Back to Journals » Clinical Ophthalmology » Volume 14
The Effect of Brimonidine 0.1% on Disc Hemorrhage in Primary Open-Angle Glaucoma Patients
Authors Nitta K , Shimamoto S, Wajima R, Tachibana G, Yamada Y, Domoto M, Takeda R , Takahashi Y, Sugiyama K
Received 9 November 2019
Accepted for publication 8 January 2020
Published 23 January 2020 Volume 2020:14 Pages 213—219
DOI https://doi.org/10.2147/OPTH.S237969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Koji Nitta,1,2 Shiro Shimamoto,3 Ryotaro Wajima,2 Gaku Tachibana,2 Yutaro Yamada,1,2 Miyuki Domoto,1 Ryuji Takeda,4,5 Yoshinari Takahashi,6 Kazuhisa Sugiyama2
1Fukui-ken Saiseikai Hospital, Fukui, Japan; 2Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan; 3Shimamoto Ophthalmology Clinic, Fukui, Japan; 4Department of Nutritional Sciences for Well-Being, Faculty of Health Sciences for Welfare, Kansai University of Welfare Sciences, Osaka, Japan; 5Department of Applied Biological Chemistry, Faculty of Agriculture, Kindai University, Nara, Japan; 6Inary Co., Ltd., Tokyo, Japan
Correspondence: Koji Nitta
Department of Ophthalmology, Fukui-ken Saiseikai Hospital, 7-1 Funabashi, Wadanaka-cho, Fukui 918-8503, Japan
Tel +81 776 23 1111
Fax +81 776 28 8515
Email [email protected]
Background: This retrospective study evaluated the effect of adjunctive administration of brimonidine 0.1% on disc hemorrhage (DH) in patients with primary open-angle glaucoma or normal-tension glaucoma who were already treated with other anti-glaucoma drugs.
Methods: Patients with DH, before adjunctive therapy with brimonidine, were enrolled. Subjects were excluded if their treatment regimen was changed within 1 year after initiation of adjunctive therapy with brimonidine. We investigated the frequency of DH and intraocular pressure (IOP). Both parameters were compared before and after adjunctive administration of brimonidine.
Results: The frequency of DH before and after brimonidine administration was 0.67± 0.68 and 0.31± 0.72 times/year, respectively, with a significant decrease (P=0.01), and the mean IOP before and after brimonidine administration was 12.5± 1.9 and 11.2± 2.2 mmHg, respectively, (P=0.0006) with a significant reduction after adjunctive administration.
Conclusion: The results of this study supported the hypothesis that the frequency of DH is reduced by brimonidine alongside lowering of IOP.
Keywords: disc hemorrhage, intraocular pressure, brimonidine, primary open-angle glaucoma, normal-tension glaucoma
Background
The highest prevalence of normal-tension glaucoma (NTG) has been reported in Asian countries, including Japan.1 One of the risk factors for the progression of visual field defects is disc hemorrhage (DH). Several reports have detailed the correlation between the occurrence of DH and glaucoma progression.2–7 The progression of visual field defects in NTG with DH was reported to be significantly faster than in NTG without DH.8 The site of a DH is strongly associated with the site of deterioration within the visual field.9 A retinal nerve fiber layer defect (RNFLD) usually enlarges from the site where the DH occurred.10
The mechanism underlying DH has not yet been clarified. However, it has been reported that gross deformation of the lamina cribrosa (LC), such as thinning and posterior displacement and enlargement of the RNFLD, causes loss of the capillary network around the nerve fiber.10 It has also been reported that changes in the degenerative retinal nerve fiber layer (RNFL) and damage to the capillary network surrounding the border of the RNFL could produce DH.11 Therefore, vascular and mechanical changes in LC in glaucomatous eye may contribute to DH.12
In addition, although it has been reported that low-pressure glaucoma patients treated with brimonidine 0.2% are less likely to have progressed visual field defects than patients treated with timolol 0.5%, even though there was no significant difference in intraocular pressure (IOP) lowering effect between them.13 To best of our knowledge, there are no reports of the effect on DH occurrence of adjunctive administration of brimonidine 0.1%. Therefore, we carried out a retrospective study in which brimonidine, primarily used for adjunctive treatment in Japan, was investigated to determine whether adjunctive brimonidine decreased the occurrence of DH.
Methods
Of the NTG or primary open-angle glaucoma (POAG) patients undergoing treatment at Fukui-ken Saiseikai Hospital and Shimamoto Ophthalmology Clinic (Fukui City), patients satisfying the following criteria were included in the present study. The eligible patients: (1) had been diagnosed with NTG or POAG; (2) had had a DH at least once between glaucoma diagnosis and brimonidine administration; (3) had been monitored for at least 1 year without any change in their glaucoma treatment after adjunctive brimonidine administration; (4) had been observed 4 times or more per year; (5) had no retinal disease or corneal opacity; (6) had not undergone laser in situ keratomileusis or glaucoma surgery during the evaluation period after adjunctive brimonidine administration; and (7) had a baseline mean deviation (MD) value of −25 dB or more. The diagnostic criteria for POAG were 1) more than 21 mmHg IOP mostly three times without any glaucoma medication. 2) open-angle, 3) Glaucomatous optic neuropathy (GON) with corresponding visual field loss, and 4) the absence of other optic neuropathies. The diagnostic criteria for NTG were 1) 21 mmHg or less IOP mostly three times without any glaucoma medication. 2) open-angle, 3) GON with corresponding visual field loss, and 4) the absence of other optic neuropathies. POAG and NTG were diagnosed according to the guidelines of the Japan Glaucoma Society and the European Glaucoma Society.14,15
The follow-up period consisted of the pre-administration and post-administration periods. In each case, the post-administration period indicates the date from which brimonidine was adjunctively administered until administration was finished patient underwent additional treatment, or until January 31, 2016. The pre-administration period indicates the period running backward from the administration start date for the same duration as the post-administration period (Figure 1). If both eyes met the study criteria, the eye with the lower MD value at the start of the evaluation (i.e., the eye with the more advanced glaucoma) was selected as the test eye. This study was approved by the Institutional Review Board of Fukui-ken Saiseikai Hospital and adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from patients using the “opt-out” prior to the start of this study under approval from the Ethics Review Board. The clinical registration ID is UMIN000026406.
The study parameters were: the number of DH (in times), frequency of DH (in times/year), IOP, gender, age at the start of administration of brimonidine, eye medical history/surgery history, follow-up period (in years), times of visits (in times), frequency of visits (in times/year), concomitant drugs, concomitant procedures, presence of complications, and adverse events.
DH occurrence was confirmed with fundoscopy and photographs captured with a fundus camera (CR6-45BM: Canon, Japan, X-10; or nonmyd WX3: Kowa, Nagoya, Japan). The frequency of DH was the times of DH occurrences per year. Until the hemorrhage was absorbed, a given DH was considered a single DH occurrence. If there were multiple DHs at the same time in a study participant, each was counted as a single DH. IOP was measured with a Goldmann applanation tonometer.
Adverse events included complications that worsened after the administration of brimonidine, and deterioration of clinical symptoms and abnormal clinical laboratory test values obtained after the administration of brimonidine.
For statistical analysis, Wilcoxon rank-sum tests were used to evaluate the duration of evaluation (in years), number of evaluations, frequency of evaluation, and number of DH before and after treatment with brimonidine. Wilcoxon signed-rank tests were used to compare the frequency of DH and the mean IOP before and after treatment with brimonidine. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS statistical software (version 9.4 for Windows; SAS Institute, Cary, NC, USA).
Results
Patient Demographics
Twenty-two patients (9 men and 13 women) were eligible for enrollment in the present study (Table 1). The mean age of the enrolled patients was 66.2±11.5 years. The distribution of glaucoma types was 17 patients with NTG and 5 patients with POAG. Pre- and post-administration periods were 1.84±0.74 and 1.77±0.76 years, respectively. The numbers of visits in the pre- and post-administration periods were 9.82±6.28 and 9.91±7.23 times, and the frequencies of visits were 5.22±1.44 and 5.71±3.44 times/year, respectively. There were no significant differences between the periods for any of the aforementioned parameters.
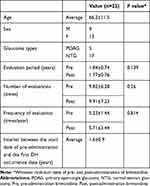 |
Table 1 Patient Demographics |
Concomitant use of other anti-glaucomatous medications included 7 patients with one other medication, 6 patients with 2, 7 patients with 3, and 2 patients with 4 (Supplemental Table).
None of the patients experienced complications. The patients’ ocular-surgery histories included cataract surgery in 6 eyes, as well as trabeculectomy, and retinal photocoagulation, each in 1 eye. No adverse events were noted in the present study.
Mean Intraocular Pressure and Frequency of DH Occurrence
The mean IOP before and after brimonidine administration was 12.5±1.9 and 11.2±2.2 mmHg, respectively (n=22, P=0.0006) (Figure 2A), and 12.2±1.9 and 11.2±2.1 mmHg, respectively (n=17, P=0.0145) in only NTG. IOP was significantly lowered after the administration of brimonidine. The frequency of DH before and after brimonidine administration was 0.67±0.68 and 0.31±0.72 times/year, respectively (n=22, P=0.0100) (Figure 2B), and 0.65±0.71 and 0.40±0.80 times/year, respectively (n=17, P=0.0420) in only NTG. The frequency of DH significantly decreased after administration of brimonidine.
Times of DH Occurrences
Figure 3 shows the times of DH occurrence before and after brimonidine administration; 6 patients with none DH, 11 patients with single DH, 4 patients with 2 times of DH, 1 patient with 3 times or more of DH before medication; 16 patients with none DH, 4 patients with single DH, 1 patient with 2 times of DH, and 1 patient with 3 times or more of DH after medication. There was a significant difference in the distribution of DH before and after brimonidine application (n=22, P=0.0062).
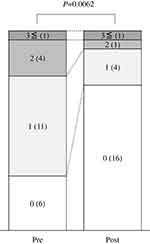 |
Figure 3 Distribution of number of DH. 3≤, DH occurrence 3 times or more; 2, DH occurrence 2 times; 1, DH occurrence once; 0, No DH occurrence. Numbers in parentheses show number of patients. |
Discussion
IOP and the frequency of DH significantly decreased after the administration of brimonidine. Miyake et al have reported that IOP reduction via trabeculectomy significantly decreased the frequency of DH in patients who had either POAG or NTG.16 After a median of 13 years of observation, in patients with ocular hypertension, the cumulative incidence of eyes experiencing at least one DH was lower for the anti-glaucomatous medication group than for the non-treatment group.4 Based on the aforementioned findings, it seemed that the main factor in decreasing DH frequency was the IOP-lowering effect of adjunctive applanation of brimonidine. It has been reported that continuous elevation of IOP leads to posterior deformation of the LC17 and causes RNFL and capillaries to be damaged particularly at the upper and lower polarized LC where the connective tissue is sparse.18 In another study, deformation of the LC was 11.1% in POAG without DH, whereas it was 88.9% in POAG with DH by optical coherence tomography (OCT), and the location of DH occurrence was related to the deformed part of the LC.19
With adjunctive administration of brimonidine, IOP and the frequency of DH decreased and distribution of DH was changed and made better. According to post reports, IOP reduced after administration brimonidine 0.2% and timolol 0.5% but there was no difference of occurrence of DH between 2 medicines. Therefore, other medicines may also make the frequency of DH significantly decreased after administration.13
Visual field defects can progress despite the lowering of IOP, it is possible that factors other than decreased IOP, such as circulatory impairment, could be involved. Brimonidine acts on α-2 receptors and suppresses the activity of adenylyl cyclase via Gi protein. It has been shown that brimonidine, in addition to lowering IOP by decreasing intracellular cyclic AMP levels, is 5 times as potent as oxymetazoline as a vasoconstrictor in human subcutaneous arteries with diameters of less than 200 µm,20 and that brimonidine can decrease nitric oxide-mediated vasodilation in small arterioles in the retina.21 With 1 week of topical administration of brimonidine, most of the vitreous samples from a group of patients contained a concentration of more than 2 nM, which has been shown in animal retinas to activate the α-2 receptor.22 The brimonidine concentrations in vitreous samples were comparable with the free concentration of brimonidine in the posterior retina/choroid in pigmented rabbits.23 However, it is ethically difficult to detect brimonidine concentrations in human posterior retina/choroid. In a comparison of patient groups with and without application of brimonidine before an intravitreal injection, subconjunctival hemorrhages were less common and less severe in the brimonidine 0.15%-treated group.24 Therefore, one action, other than IOP lowering, that decreases DH frequency could be the suppressive action of brimonidine on blood vessel dilation in the small vessels of the retina.
There are some potential limitations of our study. First, this was a retrospective study that used inclusion and exclusion criteria. There is a possible bias based on the specific inclusion and exclusion criteria applied, as well as the referral of specific patients by ophthalmologists. Second, some instances of DH may have appeared and resolved between examinations. There is a little possibility that the examinations at 3-month intervals in this study may overlook DH. Kitazawa et al demonstrated that mean duration of DH was 10.6 weeks, 92% of all instances of DH were present for at least 4 weeks.25,26 Third, the evaluation of changes in visual field defects was not possible because such changes attributable to DH are believed to occur 3 years later.5 Furthermore, the inner layer of the retina was not observed using OCT. However, the patients’ backgrounds were made as consistent as possible by, for example, adjusting the pre- and post-administration periods and selecting patients who did not change their treatment regimen after initiation of the adjunctive administration of brimonidine.
In the present study, it seemed that the decrease in the frequency of DH was due to the reduction of IOP, and it was also possible that a retinal vasoconstrictive effect caused by administration of brimonidine was involved. Because DH is a risk factor for visual field defects, in the treatment of patients with glaucoma not only must IOP be monitored but the retina, especially the peripapillary region, must also be regularly monitored and recorded using a fundus camera. When providing appropriate treatment for glaucoma, it is very important to consider the progression of visual field defects after DH and to enhance the IOP lowering effect of medical treatments.
Conclusion
The results of this study supported the hypothesis that the frequency of DH is reduced by brimonidine alongside lowering of IOP.
Abbreviations
NTG, normal-tension glaucoma; DH, disc hemorrhage; RNFLD, retinal nerve fiber layer defect; LC, lamina cribrosa; RNFL, retinal nerve fiber layer; IOP, intraocular pressure; POAG, primary open-angle glaucoma; MD, mean deviation; GON, glaucomatous optic neuropathy; OCT, optical coherence tomography.
Ethics Approval and Consent to Participate
All procedure conformed to the Declaration of Helsinki. This study was approved by the institutional Review Board of Fukui-ken Saiseikai Hospital. Written informed consent was not obtained due to the retrospective manner of the study. Alternatively, this study was carried out by the opt-out method. The medical records accessed and used in this study are not available in public databases. Patient data were de-identified upon data collection.
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
This study was funded by Senju Pharmaceutical Co., Ltd. We thank Claire Barnes, PhD, from Edanz Group for editing a draft of this manuscript.
Author Contributions
KN and KS drafted the manuscript. KN, SS, RW, GT, YY and MD examined the patient, and collected data. RT and YT performed statistical analysis. All authors read and approved the final manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by Senju Pharmaceutical. The funder had no rule in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Iwase A, Suzuki M, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi study. Ophthalmology. 2004;111:1641–1648. doi:10.1016/j.ophtha.2004.03.029
2. Kim M, Kim DM, Park KH, Kim TW, Jeoung JW, Kim SH. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol. 2013;91:e270–e275. doi:10.1111/aos.12082
3. Araie M, Shirato S, Yamazaki Y, et al. Risk factors for progression of normal-tension glaucoma under β-blocker monotherapy. Acta Ophthalmol. 2012;90:e337–e343. doi:10.1111/j.1755-3768.2012.02425.x
4. Budenz DL, Huecker JB, Gedde SJ, Gordon M, Kass M; Ocular Hypertension Treatment Study Group. Thirteen-year follow-up of optic disc hemorrhages in the ocular hypertension treatment study. Am J Ophthalmol. 2017;174:126–133. doi:10.1016/j.ajo.2016.10.023
5. Nitta K, Sugiyama K, Tanahashi T. Relationship between the frequency of disk hemorrhage and the enlargement of nerve fiver layer defects and the deterioration speed of visual field loss in normal-tension glaucoma with wedge-shaped nerve fiber layer defects. Nihon Ganka Gakkai Zasshi. 2011;115:839–847.
6. De Moraes CG, Prata TS, Liebmann CA, Tello C, Ritch R, Liebmann JM. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci. 2009;50:4727–4733. doi:10.1167/iovs.09-3446
7. Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology. 2006;113:598–602. doi:10.1016/j.ophtha.2005.12.018
8. Komori S, Ishida K, Yamamoto T. Results of long-term monitoring of normal-tension glaucoma patients receiving medical therapy: results of an 18-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2014;252:1963–1970. doi:10.1007/s00417-014-2767-3
9. Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707–714. doi:10.1016/S0002-9394(00)00441-4
10. Nitta K, Sugiyama K, Higashide T, Ohkubo S, Tanahashi T, Kitazawa Y. Does the enlargement of retinal nerve fiber layer defects relate to disc hemorrhage or progressive visual field loss in normal-tension glaucoma? J Glaucoma. 2011;20:189–195. doi:10.1097/IJG.0b013e3181e0799c
11. Takayama K, Hangai M, Kimura Y, et al. Three-dimensional imaging of lamina cribrosa defects in glaucoma using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4798–4807. doi:10.1167/iovs.13-11677
12. Park HYL, Jeong HJ, Kim YH, Park CK. Optic disc hemorrhage is related to various hemodynamic findings by disc angiography. PLoS One. 2015;10:e0120000. doi:10.1371/journal.pone.0120000
13. Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151:671–681. doi:10.1016/j.ajo.2010.09.026
14. Japan Glaucoma Society. The Japan glaucoma society guidelines for glaucoma (3rd edition). Nihon Ganka Gakkai Zasshi. 2012;116:3–46.
15. European Glaucoma Society. European glaucoma society terminology and guidelines for glaucoma, 4th edition - chapter 2: classification and terminology supported by the EGS foundation: part 1: foreword; introduction; glossary; chapter 2 classification and terminology. Br J Ophthalmol. 2017;101:73–127. doi:10.1136/bjophthalmol-2016-EGSguideline.002
16. Miyake T, Sawada A, Yamamoto T, Miyake Y, Sugiyama K, Kitazawa Y. Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma. 2006;15:164–171. doi:10.1097/00061198-200604000-00014
17. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi:10.1001/archopht.1981.03930010635009
18. Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012;119:1359–1366. doi:10.1016/j.ophtha.2012.01.034
19. Lee EJ, Kim TW, Kim M, Girard MJ, Mari JM, Weinreb RN. Recent structural alteration of the peripheral lamina cribrosa near the location of disc hemorrhage in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2805–2815. doi:10.1167/iovs.13-12742
20. Piwnica D, Rosignoli C, de Ménonville ST, et al. Vasoconstriction and anti-inflammatory properties of the selective α-adrenergic receptor agonist brimonidine. J Dermatol Sci. 2014;75:49–54. doi:10.1016/j.jdermsci.2014.04.002
21. Rosa RH
22. Takamura Y, Tomomatsu T, Matsumura T, et al. Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans. J Ocul Pharmacol Ther. 2015;31:282–285. doi:10.1089/jop.2015.0003
23. Shinno K, Kurokawa K, Kozai S, Kawamura A, Inada K, Tokushige H. The relationship of brimonidine concentration in vitreous body to the free concentration in retina/choroid following topical administration in pigmented rabbits. Curr Eye Res. 2016;42:748–753. doi:10.1080/02713683.2016.1238941
24. Kim CS, Nam KY, Kim JY. Effect of prophylactic topical brimonidine (0.15%) administration on the development of subconjunctival hemorrhage after intravitreal injection. Retina. 2011;31:389–392. doi:10.1097/IAE.0b013e3181eef28e
25. Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology. 1986;93:853–857. doi:10.1016/S0161-6420(86)33658-3
26. Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res. 2002;21:35–56. doi:10.1016/S1350-9462(01)00019-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


