Back to Journals » Clinical Interventions in Aging » Volume 16
The Effect of Daily Teriparatide versus One-Time Annually Zoledronic Acid Administration After Transforaminal Lumbar Interbody Fusion in Osteoporotic Patients
Authors Wang Z, Zhuang C, Chen W, Li Z, Li J, Lin H, Dong J
Received 8 August 2021
Accepted for publication 27 September 2021
Published 5 October 2021 Volume 2021:16 Pages 1789—1799
DOI https://doi.org/10.2147/CIA.S333207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Zixiang Wang, 1,* Chenyang Zhuang, 1,* Weisin Chen, 1 Zefang Li, 2 Juan Li, 1, 3 Hong Lin, 1, 3 Jian Dong 1
1Department of Orthopedics, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China; 2Department of Orthopedics, Qianjiang Central Hospital of Chongqing, Chongqing, 409000, People’s Republic of China; 3Department of Orthopedics, Shanghai Geriatrics Medical Center, Fudan University, Shanghai, 201100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Lin; Jian Dong
Department of Orthopedics, Zhongshan Hospital, Fudan University, Fenglin Road 180, Shanghai, 200032, People’s Republic of China
Tel +86-13636526656
; +86-13916930859
Email [email protected]; [email protected]
Purpose: The research aimed to compare the therapeutic effect of teriparatide (TPTD) and zoledronic acid (ZOL) therapy on bone formation and spinal fusion in patients with osteoporosis (OP) who underwent transforaminal lumbar interbody fusion (TLIF).
Methods: On the basis of different anti-OP treatment options, the TPTD group was treated daily with TPTD (20 μg. ih. qd) for at least 6 months, while the ZOL group was treated with a single dose of ZOL (5 mg. ivgtt. st) postoperatively. The visual analogue scale (VAS), Oswestry Disability Index (ODI), bone mineral density (BMD), and concentration of bone turnover markers before, 6, and 12 months after surgery were evaluated. X-ray and three-dimensional computed tomography scans were performed at 6 and 12 months postoperatively to assess interbody fusion.
Results: The number of patients in the TPTD and ZOL groups was 29 and 38 patients, respectively. The VAS and ODI scores in both groups were significantly reduced at 6 and 12 months after TLIF. Compared with that of baseline, the lumbar spine BMD of TPTD patients increased significantly from 0.716± 0.137 g/cm 2 to 0.745± 0.124 g/cm 2 and 0.795± 0.123 g/cm 2 at 6 and 12 months, respectively, and was significantly higher than that of the ZOL group at 12 months (0.720± 0.128 g/cm 2). The bone formation marker, P1NP, in the TPTD group increased significantly (145.48± 66.64 ng/mL and 119.55± 88.27 ng/mL) compared with baseline (44.67± 25.15 ng/mL) and in the ZOL group (28.82± 19.76 ng/mL and 29.94± 20.67 ng/mL) at 6 and 12 months, respectively. The fusion rates in the TPTD and ZOL groups were 57% and 45% at 6 months, without statistical significance. However, TPTD had a more statistically significant positive influence on fusion rate than ZOL at 12 months (86% vs 70%).
Conclusion: TPTD was more efficient than ZOL in bone formation and spinal fusion in OP patients who underwent TLIF.
Keywords: teriparatide, zoledronic acid, osteoporosis, TLIF
Introduction
With the ageing of the population, the prevalence of age-related diseases is increasing in all regions of the world, especially for degenerative spinal diseases.1,2 Treating lumbar degenerative diseases (LDDs) is one of the main clinical challenges for spine surgeons. Transforaminal lumbar interbody fusion (TLIF), a fundamental therapy for intervertebral disc and facet joint degeneration, has been proven to be an effective therapy that reduces pain and leads to neurological recovery.3 However, fusion failure is one of the most devastating complications of this technique. Osteoporosis (OP) is characterised by reduced bone mass and extensive deterioration of the osseous microarchitecture, resulting in increased bone fragility. It is a major risk factor for fusion failure, which results not only in non-union, but also in pedicle screw loosening, adjacent segment fractures, and proximal junctional kyphosis.4,5 Zou reported that OP was quite common among patients aged ≥50 years who underwent lumbar fusion (48.9% for women and 27.1% for men). Patients primarily diagnosed with degenerative lumbar scoliosis are more likely to have OP.6 Therefore, further research is required to improve the fusion rate of patients with osteoporosis.
Broadly classified into osteoanabolic agents (teriparatide (TPTD), romosozumab, etc.) and antiresorptive agents (bisphosphonates, denosumab, etc.), several pharmaceutical therapies are available for the treatment of primary or secondary OP.7 Zoledronic acid (ZOL), a classical and highly efficient aminobisphosphonate, localises and accumulates at high bone turnover sites rapidly after intravenous administration, inhibits farnesyl pyrophosphate synthase (FPPS) of osteoclasts, and prevents protein prenylation to decrease bone resorption, with a considerably long duration of action.8,9 It was verified to be capable of shortening the period of fusion, preventing subsequent vertebral compression fracture (VCF) and decreasing the rate of pedicle screw loosening after spinal interbody fusion in a large number of studies.10,11 However, the impact of fusion rate.10,11 An animal study conducted by our department in 2015 showed that ZOL has a positive effect on vertebral fusion rate at a dose higher than that used in clinics.12 TPTD, a recombinant human parathyroid hormone (rhPTH (1–34)), stimulates osteoblast receptors to promote differentiation and prolong their lifespan after intermittent daily injection, induce the formation of cancellous bone to increase bone mineral density (BMD), and reduce the incidence of osteoporotic fractures.13 In recent years, the effect of TPTD on postoperative spinal fusion with OP has attracted increasing attention. Sugiura attained lumbar fusion in 89% of osteoporotic animal models receiving TPTD, compared with only 56% in the control group.14 In addition, we previously demonstrated in an animal study that teriparatide could solidly promote non-instrumented intervertebral fusion, which is more effective than zoledronic acid.15 Nevertheless, only a few clinical researchers have described the fusion rate with TPTD on spinal fusion after surgery, and none have compared the influence of ZOL to that of TPTD.
Hence, the objective of this study was to compare the effects on BMD, bone metabolism, and fusion rate between patients with OP who received daily injected TPTD and one-time annually administered ZOL retrospectively, to provide clinical evidence and advise for the perioperative anti-osteoporosis strategy.
Patients and Methods
This is a retrospective cohort analysis of radiologic and laboratory data with level 3 evidence. It was reviewed and approved by the institutional review board of Zhongshan Hospital, Fudan University, and the independent ethics committee. All eligible participants included in the study provided written informed consent.
Patients
Patients with osteoporosis who underwent TLIF at our hospital between January 2013 and December 2015 were enrolled in this study. The inclusion criteria were as follows: 1) underwent TLIF because of LDD (lumbar disc herniation, lumbar spinal stenosis, scoliosis, degenerative lumbar instability, or spondylolisthesis); 2) diagnosis of primary OP by dual-energy X-ray absorption (DXA) measurement with T-scores of −2.5 or less at the lumbar spine, femoral neck, or total hip, and/or previous VCF, proximal femoral or other fragility fractures;16 3) 50 years ≤ age < 80 years; 4) TPTD was used for more than six months or ZOL was administered at least once after surgery. The exclusion criteria were as follows: 1) long-term or recent use of glucocorticoids, heparin, anti-OP medication, or other drugs that may affect bone metabolism; 2) suffer from diseases such as chronic hepatic/renal insufficiency or malignant tumour that may affect bone metabolism; 3) with severe trauma due to violence, such as car accidents, high fall, etc.; 4) accepted other spinal surgery previously within six months; 5) baseline measurement showed abnormal blood calcium level: greater than 2.75 mmol/L or less than 2.00 mmol/L, or abnormal 25-hydroxyvitamin D level: <15 ng/mL. Patients enrolled in the study were divided into the TPTD and ZOL groups according to postoperative anti-OP treatment.
Surgery and Postoperative Care
All eligible patients underwent surgery performed by the same surgical team and chief surgeon. TLIF was used for LDD lesions, which was performed with reference to the standard surgical procedures in the previous literature.17 During the operation, pedicle screws and intervertebral fusion cages were used. The autogenous bone obtained during the operation was implanted in the cage, and the remaining autogenous bone particles were packed at the anterior intervertebral space.
Routine postoperative management includes wound drainage and dressing changes. Wound drains were removed when the output was lower than 50 mL/day. Wearing a waist brace for 6–8 weeks and gradual training and strengthening of the back muscles were performed routinely. Moreover, weightlifting and onerous physical labour within 6 months after surgery were prohibited. Additionally, during the entire follow-up period, all patients were given 1000 mg/day oral calcium and 0.25μg /day calcitriol as a basic therapy for OP and encouraged to have more sun exposure and outdoor activities.
Administration of Anti-OP Drugs
Patients in the TPTD group received teriparatide (20 μg/day, once daily, Eli Lilly, IN, USA) subcutaneously and continuously for more than 6 months starting from 1 day after surgery, while the patients in the ZOL group received zoledronic acid once intravenously for at least 15 min (5 mg/year, Novartis, NJ, USA) to 3 days after surgery. ZOL was accessed commercially as 100 mL aqueous solution in prefilled bottles.
Clinical Data
Baseline data on age, sex, body mass index (BMI), and surgical segments were recorded. Ranging from 0 to 10, the visual analogue scale (VAS) was used to grade low back pain and leg pain, with larger scores indicating more pain. The Oswestry Disability Index (ODI), which ranges from 0 to 50, was also used to assess the severity of neurological symptoms, with larger scores indicating more pain and dysfunction. Both clinical parameters were surveyed before TLIF and at 6 and 12 months after TLIF.
Radiological Evaluation
BMD was assessed by DXA examination (Hologic Discovery A device, Bedford, MA, USA) one day before, six months after, and one year after surgery. The average BMD of the non-operated lumbar spine segments and hip joint was used to estimate the bone quality of patients after surgery because of the interference of implants. Three-dimensional computed tomography (CT, Canon, 320 row computed tomography, Aquilion One, tube voltage 120 kV) images and anteroposterior and lateral lumbar X-ray plain films of excessive extension and flexion postures were obtained six months and one year postoperatively to assess the fusion status, internal fixation device loosening, cage subsidence, pseudoarthrosis, and vertebral fracture. Evaluation of fusion status (fusion rate) was performed by three experienced and independent orthopaedists in a blinded manner. Lumbar fusion should be in accordance with the following criteria.18 1) The growth of trabecular bone can be observed on the sagittal view of reconstruction CT, forming a bone bridge and reaching the upper and lower endplates through the fusion cage, without a translucent zone between the cage and the endplate. 2) There are also continuous bone trabeculae around the cage prosthesis to bridge the upper and lower endplates, with continuous bone grafting and trabecular bone formation in the intervertebral space. 3) Hyperextension and hyperflexion radiographs display a range of <5° for intervertebral activity of fusion segment(s).
Blood Examination
The N-terminal propeptide of type 1 collagen (P1NP) and β-cross-linked C-telopeptide of type 1 collagen (β-CTX) were selected as the bone metabolism indicators of formation and resorption, respectively. After an overnight fast for inpatient or outpatient follow-up, serum samples were collected before, six months, and one year after surgery to measure the concentrations of the two bone transformation markers (BTM) mentioned above. All laboratory chemiluminescence analyses were performed using a Roche e2010 analyser (Roche Diagnostics, Mannheim, Germany) at the Immunology Department of Zhongshan Hospital Affiliated to Fudan University.
Statistical Analysis
All statistical analyses were independently performed by three academic medical statisticians using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA), and all results were expressed as mean ± standard deviation. The independent variable t-test was used to compare the continuous variables of baseline characteristics, BMD, and BTM at three time points between the two groups. Paired t-tests were used to compare the two indicators before and after treatment in each group. Pearson’s chi-square test was used to compare the categorical variables of baseline characteristics and the fusion rates at six months and one year after surgery between the two groups. Based on a two-sided 95% confidence interval (CI) for differences, statistical significance was set at p<0.05.
Results
Baseline Characteristics of the Enrolled Population
Table 1 shows the demographic characteristics of the patients before TLIF and the surgical sites of patients in the TPTD and ZOL groups. A total of 67 patients who underwent TLIF with OP were included in this study, including 29 patients in the TPTD group and 38 patients in the ZOL group. In the TPTD group, 17 patients underwent single-segmental surgery, 9 underwent double-segmental surgery, and 3 underwent multi-segmental surgery (equal to or more than 3 segments). As for the ZOL group, 14 patients underwent single-segmental surgery, 18 underwent double-segmental surgery, and 6 underwent multi-segmental surgery. There was no significant difference between the two groups in the proportion of single-, double-, and multi-segmental surgery. There were no statistically significant differences in sex, age, BMI, preoperative VAS score for low back pain and leg pain, preoperative ODI score, baseline BMD, P1NP, and β-CTX values between the two groups. Notably, during the investigation year, no adverse events or serious adverse events, such as palpitation, nausea, myalgia, general fatigue, vertebral fracture, pseudoarthrosis, cage subsidence, rod fracture, or pedicle loosening was observed and recorded.
 |
Table 1 Demographic Characteristics of the Patients Before Surgery in TPTD and ZOL Groups |
Clinical Outcomes
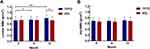
The mean VAS scores for low back pain and leg pain and the mean ODI scores significantly decreased at 6 and 12 months postoperatively in both groups (P<0.0001). Similar results for VAS and ODI were found at all three time points between the two groups (Figure 1).
BMD
The BMD of the lumbar spine did not differ between the TPTD and ZOL groups one day before surgery (0.716±0.137 vs 0.730±0.123 g/cm2, P>0.05). As shown in Figure 2A, 6 months after surgery, there was no significant change in the BMD of the lumbar spine in the ZOL group compared with baseline (P>0.05), and there was no significant difference between the TPTD and ZOL groups (P>0.05). However, there was no statistically significant change in the ZOL group at 12 months compared with baseline (0.720±0.128 g/cm2, P>0.05). Notably, 6 and 12 months after surgery, the lumbar BMD of TPTD patients increased by 3.95% and 11.10%, respectively, compared with baseline (0.745±0.124 g/cm2 and 0.795±0.123 g/cm2, P=0.0016 and P<0.0001, respectively). Regarding the effect comparison between two therapies, the lumbar BMD of the TPTD group was higher than that of the ZOL group one year after surgery (0.795±0.123 g/cm2 VS 0.720±0.128 g/cm2, P=0.0182).
As for hip BMD, there was no significant difference between the two groups before, 6 months, and 12 months after TLIF (0.689±0.112 vs 0.660±0.134 g/cm2, 0.689±0.117 vs 0.659±0.123 g/cm2, 0.706±0.127 vs 0.680±0.143 g/cm2, P>0.05, Figure 2B). There was also no significant change in hip BMD elevation in the TPTD and ZOL groups at 6 months or 12 months after surgery compared with baseline (P>0.05, Figure 2B).
BTM
The Concentration of P1NP in serum was 44.67±25.15 ng/mL and 43.72±18.23 ng/mL in the TPTD and ZOL groups before anti-OP administration, respectively; there was no statistically significant difference between the two groups (P>0.05). In Figure 3A, the P1NP in the TPTD group increased by 225% to 145.48±66.64 ng/mL at 6 months after surgery (P<0.0001) and 168% to 119.55±88.27 ng/mL at 1 year after surgery (P<0.0001) compared with baseline. While the P1NP in the ZOL group decreased by 34% to 28.82±19.76 ng/mL (P<0.0001) and 31.5% to 29.94±20.67 ng/mL (P<0.0001) at the two time points, respectively. The P1NP in the TPTD group was significantly higher than that in the ZOL group at 6 months (P<0.0001) and 1 year after surgery (P<0.0001).
The measured serum β-CTX was 0.42±0.15 ng/mL in the TPTD group and 0.42±0.19 ng/mL in the ZOL group at the beginning, and there was no significant difference between the two groups (P>0.05, Figure 3B). The β-CTX in the TPTD group increased to 0.80±0.33 ng/mL at 6 months after TLIF (P<0.0001) and 0.69±0.31 ng/mL at 1 year after TLIF (P=0.0005) compared with baseline, while that in the ZOL group decreased by 50% to 0.21±0.161 ng/mL (P<0.0001) and 0.21±0.157 ng/mL (P<0.0001) at the two time points. The β-CTX levels in the TPTD group were significantly higher than those in the ZOL group at 6 months (P<0.0001) and 1 year (P<0.0001) after TLIF.
The percent changes in BTM from baseline to 12 months are illustrated in Figure 3C. These results are similar to those shown in Figure 3A and B in a different way (%); namely, BTM increased in the TPTD group and decreased in the ZOL group. In addition, we defined the difference in percent changes between P1NP and β-CTX as the anabolic window (shaded area in Figure 3C). It remained open in the ZOL group at 12 months after a one-time administration of ZOL, as well as in the TPTD group. The area of anabolic window in the TPTD group was significantly higher than the ZOL group at 0–6 months (P<0.0001) and 0–12 months (P<0.0001) after TLIF (Figure 3D).
Fusion Status
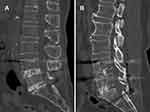
Table 2 shows the fusion outcome of the TPTD and ZOL groups at 6 and 12 months after TLIF, in which we summarised every segment with surgery considering alternative fusion status between segments in a patient with multi-segmental TLIF. Six months after surgery, the fusion rate was 57% (25/44) in the TPTD group and 45% (32/71) in the ZOL group. There was no significant difference in fusion rates between the two groups (P>0.05). At one year after surgery, the fusion rate of the TPTD group increased to 86% (38/44) and that of the ZOL group was 70% (50/71), and the difference between the two groups was statistically significant (P=0.04997). Among the six non-fusion segments in the TPTD group, one was from a three-segment surgery, four were from two double-segment surgeries, and one was from a single-segment surgery. Conversely, 21 segments were not fused in the ZOL group, among which, 4 were from four-segment surgeries (from three cases), 4 were from three-segment surgeries (from three cases), 11 were from double-segment surgeries, and 2 were from single-segment surgeries. Figure 4 shows the CT images of the representative cases of the TPTD (Figure 4A) and ZOL (Figure 4B) groups at 12 months postoperatively. L4/5 and L5/S1 both successfully reached fusion in the two groups.
 |
Table 2 Fusion Rate at 6 Months and 12 Months After Surgery of the TPTD and ZOL Groups |
Discussion
In this study, we performed a retrospective analysis of clinical, radiological, and laboratory data from 67 patients to compare the bone formation and spinal fusion effect between daily TPTD subcutaneous injection and one-time annual ZOL intravenous dripping after TLIF in osteoporotic patients. The findings illustrated that surgery could alleviate the pain indices. At 6 and 12 months after TLIF, the lumbar BMD of patients treated with TPTD was elevated, but not in ZOL patients. The therapeutic outcome for OP in daily TPTD for more than 6 months was more desirable than one-time ZOL fusion for these patients. In addition, TPTD played a stronger anabolic role during the process of osseous metabolism than ZOL, as indicated by the 1-year follow-up of BTM. Moreover, it resulted in a more ideal fusion rate improvement from TPTD than that from ZOL, which is similar to the conclusion from previous studies.19,20
For this type of study, we selected a control group with patients who were treated with ZOL for OP, as patients without medication were the ideal but not reasonable control group. Many osteoporotic therapies, such as bisphosphonates and denosumab, are widely used in clinical practice. Therefore, it is not appropriate to withhold routine treatment for patients with a definite diagnosis of OP. In contrast, concerns arose that the use of bisphosphonates after such surgery would delay or interfere with intervertebral fusion and may lead to instrumentation failure, because it is the osteoclastic activity that initiates the biological fusion process while ZOL has basic resistance to bone resorption.21 However, our previous studies and those of other investigators have stated that ZOL does not impede spinal fusion and has the potential to promote it.12,22 This could be the result of balanced osteoblastic and osteoclastic action inherent to vertebral healing. Thus, it is rational to select ZOL as the control group in this study.
Previous studies have already proven that in patients with OP, both TPTD and ZOL can improve BMD, which is a crucial indicator of bone quantity and stability of fusion instrumentation.23,24 Nevertheless, our research unexpectedly found that the BMD of the ZOL group did not increase significantly within 1 year after TLIF, and patients in the TPTD group had an 11% increase in lumbar spine density at one year after surgery. Compared with normal OP patients without surgical treatment, the activity frequency and BMD of patients included in this study were apparently lower, on account of doctors not recommending those patients to return to normal exercises prematurely because strenuous activities would affect the firmness of the implants. The newly formed bone induced by the drug was barely enough to offset the density loss caused by the lack of activity, so the BMD did not change significantly in the half year after surgery compared with that before drug administration. This unexpected finding further indicates that anti-OP therapy is particularly vital for patients planning to undergo instrumented spinal surgery. In addition, when selecting patients, we decided that the aim and indication of our study were for those with severe OP to show the efficacy of the two medicines, so the baseline BMD of the included patients was lower than that of previous researches.19,20 Meanwhile, the proportion of patients who underwent multi-segmental surgery was greater than that in previous studies20,25 (more than 40% of patients in both groups underwent two or more segmental TLIF). As a result, this study further demonstrated that TPTD can promote BMD and spinal fusion in severe OP, and multi-level surgery are risk factors for postoperative fusion.26
BTM can be used to observe the influence of anti-osteoporosis therapy. The International Osteoporosis Foundation has suggested that the markers for reference are P1NP for bone formation and β-CTX for bone resorption.27 TPTD increased the levels of P1NP and β-CTX. In contrast, after ZOL intravenous fusion, β-CTX decreased and P1NP decreased slightly.28,29 Our study showed the corresponding results. Bone metabolism is a process of coupling and balancing bone formation and resorption, and analysis of the two together could illustrate the influence of anti-OP drugs more comprehensively. Therefore, the concept of “anabolic window” was proposed.30 In the anabolic space shown in Figure 3, more bone mass was formed rather than resorbed, resulting in a rise in bone volume and a probable improvement in microarchitecture. These ultimately might result in enhanced bone strength.31 McClung et al demonstrated that TPTD had a larger anabolic window than alendronate and thus had a stronger effect on BMD. Another randomised controlled trial showed that the augmentation in bone formation markers including P1NP at 3 months could predict the improvement in BMD at 1 year.32,33 Similarly, our study confirmed that TPTD had a remarkably larger anabolic window than ZOL, and both windows remained open at 12 months after surgery, which may also explain the difference in impact on spinal fusion rate between the two drugs.
Preclinical experiments have shown increased cortical bone intensity and Haversian system remodelling after intermittent parathyroid hormone administration in animals.14,34 Based on this, several available clinical evidence and the present results suggested that TPTD offers the opportunity to raise the fusion rate and reduce complications OP patients after lumbar surgery.35,36 In a meta-analysis conducted by Fatima et al with 771 patients, the bony fusion rate was significantly higher in the TPTD group than in the placebo and bisphosphonate group, with respectively 2.23-fold and 2.12-fold higher likelihood of fusion at one-year follow-up. The TPTD group also showed reduced spinal VAS, limb VAS, and an 84% lower probability of subsequent vertebral fracture compared to the non-TPTD group.37 The different surgical techniques for cartilage endplate may affect the fusion, but this influencing factor was ruled out in our study by the same experienced surgeon. We achieved an 86% fusion rate without any cases of vertebral fracture, cage subsidence, rod fracture, or pedicle loosening in the TPTD group. The choice of fusion segments and the type of bone graft in some patients, which can surely affect the fusion rate, also differ between doctors and studies, along with different living habits and postoperative rehabilitation measures. Yagi et al proved that prophylactic TPTD could improve BMD and decrease the incidence of proximal junctional kyphosis from vertebral fracture in adults with spinal deformity and OP after posterior instrumented fusion with at least five fused vertebrae.35 A multicentre retrospective study conducted by Kawabata et al demonstrated that for patients with osteoporotic VCF after posterior instrumented fusion, the screw backout rate was significantly lower after TPTD administration compared with bisphosphonate (1.8% vs 12.5%, P<0.05). However, there was no difference in screw loosening, subsequent vertebral fracture, VAS, or ODI.38 Such findings heavily affected the preferred therapy algorithm, especially when bisphosphonates, including ZOL, are still the first-line choice. However, the detailed instructions and practical application of TPTD vary from study to study. As approved by the FDA, the subcutaneous administration of 20 mg/d TPTD was used in the study.36,39 In contrast, weekly schedules have also been described with different dosages, and a relative study for spinal fusion with OP pointed out equivalent performance between weekly dosing and daily dosing.25 Furthermore, the best time to start TPTD treatment differed among studies. Ohtori et al started it 2 months and 3 months ahead of surgery in two different studies, and Inoue had variable times with a mean value of 61 days before the operation.40–42 Ebata et al, Kaliya-Perumal et al, and the present study all initiated therapy one day postoperatively.25,43 Moreover, given that TPTD is relatively expensive globally and has limited on-label indications, payment from patients can be financially burdensome. The considerable expense of TPTD should be considered, particularly for those undertaking the costs on their own. In brief, the management and prescription of TPTD may still lack deep exploration and thought by medical teams.
The study has some limitations. First, further randomised controlled trials are required to explore the nearly constant hip BMD for 12 months, even with a substantial elevation in spinal BMD. Second, changes in bone density caused by different lumbar spine segments measured by DXA before and after surgery cannot be eliminated. Third, we were not able to evaluate an unmedicated control group to further prove the efficacy of ZOL. However, a variety of previous studies have been carried out to investigate it, including the one conducted by our department, and we made certain comparisons.10,11,22–24,44 Additionally, the sample size of this retrospective cohort study may be slightly small.
Conclusions
Daily 20 μg TPTD for at least 6 months showed a more rapid increase in bone formation and spinal fusion in Chinese OP patients who underwent TLIF operation than administration of one-time dose of 5 mg ZOL. With considerable expense taken into account, large-scale clinical trials are required to further confirm the role of daily TPTD for patients with OP who underwent lumbar operation.
Abbreviations
β-CTX, β cross-linked C-telopeptide of type 1 collagen; BMD, bone mineral density; BMI, body mass index; BTM, bone transformation marker; CI, confidence interval; CT, computed tomography; DXA, dual-energy X-ray absorption; FPPS, farnesyl pyrophosphate synthase; LDDs, lumbar degenerative diseases; P1NP, N-terminal propeptide of type 1 collagen; rhPTH (1-34), recombinant human parathyroid hormone (1-34); TLIF, transforaminal lumbar interbody fusion; TPTD, teriparatide; ODI, Oswestry Disability Index; OP, osteoporosis; VAS, visual analogue score; VCF, vertebral compression fracture; ZOL, zoledronic acid.
Data Sharing Statement
De-identified data can be available upon request from qualified academic investigators.
Ethics Approval and Consent to Participate
All participants provided written informed consent, and the study methods were conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All participants provided publication consent of recordings and images.
Author Contributions
Research design: ZX Wang, CY Zhuang, H Lin, J Dong, etc.; collection of data: ZF Li, J Li, etc.; analysis and interpretation of data: ZX Wang, WX Chen, ZF Li, etc.; drafting and revision of paper: ZX Wang, CY Zhuang, H Lin, J Dong, etc. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Non-profit Central research Institute Fund of Chinese academy of Medical sciences (Grant Number: 2020-JKCS-013) and Natural Science Foundation of Shanghai (Grant Number: 21ZR1455800).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boskey AL, Imbert L. Bone quality changes associated with aging and disease: a review. Ann N Y Acad Sci. 2017;1410(1):93–106.
2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196.
3. Schnake KJ, Rappert D, Storzer B, Schreyer S, Hilber F, Mehren C. [Lumbar fusion-Indications and techniques]. Der Orthopade. 2019;48(1):50–58. German.
4. Lubelski D, Choma TJ, Steinmetz MP, Harrop JS, Mroz TE. Perioperative medical management of spine surgery patients with osteoporosis. Neurosurgery. 2015;77(Suppl 4):S92–97.
5. Uei H, Tokuhashi Y, Maseda M, et al. Exploratory analysis of predictors of revision surgery for proximal junctional kyphosis or additional postoperative vertebral fracture following adult spinal deformity surgery in elderly patients: a retrospective cohort study. J Orthop Surg Res. 2018;13(1):252.
6. Zou D, Jiang S, Zhou S, et al. Prevalence of osteoporosis in patients undergoing lumbar fusion for lumbar degenerative diseases: a combination of DXA and Hounsfield units. Spine. 2020;45(7):E406–e410.
7. Langdahl BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178(9):1891–1906.
8. Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367–401.
9. Coxon FP, Helfrich MH, Van’t Hof R, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Mineral Res. 2000;15(8):1467–1476.
10. Ding Q, Chen J, Fan J, Li Q, Yin G, Yu L. Effect of zoledronic acid on lumbar spinal fusion in osteoporotic patients. Eur Spine J. 2017;26(11):2969–2977.
11. Tu CW, Huang KF, Hsu HT, Li HY, Yang SS, Chen YC. Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J Surg Res. 2014;192(1):112–116.
12. Yasen M, Li X, Jiang L, Yuan W, Che W, Dong J. Effect of zoledronic acid on spinal fusion outcomes in an ovariectomized rat model of osteoporosis. J Orthop Res. 2015;33(9):1297–1304.
13. Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016;27(8):2395–2410.
14. Sugiura T, Kashii M, Matsuo Y, et al. Intermittent administration of teriparatide enhances graft bone healing and accelerates spinal fusion in rats with glucocorticoid-induced osteoporosis. Spine J. 2015;15(2):298–306.
15. Yishake M, Yasen M, Jiang L, et al. Effects of combined teriparatide and zoledronic acid on posterior lumbar vertebral fusion in an aged ovariectomized rat model of osteopenia. J Orthop Res. 2018;36(3):937–944.
16. Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–839.
17. Resnick DK, Watters WC
18. Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine. 2000;25(11):1437–1446.
19. Seki S, Hirano N, Kawaguchi Y, et al. Teriparatide versus low-dose bisphosphonates before and after surgery for adult spinal deformity in female Japanese patients with osteoporosis. Eur Spine J. 2017;26(8):2121–2127.
20. Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine. 2013;38(8):E487–492.
21. Huang RC, Khan SN, Sandhu HS, et al. Alendronate inhibits spine fusion in a rat model. Spine. 2005;30(22):2516–2522.
22. Li C, Wang HR, Li XL, Zhou XG, Dong J. The relation between zoledronic acid infusion and interbody fusion in patients undergoing transforaminal lumbar interbody fusion surgery. Acta Neurochir. 2012;154(4):731–738.
23. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–240.
24. Wang C. Efficacy and safety of zoledronic acid for treatment of postmenopausal osteoporosis: a meta-analysis of randomized controlled trials. Am J Ther. 2017;24(5):e544–e552.
25. Ebata S, Takahashi J, Hasegawa T, et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders: a multicenter, prospective randomized study. J Bone Joint Surg Am. 2017;99(5):365–372.
26. Anderson JT, Tye EY, Haas AR, et al. Multilevel lumbar fusion is a risk factor for lower return to work rates among workers’ compensation subjects with degenerative disc disease. J Surg Orthop Adv. 2018;27(3):209–218.
27. Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420.
28. Eastell R, Mitlak BH, Wang Y, Hu M, Fitzpatrick LA, Black DM. Bone turnover markers to explain changes in lumbar spine BMD with abaloparatide and teriparatide: results from ACTIVE. Osteoporos Int. 2019;30(3):667–673.
29. Liang BC, Shi ZY, Wang B, et al. Intravenous zoledronic acid 5 mg on bone turnover markers and bone mineral density in East China subjects with newly diagnosed osteoporosis: a 24-month clinical study. Orthop Surg. 2017;9(1):103–109.
30. Rubin MR, Cosman F, Lindsay R, Bilezikian JP. The anabolic effects of parathyroid hormone. Osteoporos Int. 2002;13(4):267–277.
31. Gopalaswamy V, Dhibar DP, Gupta V, et al. Anabolic bone window with weekly teriparatide therapy in postmenopausal osteoporosis: a pilot study. Endocrine Pract. 2017;23(6):657–661.
32. McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–1768.
33. Anastasilakis AD, Goulis DG, Polyzos SA, et al. Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: a randomised trial. Int J Clin Pract. 2008;62(6):919–924.
34. O’Loughlin PF, Cunningham ME, Bukata SV, et al. Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine. 2009;34(2):121–130.
35. Yagi M, Ohne H, Konomi T, et al. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos Int. 2016;27(12):3495–3502.
36. Chen CM, Lin PY, Chen YC, et al. Effects of teriparatide on lung function and pain relief in women with multiple osteoporotic vertebral compression fractures. Surg Neurol Int. 2014;5(Suppl 7):S339–342.
37. Fatima N, Massaad E, Hadzipasic M, Shankar GM, Shin JH. Assessment of the efficacy of teriparatide treatment for osteoporosis on lumbar fusion surgery outcomes: a systematic review and meta-analysis. Neurosurg Rev. 2020.
38. Kawabata A, Yoshii T, Hirai T, et al. Effect of bisphosphonates or teriparatide on mechanical complications after posterior instrumented fusion for osteoporotic vertebral fracture: a multi-center retrospective study. BMC Musculoskelet Disord. 2020;21(1):420.
39. Cheng ML, Gupta V. Teriparatide - Indications beyond osteoporosis. Indian J Endocrinol Metab. 2012;16(3):343–348.
40. Ohtori S, Inoue G, Orita S, et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine. 2012;37(23):E1464–1468.
41. Ohtori S, Orita S, Yamauchi K, et al. More than 6 months of teriparatide treatment was more effective for bone union than shorter treatment following lumbar posterolateral fusion surgery. Asian Spine J. 2015;9(4):573–580.
42. Inoue G, Ueno M, Nakazawa T, et al. Teriparatide increases the insertional torque of pedicle screws during fusion surgery in patients with postmenopausal osteoporosis. J Neurosurg Spine. 2014;21(3):425–431.
43. Kaliya-Perumal AK, Lu ML, Luo CA, et al. Retrospective radiological outcome analysis following teriparatide use in elderly patients undergoing multilevel instrumented lumbar fusion surgery. Medicine. 2017;96(5):e5996.
44. Park YS, Kim HS, Baek SW, Kong DY, Ryu JA. The effect of zoledronic acid on the volume of the fusion-mass in lumbar spinal fusion. Clin Orthop Surg. 2013;5(4):292–297.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.