, Alhammoud A, Lance MD
Received 25 October 2020
Accepted for publication 27 November 2020
Published 15 December 2020 Volume 2020:13 Pages 207—215
DOI https://doi.org/10.2147/LRA.S288726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Mohammad K Al Nobani, Mohammed A Ayasa, Tarek A Tageldin, Abduljabbar Alhammoud, Marcus Daniel Lance
Hamad Medical Corporation, Doha, Qatar
Correspondence: Mohammad K Al Nobani
Hamad Medical Corporation, Al Rayyan Street, Hamad Medical City, Doha, Qatar
Tel +974 55700142
Email [email protected]
Background: Dexmedetomidine is a sedative and analgesic medication which has gained an increased usage as an adjuvant to both general and regional anaesthesia in recent years. In this systematic review and meta-analysis, we examined the changes to the characteristics of subarachnoid block when accompanied with intravenous dexmedetomidine. Our aim is to evaluate the effects of different doses of intravenous dexmedetomidine on the sensory and motor blockade duration of a single shot spinal anaesthetic and the incidence of any associated side effects.
Methods: We searched published randomized clinical trials (RCTs) from January 1992 to April 2019 that investigated the use of IV dexmedetomidine with spinal anaesthesia. After considering our inclusion and exclusion criteria, we included 15 RCTs with 985 patients. We analyzed the duration of sensory and motor blockade and the related adverse effects in relation to different doses of IV dexmedetomidine.
Results: Intravenous dexmedetomidine, with loading dose of 1 mcg/kg, prolonged the sensory blockade duration of spinal anaesthesia by a mean difference of 49.6 min, P< 0.001, and motor blockade duration by a mean difference of 44.7 min, P< 0.001, while a loading dose of 0.5 mcg/kg prolonged the sensory blockade by a mean difference of 43.06 min, P< 0.001, and motor blockade duration by a mean difference of 29.09 min, P< 0.001. Dexmedetomidine-related side effects were higher in patients receiving larger doses; the incidence of bradycardia was higher (OR=3.53, P< 0.001) and incidence of hypotension showed a 1.29 fold increase when compared to the control group (P=0.065).
Conclusion: The administration of intravenous dexmedetomidine in conjunction with spinal anaesthesia can significantly prolong the duration of both sensory and motor blockade. The use of larger loading doses of dexmedetomidine was associated with a larger side-effect profile with minimal beneficial changes when compared to lower loading doses.
Keywords: dexmedetomidine, spinal anesthesia, adjuvant medication, subarachnoid block, prolongation of spinal anesthetic
Background
Dexmedetomidine is a centrally acting selective α-2 receptor agonist that has hypnotic and analgesic properties.1 Since its introduction into anaesthesia practice, it has been widely used as an adjuvant to both general and regional anaesthesia, both as a sedative and as part of multimodal analgesia models. In recent years, multiple randomized controlled trials (RCTs) have emerged studying the effect of dexmedetomidine via multiple routes on the properties of subarachnoid block.2,3
In 2013, Abdullah4 and his colleagues published a meta-analysis of 7 RCTs studying the effects of intravenous dexmedetomidine combined with spinal anaesthesia, showing clinically significant prolongation of sensory and motor blockade duration. However, in the past 6 years, multiple new RCTs have also investigated the same question.
In our systematic review and meta-analysis, we have included 985 patients in 15 RCTs to study the effect of different doses of intravenous dexmedetomidine on spinal anaesthesia in terms of sensory blockade duration, motor blockade duration and the incidence of related side effects, such as hypotension, bradycardia, respiratory depression and postoperative sedation.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-P) guidelines and checklist (Supplementary Materials) for the preparation of this article.5
Study Selection
We searched all published RCTs from Medical Literature Analysis and Retrieval System Online database (MEDLINE via Pubmed), Cochrane Database of Systematic Reviews, Google Scholar database and the Excerpta Medica database (EMBASE) from the period January 1992 to April 2019.
We used the following search terms in combination with “Dexmedetomidine” or “Medetomidine”: Regional, Spinal, Subarachnoid, Intrathecal, Intravenous, IV, Systematic.
We also manually cross-referenced previous relevant reviews and identified RCTs that met our inclusion criteria.
Inclusion and Exclusion Criteria
We included RCTs that investigated the characteristics of a single shot subarachnoid blockade with the use of intravenous dexmedetomidine as a bolus and/or infusion.
We excluded RCTs that: (1) were non-English language articles, (2) were animal studies, (3) used intrathecal injections of dexmedetomidine, or non-intravenous routes of dexmedetomidine, (4) used dexmedetomidine sedation for other type of procedures, (5) compared dexmedetomidine with other drugs that might affect the properties of subarachnoid block, (6) did not use a placebo arm, (7) were unpublished trials.
Data Collection
The quality of the articles were assessed using JADAD6 scoring methodology, and the risk of bias was assessed by two independent authors (MNO and MAY). Articles with JADAD score < 3 were excluded from the analysis, and then the following data was extracted: type and dosage of the local anaesthetic used, dexmedetomidine dosage, sensory block duration and onset, motor block duration and onset, time to first analgesia use, and dexmedetomidine-related side effects (hypotension, bradycardia, respiratory depression and post-operative sedation).
One randomized controlled trial7 was excluded by consensus among the three authors, based on the risk of outcome bias (duration of both sensory and motor block). Moreover, we failed to contact the corresponding author.
With regard to outcome, we analyzed the following data: sensory and motor block duration in relation to different intravenous dexmedetomidine doses and related side effects.
The data were recorded and checked for any discrepancies by the three authors and entered into a preformed data spreadsheet. The discrepancies were resolved by re-examining the articles’ data.
Data Analysis
Open meta-analysis software using a random-effect model was used for the data analysis, including subgroups analysis. The standardized Mean Difference (SDM) was used for the continuous variables, whereas the Relative Risk (RR) and 95% confidence intervals were used for the dichotomous variables. I2 was used to check the statistical heterogeneity across the studies.
Articles Demographic Characteristics
Tables 1–3 summarize the demographic characteristics of the RCTs.
 |
Table 1 Demographic Characteristics of the RCTs |
 |
Table 2 Group Characteristics of the RCTs |
 |
Table 3 Outcomes of the RCTs |
Results
15 intermediate to high quality trials met our inclusion criteria8–23 and investigated the effects of intravenous dexmedetomidine on the properties of a single shot subarachnoid block.
The analysis included 985 patients divided equally between the intervention group and the placebo control group.
Figure 1, a PRISMA flow chart, summarizes the results of the screened, excluded and included RCTs in the final analysis.
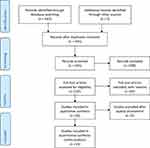 |
Figure 1 PRISMA flowchart. Note: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.24 |
Tables 1, –3 show the demographic characteristics and outcomes retrieved from the included trials.
Sensory Block Duration
Of the 15 RCTs we analyzed, four of the trials had 2 interventional arms of different doses of dexmedetomidine, making a total of 19 interventional arms.
Our analysis showed that administration of intravenous (IV) dexmedetomidine in conjunction with spinal anaesthesia significantly prolonged the duration of the sensory blockade regardless of the administered dose of dexmedetomidine or the type and dose of the used local anesthetic utilized, by a mean difference of 47.583 min, 95% CI (33.133–62.033), P<0.001, I2 = 95.7%.
Subgroup analysis of the duration of the blockade using IV dexmedetomidine 1 mcg/kg loading dose, showed prolongation of the duration of the block by a mean difference of 49.6 min, 95% CI (24.7–74.5), P<0.001, I2 = 97.3%. While using IV dexmedetomidine 0.5 mcg/kg loading dose, the duration was prolonged by a mean difference of 43.06 min, 95% CI (27.8–58.2), P<0.001, I2 = 83.9%.
Figure 2 and Table 4 summarize these findings.
 |
Table 4 Results for Sensory and Motor Block Duration |
 |
Figure 2 Sensory block duration. |
Motor Block Duration
Of the 15 RCTs we analyzed, four of the trials had 2 interventional arms of different doses of dexmedetomidine, making a total of 19 interventional arms.
The IV administration of dexmedetomidine showed prolongation of motor blockade duration by a mean difference of 43.2 min, 95% CI (23.63–62.77), P<0.001, I2 = 97.5% regardless of the amount of administered loading dose of dexmedetomidine or type and dose of local anesthetic used.
Subgroup analysis using IV dexmedetomidine 1 mcg/kg loading dose showed prolongation of the duration of the blockade by a mean difference of 44.7 min, 95% CI (23.2–66.3), P<0.001, I2 = 97.03%. While using IV dexmedetomidine 0.5 mcg/kg loading dose, the duration was prolonged by a mean difference of 29.09 min, 95% CI (9.3–48.7), P=0.004, I2 = 83.8%.
Figure 3 and Table 4 summarize these findings.
 |
Figure 3 Motor block duration. |
Dexmedetomidine-Related Adverse Effects
Since there was an absence of any specific standard definition of dexmedetomidine-related adverse effects, we present the results as reported in the RCTs.
Table 5 summarizes the findings.
 |
Table 5 Dexmedetomidine-Related Adverse Effects |
Bradycardia
We analyzed 15 RCTs with a total of 19 interventional arms, having 985 patients in all arms.
The probability of bradycardia was higher in the patients receiving dexmedetomidine regardless of the dose administered (RR=3.57; 95% (2.48–5.12); P<0.001, I2 = 0%).
Subgroup analysis using IV dexmedetomidine 1 mcg/kg loading dose showed a higher probability of developing bradycardia (RR=4.27; 95% (2.72–6.7); P<0.001, I2 = 0%). While using IV dexmedetomidine 0.5 mcg/kg loading dose showed a higher probability of bradycardia compared to placebo (RR=2.77; 95% CI: (1.42–5.39), P<0.003, I2 = 0%)
There were no reported cases of prolonged or delayed bradycardia in the studied RCTs.
Hypotension
The relative risk of hypotension in the interventional group was 1.23-fold higher compared to the control group, regardless of the administered dose of dexmedetomidine (95% CI: 0.86–1.77; P=0.247, I2 =16.61%). Table 5 summarizes the subgroup analysis results.
Other Related Adverse Effects
Respiratory depression was reported in 15 RCTs. 13 cases were reported in the interventional group and 9 cases in the control group. There was no statistical significance noted between both arms.
Postoperative sedation was reported in 10 arms among 6 RCTs. The group that received dexmedetomidine was 5.86 times more likely to develop postoperative sedation (95% CI [3.35–10.25]; p value <0.001; I2 =0%). It should be noted that higher doses of dexmedetomidine were associated with a higher relative risk of postoperative sedation, loading dose of 1mcg/kg was 7 times more likely to be associated with postoperative sedation while loading dose of 0.5mcg/kg was 5.66 times higher compared to the control group (p value <0.001; I2 =0% for both).
Discussion
The result of this review confirms the outcome of a smaller previously published systematic review,4 that the administration of IV dexmedetomidine in patients receiving subarachnoid blockade prolongs the duration of sensory and motor block. Higher doses (1mcg/kg loading dose) of dexmedetomidine were associated with a longer duration of both sensory and motor block when compared to lower doses of dexmedetomidine (0.5 mcg/kg loading dose or less), but also with a higher incidence of bradycardia and postoperative sedation. It should be noted that loading doses with 1mcg/kg dexmedetomidine when compared to 0.5mcg/kg or 0.25mcg/kg did not show a large difference in sensory block duration, while motor block duration was more significantly prolonged. There was no significant difference in the incidence of hypotension or respiratory depression between the IV dexmedetomidine group and the placebo-controlled group.
As an adjuvant medication, dexmedetomidine has been used by different routes to prolong the duration of local anaesthetics. It has been shown to prolong the duration of regional blocks when administered perineurally25,26 and shown to prolong the duration of subarachnoid block when administered via the intrathecal route,2,27–29 suggesting both peripheral and central mechanisms of action for dexmedetomidine. It has high selectivity towards α2-adrenergic receptors30 acting at the presynaptic C-fibers, postsynaptic dorsal horn neurons and locus ceruleus of the brain stem.31
There are several limitations in this review. We have included multiple RCTs looking into different outcomes as well as using different protocols of dexmedetomidine administration. Some studies used only a loading dose of dexmedetomidine while others followed it with maintenance infusions ranging from 0.2–0.6 mcg/kg/hr lasting for different durations. Some protocols continued the infusion until the end of surgery, while others infused the maintenance dose for a specific duration. Moreover, loading doses were administered over different durations, ranging from 5−15 minutes and were administered at different times in relation to the spinal anaesthetic injection. A small number of RCTs began the loading dose before the spinal injection, while others started it after establishment of the subarachnoid block.
In addition, different local anesthetic drugs with variable doses, were used for the spinal anaesthesia. One trial used prilocaine,15 two trials used ropivacaine13,20 while the remaining trials have used different doses of bupivacaine. Intrathecal fentanyl was used in two trials as an adjuvant to the local anesthetic.32,33
Finally, the end point for sensory block duration was defined differently in the trials, as some of them used time for two segment regression to cold or to pinprick sensation, while others did not specifically define how they assessed the sensory block duration. The absence of a standardized method of assessment was also observed when reporting the motor block duration and dexmedetomidine-related adverse effects were not clearly elucidated in all the clinical trials.
Conclusion
We conclude that the administration of intravenous dexmedetomidine in conjunction with spinal anaesthesia can significantly prolong the duration of both sensory and motor blockade. Considering both advantages and disadvantages, the use of 1mcg/kg loading dose of dexmedetomidine was associated with a larger side-effect profile, while the beneficial changes to the characteristics of the subarachnoid blockade were minimal when compared to lower loading doses. In that sense, a lower loading dose should be preferred.
Abbreviations
RCT, Randomized controlled trials; IV, intravenous; PRISMA-P, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; SDM, Standardized mean difference; RR, Relative Risk; Mcg, micrograms; Kg, kilogram.
Data Sharing Statement
Data material is available upon request.
Acknowledgments
We would like to acknowledge the contributions of Dr. Mark Hitchcock for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work
Funding
Open Access funding provided by Qatar National Library.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Weinger MB, Segal IS, Maze M. Dexmedetomidine, acting through central alpha-2 adrenoceptors, prevents opiate-induced muscle rigidity in the rat. Anesthesiology. 1989;71(2):242–249. doi:10.1097/00000542-198908000-00013
2. Zhang C, Li C, Pirrone M, Sun L, Mi W. Comparison of dexmedetomidine and clonidine as adjuvants to local anesthetics for intrathecal anesthesia: a meta-analysis of randomized controlled trials. J Clin Pharmacol. 2016;56(7):827–834. doi:10.1002/jcph.666
3. Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196. doi:10.1097/AAP.0000000000000564
4. Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013;117(1):271–278. doi:10.1213/ANE.0b013e318290c566
5. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1
6. Halpern SH, Douglas MJ. Jadad scale for reporting randomized controlled trials. Evid Based Obstet Anesth. 2005;237–238. doi:10.1002/9780470988343
7. Zhang H, Li M, Zhang SY, Fu M, Zhang SY. Intravenous dexmedetomidine promotes spinal bupivacaine anesthesia and postoperative analgesia in lower limb surgery: a double-blind, randomized clinical consort study. Med (United States). 2016;95(8). doi:10.1097/MD.0000000000002880
8. Subramanian A, Agrawal D, Sharma S, Mukhopadhayay A, Chhabra G, Rangarajan K. Hypofibrinogenemia in isolated traumatic brain injury in Indian patients. Neurol India. 2010;58(5):756. doi:10.4103/0028-3886.72175
9. Kim MH, Jung SY, Shin JD, et al. The comparison of the effects of intravenous ketamine or dexmedetomidine infusion on spinal block with bupivacaine. Korean J Anesthesiol. 2014;67(2):85–89. doi:10.4097/kjae.2014.67.2.85
10. Al-Mustafa MM, Badran I, Abu-Ali HM, Al-BarazangI BA, Massad IM, Al-ghanem SM. Intravenous dexmedetomidine prolongs bupivacaine spinal analgesia. Middle East J Anesthesiol. 2009;20(2):225–231.
11. Reddy VS, Shaik NA, Donthu B, Sannala VKR, Jangam V. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: a randomized double-blind study. J Anaesthesiol Clin Pharmacol. 2013;29(3):342–347. doi:10.4103/0970-9185.117101
12. Kaya FN, Yavascaoglu B, Turker G, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anesth. 2010;57(1):39–45. doi:10.1007/s12630-009-9231-6
13. Elcicek K, Tekin M, Kati I. The effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaine anesthesia. J Anesth. 2010;24(4):544–548. doi:10.1007/s00540-010-0939-9
14. Jung SH, Lee SK, Lim KJ, et al. The effects of single-dose intravenous dexmedetomidine on hyperbaric bupivacaine spinal anesthesia. J Anesth. 2013;27(3):380–384. doi:10.1007/s00540-012-1541-0
15. Tekin M, Kati I, Tomak Y, Kisli E. Effect of dexmedetomidine IV on the duration of spinal anesthesia with prilocaine: a double-blind, prospective study in adult surgical patients. Curr Ther Res. 2007;68(5):313–324.
16. Harsoor SS, Rani DD, Yalamuru B, Sudheesh K, Nethra SS. Effect of supplementation of low dose intravenous dexmedetomidine on characteristics of spinal anaesthesia with hyperbaric bupivacaine. Indian J Anaesth. 2013;57(3):265–269. doi:10.4103/0019-5049.115616
17. Hong JY, Kim WO, Yoon Y, Choi Y, Kim SH, Kil HK. Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anaesthesia in elderly patients. Acta Anaesthesiol Scand. 2012;56(3):382–387. doi:10.1111/j.1399-6576.2011.02614.x
18. Dinesh CN, Sai Tej NA, Yatish B, Pujari VS, Mohan Kumar RM, Mohan CVR. Effects of intravenous dexmedetomidine on hyperbaric bupivacaine spinal anesthesia: a randomized study. Saudi J Anaesth. 2014;8(2):202–208. doi:10.4103/1658-354X.130719
19. Kumari R, Kumar A, Kumar S, Singh R. Intravenous dexmedetomidine as an adjunct to subarachnoid block: a simple effective method of better perioperative efficacy. J Anaesthesiol Clin Pharmacol. 2017;33(2):203–208. doi:10.4103/joacp.JOACP_367_15
20. Rekhi BK, Kaur T, Arora D, Dugg P. Comparison of intravenous dexmedetomidine with midazolam in prolonging spinal anaesthesia with ropivacaine. J Clin Diagn Res. 2017;11(2):UC01–UC04. doi:10.7860/JCDR/2017/23874.9344
21. Lee MH, Ko JH, Kim EM, Cheung MH, Choi YR, Choi EM. The effects of intravenous dexmedetomidine on spinal anesthesia: comparision of different dose of dexmedetomidine. Korean J Anesthesiol. 2014;67(4):252–257. doi:10.4097/kjae.2014.67.4.252
22. Kavya UR, Laxmi S, Ramkumar V. Effect of intravenous dexmedetomidine administered as bolus or as bolus-plus-infusion on subarachnoid anesthesia with hyperbaric bupivacaine. J Anaesthesiol Clin Pharmacol. 2018;34(1):46–50. doi:10.4103/joacp.JOACP_132_16
23. Park SH, Shin YD, Yu HJ, Bae JH, Yim KH. Comparison of two dosing schedules of intravenous dexmedetomidine in elderly patients during spinal anesthesia. Korean J Anesthesiol. 2014;66(5):371–376. doi:10.4097/kjae.2014.66.5.371
24. Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10).
25. Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181. doi:10.1093/bja/aew411
26. Andersen JH, Jaeger P, Grevstad U, et al. Systemic dexmedetomidine is not as efficient as perineural dexmedetomidine in prolonging an ulnar nerve block. Reg Anesth Pain Med. 2019;44(3):333–340. doi:10.1136/rapm-2018-100089
27. Gupta M, Gupta P, Singh DK. Effect of 3 different doses of intrathecal dexmedetomidine (2.5µg, 5µg, and 10 µg) on subarachnoid block characteristics: a prospective randomized double blind dose-response trial. Pain Physician. 2016;19(3):E411–E420.
28. Yektaş A, Belli E. The effects of 2 μg and 4 μg doses of dexmedetomidine in combination with intrathecal hyperbaric bupivacaine on spinal anesthesia and its postoperative analgesic characteristics. Pain Res Manag. 2014;19.
29. Abdallah FW, Dwyer T, Chan VWS, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016;124(3):683–695. doi:10.1097/ALN.0000000000000983
30. Kaur M, Singh P. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5(2):128. doi:10.4103/0259-1162.94750
31. Halder S, Das A, Mandal D, et al. Effect of different doses of dexmedetomidine as adjuvant in bupivacaine -induced subarachnoid block for traumatized lower limb orthopaedic surgery: a prospective, double-blinded and randomized controlled study. J Clin Diagn Res. 2014;8(11):GC01.
32. Agrawal A, Agrawal S, Payal YS. Comparison of block characteristics of spinal anesthesia following intravenous dexmedetomidine and clonidine. J Anaesthesiol Clin Pharmacol. 2016;32(3):339–343. doi:10.4103/0970-9185.188830
33. Chan IA, Maslany JG, Gorman KJ, O’Brien JM, McKay WP. Dexmedetomidine during total knee arthroplasty performed under spinal anesthesia decreases opioid use: a randomized-controlled trial. Can J Anesth. 2016;63(5):569–576. doi:10.1007/s12630-016-0597-y
 © 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
