Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The Effect of Chilgoza Pine Nut (Pinus gerardiana Wall.) on Blood Glucose and Oxidative Stress in Diabetic Rats
Authors Hosseini SA, Vali M, Haghighi-Zade MH, Siahpoosh A, Malihi R
Received 19 February 2020
Accepted for publication 24 June 2020
Published 7 July 2020 Volume 2020:13 Pages 2399—2408
DOI https://doi.org/10.2147/DMSO.S250464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Seyed Ahmad Hosseini,1 Maryam Vali,2 Mohammad Hossein Haghighi-Zade,3 Amir Siahpoosh,4 Reza Malihi5
1Nutrition and Metabolic Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 2Department of Nutrition, School of Paramedical Sciences, Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 3School of Public Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 4Department of Pharmacognosy, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 5Department of Nutrition, Abadan Faculty of Medical Sciences, Abadan, Iran
Correspondence: Reza Malihi Tel +989 166051161
Email [email protected]
Purpose: Diabetes can increase oxidative stress in various tissues of the body, and the progress of this process is associated with intensification of the complications of diabetes. The current study purposed to evaluate the protective effect of Pinus gerardiana (PG) seed on oxidative stress induced by diabetes in the liver and serum of streptozotocin (STZ)-induced diabetic rats.
Materials and Methods: This interventional study was performed on 36 male Wistar rats. The rats were randomly divided into 6 groups (healthy controls, healthy treated with 3% and 6% (PG), diabetic control, diabetic treated with 3% and 6% (PG) doses). After 6 weeks of intervention, weight, glucose, and oxidative stress parameters in serum and liver including total antioxidant capacity, malondialdehyde, total thiol and superoxide dismutase activity were measured. Data analysis was done by statistical software version 16 and Tukey’s one-way ANOVA tests.
Results: Diabetic rats showed significantly higher malondialdehyde and fasting glucose levels (12± 1.2 mmol/L) and significant reductions in fasting insulin serum, weight (− 37%), and activity of superoxide dismutase enzymes, total thiol groups, and total antioxidant capacity of serum and liver (about +49% in liver and +16% in serum) (p < 0.001) compared with the healthy groups. Oral administration of PG nuts to diabetic rats caused a significant reduction in malondialdehyde and fasting glucose levels (− 43%) and weight loss (+15%), and a significant increase in activity of superoxide dismutase enzymes, total thiol groups, and total antioxidant capacity of serum and liver (p < 0.001).
Conclusion: The present study concluded that PG can decrease fasting blood glucose, improve insulin resistance, reduce weight loss, and improve oxidative stress indices in the serum and liver of STZ-induced diabetic rats. It is a potential therapeutic food supplement for the treatment and prevention of hyperglycemia and high oxidative stress of diabetes.
Keywords: diabetes mellitus, Pinus gerardiana, oxidative stress, blood glucose, rat
Introduction
According to the World Health Organization (WHO), interventions for lifestyle modification and the prevention of non-communicable diseases such as diabetes are of great importance for developing countries; clearly, such interventions should be commensurate with the limited financial resources of these countries.1 Diabetes is a common multi-factor metabolic disease that leads to impaired glucose homeostasis in the body. Based on reports by the International Diabetes Federation (IDF) in 2015, 415 million people suffer from diabetes throughout the world, and this number is expected to reach 642 million by 2040. The prevalence of diabetes in the Middle East and the Horn of Africa is estimated to be 35.4 million (9.1% of the adult population) and is predicted to double by 2035.2 Diabetes induces oxidative stress in different tissues of the body, and the extension of this process exacerbates diabetes-associated complications. It also creates an imbalance between the body’s antioxidant defense system and the production of free radicals, increasing the amount of these compounds (eg, reactive oxygen species) in the body. These compounds are involved in cellular reactions and increase the production of hydrogen peroxide, which is a more toxic substance than free radicals.3 The antioxidant defense system of the body is categorized into the enzymatic group (such as SOD, glutathione peroxidase and catalase) and the non-enzymatic group (such as vitamin E, carotenoids, ascorbic acid, and bilirubin). This system can counteract the damage of free radicals by preventing their formation, repairing the damage caused by free radical activities, increasing the removal of damaged molecules, and minimizing cell mutation.4 The first line of treatment for diabetes is to use blood-sugar-lowering drugs.5 Because diabetes mellitus causes a series of changes in the basal metabolism and oxidative stress conditions of the body, it is not possible to expect control of other complications only by lowering blood sugar with medication. Moreover, these treatments have been shown to have certain adverse effects, such as weight gain, hypoglycemia, vomiting, edema, gastrointestinal disorders, and liver injury after long-term use.6 Herbal intervention is considered to be natural, and the practice may have been part of the culture for many generations. Therefore, research to find plant compounds that have beneficial effects in improving the diabetic condition is absolutely necessary.7
Nuts are known to be rich sources of fats and provide a wide range of unsaturated fatty acids, phytosterols, and other health-promoting substances.8 In general, pine nuts are a valuable source of nutrients. Hence, they are expected to inherently contain high levels of antioxidants capable of reducing the level of oxidation.9 One of the most important species of pine is the chilgoza pine (Pinus gerardiana Wall.), which is considered as an important nutritional source in its native distribution areas. In terms of nutritional value, Chilgoza pine nuts contain 51.3% fat (fatty acids), 8.7% water, 13.6% protein, 22.5% starch, 0.9% fiber, and 3% minerals and ash, and their fatty acids include stearic acid (1.2%), pinolenic acid (19%), oleic acid (2.3%), and linoleic acid (2.8%).10 The aqueous and dichloromethane extracts from this pine nut contain high levels of catechins, gallocatechins, luteins, lycopenes, carotenoids, and tocopherols. The pine nut also contains healthy amounts of essential minerals such as manganese, potassium, calcium, iron, magnesium, zinc, and selenium. It is one of the richest sources of manganese (8.802 mg/100 g, or about 383% RDI), an important cofactor for antioxidant enzymes such as superoxide dismutase.11 The liver is comprised of tissues that are greatly affected by the damage caused by oxidative stress.
Given that no study has ever investigated the association between the antioxidant properties of chilgoza pine nuts and diabetes and oxidative stress, the current study investigated the effects of chilgoza pine nuts on diabetes-induced oxidative stress in the liver and serum of STZ-induced diabetic male rats.
Materials and Methods
Plant Preparation
Pinus gerardiana Wall. (PG) nuts were obtained from the forests of eastern Afghanistan and identified by a pharmacognosist at the Medicinal Plants Research Center of Ahwaz Jundishapur University of Medical Sciences in Iran (Herbarium Code: A017001001p). The nuts were peeled by industrial lender and powdered. The resulting powder was mixed with the standard powdered chow of the rat (3% and 6% w/w), then returned to the normal form of the rat food, and freely provided to the rats in the intervention groups.
Laboratory Animals
Thirty-six male, white Wistar rats (8 weeks old) weighing about 180 to 200 grams were placed in special cages (6 rats in each cage) with sawdust substrate for 2 weeks under a cycle of 12-hour light/12-hour darkness at 22 ± 1°C and humidity levels recommended for laboratory animals with automatic ventilation until compatible with laboratory conditions. The rats were fed during this period with a standard laboratory chow (Pars Dam Co, Tehran, Iran) containing 46% nanofibrillated cellulose (NFC), 25% neutral detergent fiber (NDF), 19% protein, and 10% lipid. They also had ad libitum access to drinking water.
Water consumption, food, and animal weight in each group were measured weekly. The weekly water and food intake was calculated using the following formulas. Daily food/water intake was expressed per 100 g body weight (bw): Amount of water consumed (mg/100g bw) = [total water consumption of each group during one week (mL)/total weight of rats in each group at the end of the week (g)]×100. And amount of food consumed (mg/100g bw) = [total food consumption of each group during one week (mL)/total weight of rats in each group at the end of the week (g)]×100.
At the end of the study, rats were rendered unconscious with 50 mg/kg ketamine intraperitoneally. The abdomen was opened, and after the viscera was removed, the inferior vena cava was accessed and blood was drawn using a 10-mL syringe.
Diabetes Induction
Diabetes was induced by an intraperitoneal injection of NA (nicotinamide, 120 mg/kg b. wt., dissolved in normal saline) 15 minutes before the single-dose administration of STZ (Streptozotocin, 55 mg/kg b. wt., freshly prepared in citrate buffer, pH 4.5). The development of diabetes was confirmed 7 days later. Following diabetes induction, blood was drawn from the tail veins of the experimental rats to determine their fasting blood glucose levels. Rats with fasting blood glucose levels higher than 13.9 mmol/L were considered diabetic. After confirmation of the diabetic condition, the treatment period started with the desired doses of PG in the target groups and lasted for 6 weeks.
Study Design
After two weeks of adaptation to new conditions, the animals were randomly divided into six groups to begin the study.
Group 1: Healthy Controls (C)
Group 2: Controls receiving 3% PG
Group 3: Controls receiving 6% PG
Group 4: Diabetic controls (D)
Group 5: Diabetic rats receiving 3% PG
Group 6: Diabetic rats receiving 6% PG
Homogenized Liver Preparation
At the end of the experiment, liver tissue was removed and minced with a small scissor in a cold mannitol solution containing 0.225 M D-mannitol, 75 mM sucrose, and 0.2 mM ethylene-diamine-tetra-acetic acid (EDTA). The minced liver was gently homogenized, in a homogenizer with a Teflon pestle, and then centrifuged. The supernatant was used to evaluate the parameters.
Measuring Parameters
Fasting Blood Glucose, Fasting Insulin and HOMA-IR
A glucometer was used to confirm diabetes in the rats. At the end of the study, the rat-tail vein blood samples were tested with the enzymatic method to achieve an accurate measurement of fasting blood sugar (FBS).
Fasting serum insulin was assayed by ELISA kit (ZellBio Co., Germany) following the manufacturer’s protocol. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated to measure the insulin sensitivity of rats using the following formula: [fasting plasma insulin (mU/L) × fasting blood glucose (mmol/L)]/22.5.
Malondialdehyde (MDA)
The MDA level is an appropriate index for assessing lipid peroxidation under oxidative stress conditions. In this study, the MDA level was measured using spectrofluorometry. Thiobarbituric acid reactive substances (TBARS) assay was used to measure lipid peroxidation which is based on the reaction of malondialdehyde (MDA) and thiobarbituric acid (TBA) in the glacial acetic acid medium. Samples were first mixed with 1.5 mL tri-chloro-acetic acid (20% w/v), then centrifuged at 3000g. The taken pellets were resuspended with 1.5 mL 2-thiobarbituric acid (0.2% w/v) and 1.5 mL H2SO4 (0.05 M), following by incubation in boiling water for 45 min. Afterwards, samples were first mixed with 2 mL n-butanol, allowed to cool, then centrifuged and their absorption was read at 532 nm. The tetraethoxypropan standard curve was used to determine the concentrations.12
Total Antioxidant Capacity (TAC)
TAC was measured using the FRAP method. The basic principle of this method is the reduction of ferric tripyridyl triazine (Fe III-TPTZ) complex to Fe II-TPTZ by biological antioxidants. The change in absorbance of samples at a wavelength of 600 nm was compared with the change in the standard absorbance (FeSO4.7H2O).13
Total Protein Assay
The Protein concentration in the samples was measured by the Bradford method. 750 uL diluted coomassie blue was mixed with 50 μL of samples. After 10 min incubation in room temperature, absorbance of samples was measured in 595 nm. A standard curve was created using bovine serum albumin ranging from 0.25 mg/mL to 1 mg/mL.14
Total Thiol Molecules (TT)
Total thiol molecules are also a marker of damage from free radicals. These factors are susceptible to oxidative damage and decrease as a result of these injuries. The colorimetric method with DTNB (5,5ʹ-dithio-2- nitrobenzoic acid) was used to evaluate TT. DNTB combined with these groups forms a yellow complex that has a maximum absorption at 412 nm.15
Superoxide Dismutase (SOD) Activity
SOD activity was measured using a calorimetrically enzymatic assay kit (ZellBio GmbH, Ulm, Germany). In this assay, the SOD activity unit was considered as the amount of the sample that will catalyze the decomposition of 1 mmol of O2 to H2O2 and O2 in 1 minute. The SOD activity was determined colorimetrically at 420 nm.
Statistical Analysis
Statistical analysis was performed using SPSS software package (Version 16.0, IBM, Armonk, NY, USA). The normal distribution of data was evaluated using the Kolmogorov–Smirnov (KS) test. After ensuring that the data distribution was normal, one-way ANOVA with Tukey’s post hoc test was used to compare the means of the variables studied between the groups. A p-value <0.05 was considered statistically significant in all tests.
Ethical Considerations
The initial plan of the study was approved by the ethics committee of the Research Center of Laboratory Animals, Ahvaz Jundishapur University of Medical Sciences, with code No. IR.AJUMS.REC.1396.265 and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23, revised 1996).
Results
Body and Liver Weight, Total Protein and Intake of Water and Food
The groups had no statistically significant difference in mean body weight at baseline. At the end of the study, the mean weight of diabetic rats was decreased significantly compared to the control group (p <0.001) (Table 1). The mean weight of rats in the diabetic groups treated with doses of 3% PG and 6% PG was increased at the end of treatment compared with the diabetic control group (p <0.001) (Table 1).
 |
Table 1 Effect of Pinus gerardiana on Rats’ Body Weight, Liver Weight/Index and Total Protein in Different Studied Groups |
After 6 weeks of intervention, the healthy and healthy control groups receiving 3% and 6% PG showed weight gains of 20%, 17%, and 17%, respectively, compared to the start of the study. Weight changes in the diabetic control and diabetic groups receiving 3% and 6% PG were −17%, −7%, and −6%, respectively, a decrease compared to the initial weight. Body weight changes in the healthy groups showed that the rate of weight gain was 3% lower in the two healthy groups receiving 3% and 6% PG than in the healthy controls, but this difference was not statistically significant (p >0.05).
In general, diabetic rats received more water and food than healthy ones due to the effects of diabetes (p < 0.001). Food intake in the diabetic control group was at least 24% higher than in the healthy control group (p <0.001). The C+PG 3% and C+PG 6% groups had similar food intake to the healthy control group, and both groups showed a tendency to decrease gradually and dosage-dependently, but not significantly (p >0.01). Compared with the DM group, the PG-administered groups had a significant reduction in food intake (p <0.01) (Figure 1). The water consumption of the diabetic rats was increased. The average water consumption of the D group was 117.4 ± 2.6 mL compared to 96 ± 1.7 mL in the healthy control group (p <0.001). The polydipsia symptoms of the PG 6% group had the greatest relief, with an average of 105 ± 2.3 mL, which was about 10% lower than that of the D group (p <0.001) (Figure 1).
Liver weight was decreased in the D group when compared with the non-diabetic groups (p <0.001), but this reduction was significantly lower in PG 6% groups than in the diabetic group (6.5±0.2 g Vs 5.7±0.2g) (p <0.001), as shown in Table 1. The liver index was increased in the D group compared with the non-diabetic groups (p <0.01), but this increase was not significant in the other diabetic groups (Table 1).
The induction of diabetes led to a 21% reduction in total protein compared to the healthy groups (p <0.001). Treatment with PG increased the level of total protein compared with the disease control group (p <0.001), as shown in Table 1.
Fasting Serum Glucose, Fasting Serum Insulin and HOMA-IR
The findings for serum glucose, serum insulin, and HOMA-IR are depicted in Figure 2. STZ injections increased blood glucose levels in the diabetic group by an average of 12 ±1.2 mmol/L over that of the healthy control group (p <0.001). In the diabetic groups treated with 3% PG (p <0.01) and 6% PG (p <0.001), blood glucose at the end of the study showed a significant decrease compared to the diabetic control group (p <0.001). No difference in blood glucose was observed in the healthy control group receiving PG. Diabetes induction can lead to significant insulin resistance in the diabetes group (p <0.001). The HOMA-IR of the D group was 17.9±1.7, which was much higher than the other groups, especially the C group; it was almost 2 times that of the C group. The PG 6% group had a significant improvement over the D group (p <0.01). The findings regarding fasting serum insulin were similar to those of the two factors mentioned earlier (Figure 1).
Antioxidant Barrier (SOD, TAC)
Measurements of the antioxidant barrier parameters of the liver and serum are shown in Table 2. Diabetes significantly reduced the TAC (about 49% in the liver and 16% in serum) and SOD activity levels (about 22% in liver and 28% in serum) in the liver and serum compared to the healthy control (p <0.001). The amount of hepatic TAC in the D group was half that of the C group. On the other hand, treatment with PG 6% significantly improved these factors compared to the diabetic control group (p <0.05) in the liver and serum. PG 3% only improved the hepatic TAC level compared to the diabetic control group. Table 3 shows that there is a strong relationship between serum and liver levels of SOD and TAC (p <0.001).
 |
Table 2 Effect of Pinus gerardiana on Antioxidant Barrier Parameters (TAC, SOD) |
 |
Table 3 Coefficient (r) and Significance of the Correlation Between the Tissue and Serum Antioxidant Barrier Parameters |
Oxidative Damage (MDA, TT)
Measurements of the oxidative damage parameters of the serum and liver are presented in Figure 3. Diabetes increased (about 111% in liver and 43% in serum) the MDA oxidative stress marker level and significantly decreased (about 43% in liver and 39% in serum) the TT levels in the serum and liver compared to the healthy control (p <0.001). On the other hand, treatment with PG 6% significantly improved these factors compared to the diabetic control group (p <0.001). PG 3% only improved the serum levels of TT and MDA compared to the diabetic control group (p <0.05). Table 4 shows that there is a strong relationship between serum and liver levels of MDA and TT (p <0.001).
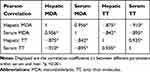 |
Table 4 Coefficient (r) and Significance of the Correlation Between the Tissue and Serum Oxidative Damage Parameters |
Discussion
The current study evaluated the protective effects of Pinus gerardiana Wall. nuts against diabetes-induced complications, such as weight loss, hyperglycemia, oxidative stress, and other pathological changes in the serum and liver of male diabetic rats. As shown in a previous study, PG contains quercetin, gallic acid, vanillic acid, benzoic acid, syringic acid, m-coumaric acid, and other phenolic compounds. Methanolic plant extract had greater total flavonoid contents compared to ethyl acetate extract. Methanol extract also contained higher total phenolic contents than the corresponding ethyl acetate extract. In a study by Lee et al, the most abundant phenolic compounds in PG were found to be gallocatechin, gallic acid, and ellagic acid, respectively.11
The present study suggests that the use of PG will prevent body weight loss during diabetes and decreased blood sugar in a dose-dependent manner (p <0.001). In STZ-induced diabetic rats, because the body cannot consume blood glucose because of insufficient insulin secretion, it uses other sources such as fats and proteins, resulting in an increase in catabolism, muscle wasting, protein depletion, and weight loss.16 However, treatment with PG in the diabetic groups showed a significant (p <0.001) dose-dependent decrease in weight loss compared to the animals in the diabetic control group. In fact, the inhibition of weight loss in diabetic rats treated with PG might be explained by enhanced glycemic control and increased synthesis of structural proteins.
Water and food consumption was increased in diabetic rats compared to the healthy controls (p < 0.001). Compared with the D group, the PG-administered groups had a significant reduction in food (p <0.01) and water (p <0.001) intake. Diabetes is generally associated with symptoms of polydipsia and polyphagia. Reductions in food and water intake affected by PG can be attributed to better control of blood sugar and its high content of protein and lipoic acid, which is effective in reducing appetite.
The current findings showed that liver weight in diabetic rats decreased significantly compared to healthy rats; in contrast, the liver index increased. The PG 6% treatment showed a tendency to normalize these parameters, but it was only significant in preventing liver weight loss (p <0.001). The study results suggest that this condition may be due to PG-reduced weight loss in diabetic rats, increased antioxidant capacity, and reduced liver damage.
The observations of the present study demonstrated that streptozotocin was effective in producing hyperglycemia in rats compared to healthy rats. The decline in blood glucose level, increase in serum insulin, and improved insulin homeostasis in PG-treatment groups were significant in comparison to the diabetes control group (p < 0.01).
The current findings showed that the STZ injection significantly increased blood glucose levels in all three groups of diabetic rats compared to the healthy controls. Conversely, a significant inverse dosage-dependent decrease was observed in the blood glucose of diabetic rats treated with PG, possibly due to the presence of anti-hyperglycemic constituents such as gallic acid and ellagic acid. The mechanism of action of gallic acid on glucose metabolism includes decreasing the expression of PPARγ gene and activation of Akt (protein kinase B).17 The mechanism of action of the antidiabetic ellagic acid is to affect the beta cells of the pancreas and induce insulin secretion, reducing glucose intolerance.18 Previous studies have demonstrated that STZ injection significantly reduces body weight, which is associated with an increase in serum glucose level and impaired insulin and lipid profiles.19
In this study, SOD activity levels in the serum and liver of diabetic rats were decreased significantly compared to the control group, but a significant dosage-dependent increase was observed in the diabetic rats treated with PG compared to the control group. STZ-induced diabetes exacerbated tissue damage by reducing the activity of endogenous antioxidant enzymes. Oxidative stress and reactive oxygen species (ROS) have been proven to affect the pathogenesis of diabetes.20 SOD, catalase, and glutathione peroxidase are of the antioxidant enzymes that form a defensive system against ROS.21 The SOD activity in diabetic rats was observed to be low in this study, which may be due to the increased lipid peroxidation and production of free oxygen radicals, which is consistent with the results of previous studies.22,23 The amount of manganese in PG is 8.8 mg/100 gr, which is about 4 times more than the required daily intake of this element. Manganese is required as a rare metal ion for the activity of the SOD enzyme. This element eliminates some free radicals, such as anion superoxide, by reacting with them and converting them into Mn3+.11 Significant increases in SOD activity in the PG-treated diabetic rats may indicate the effects of manganese and other antioxidants present in PG on reducing or suppressing oxidative stress in the liver and serum of diabetic rats by elevating antioxidant levels.
The findings showed that TAC levels in the liver and serum of diabetic rats were significantly decreased compared to the healthy rats after the induction of diabetes by STZ. After treatment of diabetic rats with PG, a significant directly dosage-dependent increase in TAC was observed in the liver and serum of both groups of rats in comparison with the diabetic control group. In a study by Ranjbar et al, the activity of SOD and TAC was found to be reduced in diabetic rats compared to healthy rats.24 Kesavula et al suggested that the lipid peroxidation levels in diabetic patients were increased compared to the control group, resulting in a decrease in serum TAC levels in these patients.25 The current results are consistent with recent findings; the production of free radicals was observed to be elevated in diabetes. An increase in the number of free radicals leads to the employment of the antioxidant system and a decrease in TAC levels in the serum and liver of diabetic rats. Due to the non-enzymatic antioxidant compounds, such as carotenoids and tocopherols, found widely in PG, the direct and dosage-dependent treatment of rats increases TAC in the serum and liver tissues. Numerous studies have demonstrated that catechins possess antioxidative properties, as they improve total antioxidant capacity, suppress destructive oxygen-free radicals, and prevent oxidative stress damage.26
The current study evaluated the effects of PG nuts on MDA levels in the serum and liver tissues of rats. The findings demonstrate an increase in the MDA level in diabetic rats. MDA is one of the lipid peroxidation products which is measured to evaluate lipid peroxidation levels. The results of this study indicate that oxidative stress in diabetic rats occurs as a result of high blood glucose and its respective metabolic abnormalities. This was confirmed by measuring the MDA level as an index for lipid peroxidation.27 This decrease in MDA levels can be due to the high levels of antioxidant compounds in PG. It is important to note that PG exerts its antioxidant effects mostly in diabetic rats rather than healthy rats.
Generally, the plasma total thiol groups are sensitive to oxidative damage, and a reduction in these compounds is an obvious sign of oxidative stress. In this study, the TT levels in the serum and liver of diabetic rats were decreased significantly compared to the control group; however, the TT level was observed to be dose-dependently and significantly increased in the diabetic rats treated with PG. Ranjbar et al found that STZ-induced diabetes resulted in decreased TAC and TT after 4 weeks.24 The measurement of thiol groups can be an indicator of the effect of oxidative stress on proteins. Studies have shown that the free radicals of hydroxyl and nitric oxide metabolites can react with thiol groups to neutralize oxidative stress.28,29 Sharifzadeh et al reported that catechin compounds can decrease oxidative stress and increase TT in STZ-induced diabetic rats, which is consistent with the findings of the present study.30 The current study has a limitation to consider. The lack of evaluation of more biomarkers in oxidative stress including GSH-Px, Vit E, Carotene, Vit C, Ceruloplasmin, Transderrin and lactoferrin and also, not using more methods to determine the total antioxidant capacity can be considered as limitations in our study.
Conclusion
The use of antioxidant agents plays an important role in reducing the consequences of diabetes. The use of plant-based compounds that usually have fewer side effects is of particular importance. The present study concluded that the 6-week administration of 3% and 6% PG to diabetic male Wistar rats affected a reduction in blood glucose levels, increased insulin secretion, and decreased insulin resistance. PG can reduce weight loss and food/water intake and improve oxidative stress indices in the serum and liver of STZ-induced diabetic rats, which is probably effective in preventing these complications of diabetes. Given the beneficial effects of this nut on the hyperglycemia and high oxidative stress of diabetes, it is recommended that future studies use clinical trials to investigate the effects of chilgoza pine nuts in human diabetes and cardiovascular disease patients.
Acknowledgments
This paper was based on a thesis in masters of science conducted in Ahvaz Jundishapur University of Medical Sciences (Grant No. B-9636 Approval Date: 2017-04-26). We thank Arvand International Division of Ahvaz Jundishapur University of Medical Sciences and the participants, without whom this study would not have been possible.
Disclosure
The authors declare no conflict of interest.
References
1. Marrero S, Adashi EY. Noncommunicable diseases. Semin Reprod Med. 2015;33(1):35–40. doi:10.1055/s-0034-1395277
2. Rahelic D. 7th edition of IDF diabetes atlas – call for immediate action. Lijec Vjesn. 2016;138(1–2):57–58.
3. Arora MK, Singh UK. Oxidative stress: meeting multiple targets in pathogenesis of diabetic nephropathy. Curr Drug Targets. 2014;15(5):531–538. doi:10.2174/1389450115666140321120635
4. Okoduwa SI, Umar IA, Ibrahim S, Bello F, Habila N. Age-dependent alteration of antioxidant defense system in hypertensive and type-2 diabetes patients. J Diabetes Metab Disord. 2015;14:32. doi:10.1186/s40200-015-0164-z
5. Huang YH, Chen ST, Liu FH, et al. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: a randomized, double-blind, placebo-controlled pilot trial. PLoS One. 2019;14(8):e0221199. doi:10.1371/journal.pone.0221199
6. Zhu W, Huang W, Xu Z, et al. Analysis of patents issued in china for antihyperglycemic therapies for type 2 diabetes mellitus. Front Pharmacol. 2019;10:586. doi:10.3389/fphar.2019.00586
7. Chaudhury A, Duvoor C, Dendi R, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne). 2017;8:6. doi:10.3389/fendo.2017.00006
8. Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111(2):421–427. doi:10.1016/j.foodchem.2008.04.008
9. Destaillats F, Cruz-Hernandez C, Giuffrida F, Dionisi F. Identification of the botanical origin of pine nuts found in food products by gas-liquid chromatography analysis of fatty acid profile. J Agric Food Chem. 2010;58(4):2082–2087. doi:10.1021/jf9041722
10. Zargari A. Medicinal Plants.
11. Lee YH, Choo C, Watawana MI, Jayawardena N, Waisundara VY. Evaluation of the total antioxidant capacity and antioxidant compounds of different solvent extracts of chilgoza pine nuts (Pinus gerardiana). J Funct Foods. 2015;18:1014–1021. doi:10.1016/j.jff.2014.07.009
12. Moridi H, Hosseini SA, Shateri H, et al. Protective effect of cerium oxide nanoparticle on sperm quality and oxidative damage in malathion-induced testicular toxicity in rats: an experimental study. Int J Reprod BioMed. 2018;16:261. doi:10.29252/ijrm.16.4.261
13. Tejeda L, Debiec M, Nilsson L, Peñarrieta JM, Alvarado JA. Chemical composition, antioxidant capacity and content of phenolic compounds in meals collected in hospitals in Bolivia and Sweden. Nutr Hosp. 2012;27(4):1009–1016. doi:10.3305/nh.2012.27.4.5849
14. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi:10.1016/0003-2697(76)90527-3
15. Alves M, Martins A, Rato L, Moreira P, Socorro S, Oliveira P. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta Mol Basis Dis. 2013;1832:626–635. doi:10.1016/j.bbadis.2013.01.011
16. Ashraf H, Heydari R, Nejati V, Ilkhanipoor M. Preventive effect of berberis integerrima on the serum levels of glucose and lipids in streptozotocin (STZ)-induced diabetes in rats. J Fasa Univ Med Sci. 2012;2(3):148–155.
17. Bak EJ, Kim J, Jang S, et al. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand J Clin Lab Invest. 2013;73(8):607–614. doi:10.3109/00365513.2013.831470
18. Fatima N, Hafizur RM, Hameed A, Ahmed S, Nisar M, Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur J Nutr. 2017;56(2):591–601. doi:10.1007/s00394-015-1103-y
19. Ramachandran S, Faisal TK, Anjumary J, et al. Comparative evaluation of hypoglycemic and hypolipidemic activity of various extract of Anogeissus latifolia bark in streptozotocin-induced diabetic rats. J Complement Integr Med. 2017;14(3). doi:10.1515/jcim-2016-0130.
20. Nasri S, Roghani M, Baluchnejadmojarad T, Rabani T, Balvardi M. Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology. 2011;18(4):273–278. doi:10.1016/j.pathophys.2011.03.001
21. Noyan T, Balaharoglu R, Komuroglu U. The oxidant and antioxidant effects of 25-hydroxyvitamin D3 in liver, kidney and heart tissues of diabetic rats. Clin Exp Med. 2005;May(1):31–36. doi:10.1007/s10238-005-0061-8
22. Kannadhasan R, Venkataraman S. In vitro capacity and in vivo antioxidant potency of sedimental extract of Tinospora cordifolia in streptozotocin induced type 2 diabetes. Avicenna J Phytomed. 2013;3(1):7–24.
23. Sha J, Sui B, Su X, Meng Q, Zhang C. Alteration of oxidative stress and inflammatory cytokines induces apoptosis in diabetic nephropathy. Mol Med Rep. 2017;16(5):7715–7723. doi:10.3892/mmr.2017.7522
24. Ranjbar A, Ghasemi H, Hatami M, Dadras F, Heidary Shayesteh T, Khoshjou F. Tempol effects on diabetic nephropathy in male rats. J Renal Inj Prev. 2016;5(2):74–78. doi:10.15171/jrip.2016.16
25. Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26:387–392.
26. Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem. 2018;241:480–492. doi:10.1016/j.foodchem.2017.08.117
27. Ogunyinka BI, Oyinloye BE, Osunsanmi FO, Opoku AR, Kappo AP. Protective effects of parkia biglobosa protein isolate on streptozotocin-induced hepatic damage and oxidative stress in diabetic male rats. Molecules. 2017;22(10):1654. doi:10.3390/molecules22101654
28. Fisher-Wellman KH, Gilliam LA, Lin CT, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–1208. doi:10.1016/j.freeradbiomed.2013.09.008
29. Francik R, Krośniak M, Sanocka I, Bartoń H, Hebda T, Francik S. Aronia melanocarpa treatment and antioxidant status in selected tissues in wistar rats. Biomed Res Int. 2014;2014:457085. doi:10.1155/2014/457085
30. Sharifzadeh M, Ranjbar A, Hosseini A, Khanavi M. The effect of green tea extract on oxidative stress and spatial learning in streptozotocin-diabetic rats. Iran J Pharm Res. 2017;16(1):201–209.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



