Back to Journals » Cancer Management and Research » Volume 12
The Effect and Safety of Anti-PD-1 Single/Combination Therapy in Refractory Thymic Carcinoma: A Case-Series Study
Authors Jin W , Duan JC, Wang ZJ, Lin L, Bai H, Wang J, Feng L
Received 30 July 2020
Accepted for publication 15 October 2020
Published 6 November 2020 Volume 2020:12 Pages 11351—11358
DOI https://doi.org/10.2147/CMAR.S274830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Wei Jin,1,* Jian-Chun Duan,2,* Zhi-Jie Wang,2 Lin Lin,2 Hua Bai,2 Jie Wang,2 Li Feng1
1Department of Chinese Medicine, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 2State Key Laboratory of Molecular Oncology, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Wang
State Key Laboratory of Molecular Oncology, Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17, Panjiayuan Nanli, Chaoyang District, Beijing 100021, People’s Republic of China
Tel +86 139-1070-4669
Email [email protected]
Li Feng
Department of Chinese Medicine, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17, Panjiayuan Nanli, Chaoyang District, Beijing 100021, People’s Republic of China
Tel +86 186-1814-7576
Email [email protected]
Abstract: Immunotherapy provided with checkpoint inhibitors such as the programmed cell death-1 (PD-1) receptor or its ligand-1 (PD-L1) protein has been shown to be effective for treating several types of cancer, and was recently approved for use in treating malignant melanoma, advanced non-small cell lung cancer (NSCLC), urothelial carcinoma, head and neck squamous cell carcinoma, liver cancer, and additional forms of cancer. However, there is little evidence concerning its effectiveness in treating thymic squamous cell carcinoma (TSCC). Here, we report two cases of refractory TSCC that were treated with PD-1 single/combination therapy in a clinical setting. The patients exhibited variable responses to therapy without any serious adverse events. In summary, our findings show that immunotherapy provided with an immuno-checkpoint inhibitor in combination with chemotherapy/anti-angiogenesis therapy can improve the treatment response of patients with refractory TSCC. Anti-PD-1 single/combination therapy may be used as a strategy for treating advanced refractory TC.
Keywords: thymic carcinoma, anti-PD-1, single/combination therapy
Introduction
Thymic carcinoma (TC) is a rare mediastinal malignant tumor derived from thymic epithelial cells, and accounts for 5~36% of all thymic epithelial tumors (TETs).1–3 Histological examinations have shown that the majority of TCs are either squamous carcinomas or undifferentiated carcinomas. A retrospective study by the Chinese Alliance for Research in Thymomas (ChART) showed that the most frequent histologic subtype of TCs was squamous cell carcinoma.4 A study has shown that the 5-year survival rate of patients with TC is only 30%.5 Surgical resection is the primary method used to manage early-stage TC when a complete resection is possible. However, the postoperative recurrence rate of TC is high,6,7 and the prognosis of TC patients is poor. As most cases of TC are diagnosed at an advanced stage, chemotherapy is usually administered to slow the progress of the tumor. The efficacy of conventional chemotherapy in patients with advanced-stage or relapsing tumors is poor,8 there are few published findings regarding the efficacy of second-line chemotherapy in patients with previously treated or advanced TC.
The evolution of immune-checkpoint inhibitors (ICIs) has revolutionized the field of immunotherapy. Cancer cells are known to activate different immune checkpoints, and several ICIs including pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab have recently shown promising results for suppressing cancer growth and progression.
ICIs that target PD-1/PD-L1 have been shown to be beneficial in treating multiple types of solid tumors, and to increase the overall response rate (ORR) with a low rate of immune-related adverse events. Investigations into methods for obtaining long-term immune responses and increasing patient survival times by using ICIs has become an increasingly popular area of research. Several anti-PD-1/PDL-1 drugs have recently been approved by the Food and Drug Administration for use in treating malignant melanoma, NSCLC, and other types of solid tumors. Under normal physiologic conditions, PD-1 expressed on activated T-cells is known to protect the host against autoimmunity by inhibiting effector T-cell activity and stimulating the activity of immunosuppressive regulatory T cells (Tregs).9 Expression of the PD-ligand (PD-L1) is specifically upregulated in the presence of a tumor, and that upregulation further promotes tumor invasion and growth by suppressing the T-cell response.
The thymus is a complex immune organ known to play a critical role in creating and building a T-cell repertoire. Previous studies have demonstrated PD-L1 expression in the thymus, and several studies reported the frequency of PD-L1 expression in thymomas (23%-68%) and TCs (70%-75%).10,11 A study that analyzed PD-1 and PD-L1 and their levels of mRNA expression in 68 samples of formalin-fixed paraffin-embedded thymic epithelial neoplasm tissue (63 thymomas and 5 thymic carcinomas) suggest possible involvement of the PD-1 and PD-L1 pathway in TETs’ progression.12 Although still controversial, given the immunological significance of PD-1/PD-L1 within the thymus and the recent emergence of PD-L1 as an important predictive biomarker for the effectiveness of cancer immunotherapies,13,14 anti-PD-1/PD-L1 therapies could play an important role in the management of advanced TC. It is also possible that combination treatment with conventional cytotoxic agents plus immunotherapy agents could increase the efficacy of both types of agents.15 However, the low prevalence of TC significantly limits the ability of investigators to generate epidemiological evidence regarding the efficacy of anti-PD-1/PD-L1 therapies used for advanced-stage TC. The low number of available patients could also be responsible for the current lack of consensus on how to best treat advanced-stage TC patients in clinical practice.
Here, we describe two cases of advanced-stage recurrent TSCC treated with PD-1 inhibitor single/combination therapy and provide an assessment of the efficacy and safety of the treatment.
Case Presentations
Case 1
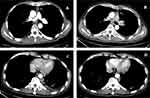
A 40-year-old male presented at the hospital and was subsequently diagnosed with squamous cell carcinoma of the thymus (Masaoka stage IV) in November 2004 (Figure 1). The patient was administered preoperative and postoperative chemotherapy with cyclophosphamide, epirubicin, and cisplatin. Because of repeated local recurrence, the patient received gamma knife treatment in 2006, and again in 2014. Shortly afterwards, the patient presented with an inoperable relapse and was administered concurrent radiochemotherapy with docetaxel and oxaliplatin. In 2015, second-line treatment with albumin paclitaxel was initiated; however, the disease continued to progress, and third-line treatment with gemcitabine plus bevacizumab was initiated. A second axillary lymph node biopsy was performed, which revealed the same histological type as the primary lesion. Immunohistochemistry (IHC) showed that the biopsy tissue was PD-1 and PD-L1 positive. In June 2016 (Figure 2A), ICI therapy was initiated consisting of 40 cycles of nivolumab. That treatment continued until May 2018 (Figure 2B) and resulted in SD with a progression-free survival (PFS) time of 38 months. In August 2019, the patient received one cycle of pembrolizumab plus vinorelbine due to re-emergence of the disease. Chest imaging revealed a right hilar lymph node lesion that showed a 10% increase in size. This finding was followed by re-initiation of treatment with 3 cycles of nivolumab in combination with nab-paclitaxel, which resulted in a partial response. Unfortunately, the treatment had to be discontinued due to complaints of chest tightness and asthma. The disease then rapidly progressed, with the right hilar lymph node lesion showing 50% enlargement. Since December 2019, the patient has been receiving oral anlotinib therapy. With the exception of itchy skin on the limbs (CTCAE grade 1), no serious adverse events were observed during these single/combination treatments.
 |
Figure 1 Histopathology from case 1. (A) ×40. (B) ×200. |
Case 2
In 2017, a 52-year-old male presented at the hospital with complaints of progressive chest pain. Chest imaging revealed an anterior mediastinum mass with enlarged lymph nodes and a left pleura metastasis. A small needle biopsy of the anterior mediastinal mass revealed poorly differentiated squamous cell carcinoma of the thymus (Figure 3). Immunohistochemistry showed that 70% of the cells were positive for PD-L1. The patient was administered first-line treatment with paclitaxel liposomes plus carboplatin; however, the disease continued to progress. Next, immunotherapy was initiated with pembrolizumab as second-line treatment, and the patient showed progressive disease after 2 cycles of immunotherapy. Subsequently, the treatment was modified to include combination therapy with albumin paclitaxel as a third-line treatment, which led to significant disease remission after 2 cycles. Upon examination, the patient showed only slight progress after the 3rd cycle (Figure 4A), and was switched to treatment with pembrolizumab plus anlotinib, which subsequently resulted in progressive disease. This was followed by the introduction of palliative radiotherapy, and concurrent administration of targeted therapy with anlotinib. Combination therapy with anlotinib plus pembrolizumab was continued for 16 cycles as a part of a sequential regimen, resulting in the maintenance of stable disease until December 2019 (Figure 4B), when an enlarged pleural tubercle and new bone metastases were observed (Figure 4C). As a consequence of these findings, the third-line chemotherapy was judged to be ineffective, and the patient was switched to one cycle of combination therapy with pembrolizumab plus gemcitabine. To our surprise, the patient exhibited a partial response (Figure 4D). Furthermore, during the entire course of pembrolizumab immunotherapy and immunocombination therapy with albumin paclitaxel/anlotinib, the patient experienced only grade 1 nausea without any additional immunotherapy-related side effects. However, the patient did experience a grade 3 skin reaction that manifested as rashes on the trunk and limbs, accompanied by itching during treatment with pembrolizumab plus gemcitabine combination therapy. Later, a marked improvement in the skin reaction was observed after initiation of hormone therapy. In summary, the duration of anti-PD-1 single therapy and immunotherapy in combination with chemotherapy/antiangiogenic therapy lasted for a total of 23 months.
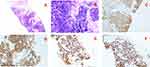 |
Figure 3 Histopathology from case 2. (A) ×40. (B) ×200. (C) CD5. (D) CD117. (E) P40. (F) P63. |
Discussion
TCs are malignant mediastinal neoplasms derived from thymic epithelial cells and have a poor prognosis. Due to the rarity of these tumors, treatment decisions are often based on findings from underpowered prospective clinical studies. Moreover, TCs are more aggressive, less responsive to chemotherapy, and have an increased likelihood of producing distant metastases when compared with other types of tumors. TSCCs are known to respond poorly to chemotherapy, with objective positive rates ranging from 22% to 36%.16,17 To date, the response of TSCCs to radiotherapy is also controversial. In addition, there is no standard second-line treatment for patients with recurrent TC. These factors emphasize the need for prognostic biomarkers and new alternative strategies for treating TC. Recently, ICIs have ushered in a new era in cancer treatment, provoking us to evaluate the prospects for using ICIs in treatment of TCs.
PD-L1 expression is the most studied biomarker for judging the efficacy of a PD-1/PD-L1 inhibitor. The efficacy of pembrolizumab was recently evaluated in a single-arm Phase II study that enrolled 40 patients with recurrent TC that had progressed after at least one cycle of chemotherapy. Only 3% of those patients achieved a complete response, 20% achieved a partial response, and 53% achieved stable disease.18 Another phase II trial enrolled 26 patients with refractory or recurrent TET. In that study, 19.2% of the patients showed a partial response, 53.8% of patients achieved stable disease, and the median PFS time was 6.1 months.19 A recent case study showed that 100% of squamous cell carcinoma specimens obtained from a Japanese patient treated with pembrolizumab expressed PD-L1. The patient’s tumor was successfully treated, as verified by its significant regression without any accompanying serious adverse side effects. These studies suggest that inhibition of the PD-1/PD-L1 axis by anti-PD-1 or anti-PD-L1 antibodies might be a strategy for treating TC.20
Both patients (case #1 and case #2) in our case-series tested positive for PD-L1 expression. Our immunohistochemical analysis of one of the patients (case #2) showed a high level of PD-L1 expression (up to 70%), and that patient received pembrolizumab as second-line therapy. However, the patient showed disease progression after only 2 cycles of treatment, suggesting a lack of efficacy. Our results are in sharp contrast with those of a previous study that reported a positive association between a high level of PD-L1 expression (>50% of cells) and a favorable prognosis.
Furthermore, in a small study that evaluated the efficacy of nivolumab in four patients with TC, 3 patients showed a partial response and the remaining patient achieved stable disease; those results suggest the potential benefits of using these inhibitors to treat TCs in real-world clinical setting.21 In our case study, one patient (case #1) who received nivolumab as monotherapy achieved stable disease and a PFS time of 38 months, which was consistent with previous reports of a durable response to immunotherapy lasting longer than one year.22,23 Due to the low incidence of TC, we were unable verify the correlation between PD-1 expression and the efficacy of TC treatment; further emphasizing the need for a larger sample size.
It is believed that immunotherapy might be even more beneficial when administered in combination with other therapies such as chemotherapy, radiotherapy, and antiangiogenic therapy. These potentially synergistic combination therapies may cause an anti-tumor immune response by releasing antigens that promote tumor cell apoptosis, and thereby restore active immunity.24,25 Consistent with literature reports, both patients with progressive disease in our study who were administered nivolumab/pembrolizumab in combination with albumin paclitaxel achieved a partial response. Furthermore, one of those patients was further treated with pembrolizumab in combination with the traditional chemotherapy drug gemcitabine, and continued to show a partial response. Knowledge regarding chemotherapy for TC has mainly been based on retrospective series with small patient samples. A retrospective analysis reported that gemcitabine achieved moderate outcomes concerning in 4(36.4%) of 11 patients with refractory TC who had previously been treated mainly with platinum-based chemotherapy, suggesting gemcitabine as a possible important drug for treating TC.26 Another study evaluated the efficacy of second-line chemotherapy in patients with previously treated advanced TC. When gemcitabine was used as the second-line chemotherapeutic agent, the response rate was 28.6%.8 However, gemcitabine was only used as a second-line treatment for TC in the above studies, and there is no standard treatment after multiple lines of chemotherapy have been used. Because of its rarity, there are no randomized studies on the effects of chemotherapy in treatment of TC, and additional evidence is required to prove the efficacy of gemcitabine when used as a single agent in treatment of TC. Our study suggests the potential clinical benefits of receiving different combinations of an ICI plus other forms of therapy. A previous retrospective study showed that elevated levels of PD-L1 and PD-1 expression after chemotherapy are markers that support the use of anti-PD-1/L1 drugs for treating TM and TC, as well as the continued use of those agents after chemotherapy.27 Our results are also consistent with that study, as they suggest the benefit of providing ICI therapy after conventional chemotherapy.
We observed a favorable response in one patient (case #2) who was administered pembrolizumab in combination with the antiangiogenic drug, anlotinib, as that patient achieved 15 months of PFS. Tumor growth requires the generation of new blood vessels (i.e. neovascularization) to maintain a continuous supply of oxygen and nutrients. While angiogenesis is critical for the growth of primary tumors, it also has a complex relationship with the immune system.28 Tumor cells evade immune surveillance by immunologically suppressing their microenvironment. VEGF produced by tumors not only plays an essential role in tumor vascularization but also inhibits the immune system by identifying and destroying immune cells that attack tumor cells.29 VEGF signaling specifically modulates immunoregulation by inhibiting the activity of T cells to reduce an antitumor response. Several Phase 2 trials have reported the activity and safety of multi-targeted kinase inhibitors, mainly targeting VEGR, as second-line treatment for thymic carcinoma. The activity of sunitinib malate, a multitargeted kinase inhibitor targeting the VEGFR, was tested in 23 patients with thymic carcinoma and 16 patients with thymoma in a phase 2 trial, in which 6 (26%) of the 23 patients with thymic carcinoma achieved a partial response.30 Another multicenter, phase 2 trial assessed the activity and safety of lenvatinib as a second-line treatment in advanced or metastatic thymic carcinoma. Those findings showed that 16 (38%) of 42 patients had a partial response.31 These results suggest that sunitinib and lenvatinib could become treatment options for patients with previously treated advanced or metastatic thymic carcinoma. However, the above trials all have the limitation of a small sample size, and their results need to be confirmed in larger trials. Anlotinib is a novel TKI that was independently developed in China. Anlotinib can bind to VEGFR-1, VEGFR-2, VEGFR-3, PDGFR, fibroblast growth factor receptor (FGFR), c-Kit, Ret, and other targets. Several clinical trials have demonstrated that anlotinib has good efficacy and safety when used in the treatment of non-small cell lung cancer, soft tissue sarcoma, small cell lung cancer, medullary thyroid carcinoma, renal carcinoma and colorectal cancer. Because of the rarity of thymic carcinoma, no clinical trials have investigated the safety and efficacy of anlotinib in treatment of TC. However, such findings support the use of antiangiogenic agents in treatment of advanced thymic malignancies. Previous success stories regarding the effectiveness of anlotinib therapy motivated us to treat two of our patients with that drug. The results were encouraging, as one of the patients reaped the benefits of immunotherapy provided in combination with anlotinib, further confirming the stimulatory effect of anti-angiogenesis drugs on the immune system.32 The use of anlotinib in combination with traditional chemotherapy, targeted therapy, and immunotherapy needs further exploration. It is hoped that immune-based combination therapy will become more widely used to treat various types of tumors in the future, and thus benefit more patients.
In conclusion, our study showed that different individuals can respond differently to immunotherapy. Although we observed a suboptimal response rate, the responses we did observe when using immunomonotherapy or an immuno-checkpoint inhibitor in combination with chemotherapy/anti-angiogenesis therapy was beneficial, and those treatments were accompanied by an acceptable level of toxicity. Our study further emphasizes the need for investigating the role that combination therapies consisting of an ICI plus chemotherapy/antiangiogenic drugs can play in treating refractory TC. Despite the limitations of our study, results for the 2 cases described here suggest that immune-based combination therapy may be used as a treatment option for patients with advanced refractory TC, a population for which no standard treatments are available. However, our results must be viewed with caution and reveal the need for conducting large-scale prospective studies that explore the role of immunotherapy in TSCC. Furthermore, prognostic biomarkers could play an essential role in realizing the possible synergistic antitumor effects that ICIs might exert when used as part of combination therapy for TC. Whether PD-L1 can be used as a potential biomarker for TC, still needs to be verified in studies with large sample sizes.
Ethics and Consent
All procedures involving human subjects were performed in accordance with ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This was a retrospective study, and written informed consent has been provided by the patients to have the case details and any accompanying images published. The study does not require institutional approval to publish the case details. The patients’ data had been de-identified prior to analysis. The study was based on data obtained from anonymized patients to protect the confidentiality and privacy of the patients.
Acknowledgment
Source of the work: Cases from the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Funding
All authors declare that no financial funding was received.
Disclosure
All authors declare having no conflicts of interest.
References
1. Wick MR, Weiland LH, Scheithauer BW, et al. Primary thymic carcinomas. Am J Surg Pathol. 1982;6(7):613–630. doi:10.1097/00000478-198210000-00003
2. Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer. 1991;67(4):1025–1032.
3. Hsu CP, Chen CY, Chen CL, et al. Thymic carcinoma. Ten years’ experience in twenty patients. J Thorac Cardiovasc Surg. 1994;107(2):615–620. doi:10.1016/S0022-5223(94)70112-1
4. Fu H, Gu Z, Fang W, et al. Long-term survival after surgical treatment of thymic carcinoma: a retrospective analysis from the Chinese alliance for research of thymoma database. Ann Surg Oncol. 2016;23(2):619–625. doi:10.1245/s10434-015-4825-4
5. Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg. 2013;95(1):299–303. doi:10.1016/j.athoracsur.2012.09.006
6. Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Ann Thorac Surg. 2003;76(3):878–884. doi:10.1016/s0003-4975(03)00555-1
7. Tseng Y-L. Thymic carcinoma: a rare cancer requiring special attention. Formos J Surg. 2011;44(4):136–140. doi:10.1016/j.fjs.2011.08.007
8. Tateishi K, Ryo K, Shukuya T, et al. Clinical outcomes of second-line chemotherapy in patients with previously treated advanced thymic carcinoma: a retrospective analysis of 191 patients from the NEJ023 study. Oncologist. 2020;25(4):e668–e674. doi:10.1634/theoncologist.2019-0593
9. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
10. Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. 2015;10(3):500–508. doi:10.1097/JTO.0000000000000429
11. Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. 2015;88(2):154–159. doi:10.1016/j.lungcan.2015.03.003
12. Rossana B, Gaia G, Alessandro B, et al. Prognostic relevance of programmed cell death protein 1/programmed death-ligand 1 pathway in thymic malignancies with combined immunohistochemical and biomolecular approach. Expert Opin Ther Targets. 2020:1–7. doi:10.1080/14728222.2020.1790529.
13. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi:10.1158/1535-7163.MCT-14-0983
14. Song Y, Li Z, Xue W, et al. Predictive biomarkers for PD-1 and PD-L1 immune checkpoint blockade therapy. Immunotherapy. 2019;11(6):515–529. doi:10.2217/imt-2018-0173
15. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis From KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi:10.1200/JCO.19.03136
16. Lemma Girum L, Lee J-W, Aisner Seena C, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29(15):2060–2065. doi:10.1200/JCO.2010.32.9607
17. Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol. 2015;26(2):363–368. doi:10.1093/annonc/mdu541
18. Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347–355. doi:10.1016/S1470-2045(18)30062-7
19. Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. 2019;37(24):2162–2170. doi:10.1200/JCO.2017.77.3184
20. Isshiki T, Isobe K, Tochigi N, et al. Successful use of pembrolizumab to treat refractory thymic carcinoma with high PD-L1 expression. Case Rep Oncol. 2018;11(3):688–692. doi:10.1159/000493187
21. Uchida N, Fujita K, Okamura M, et al. The clinical benefits of immune checkpoint inhibitor for thymic carcinomas~experience of single public hospital in Japan. Respir Med Case Rep. 2019;26:39–41. doi:10.1016/j.rmcr.2018.11.007
22. Brahmer Julie R, Drake Charles G, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi:10.1200/JCO.2009.26.7609
23. Topalian SL, Stephen Hodi F, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
24. Sara P, Angel M-VM, Niki K, et al. Integrating the molecular background of targeted therapy and immunotherapy in lung cancer: a way to explore the impact of mutational landscape on tumor immunogenicity. Transl Lung Cancer Res. 2015;4(6):721–727. doi:10.3978/j.issn.2218-6751.2015.10.11
25. Tan W-L, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol. 2016;17(8):e347–e362. doi:10.1016/S1470-2045(16)30123-1
26. Okuma Y, Hosomi Y, Watanabe K, et al. Gemcitabine in patients previously treated with platinum-containing chemotherapy for refractory thymic carcinoma: radiographic assessment using the RECIST criteria and the ITMIG recommendations. Int J Clin Oncol. 2016;21(3):531–538. doi:10.1007/s10147-015-0926-0
27. Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. doi:10.1016/j.lungcan.2016.05.007
28. Nakamura K, Smyth Mark J. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. 2017;95(4):325–332. doi:10.1038/icb.2016.126
29. Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23(2–3):263–272. doi:10.1385/IR:23:2-3:263
30. Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16(2):177–186. doi:10.1016/S1470-2045(14)71181-7
31. Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 2020;21(6):843–850. doi:10.1016/S1470-2045(20)30162-5
32. Ken G. Promising early results for immunotherapy-antiangiogenesis combination. J Natl Cancer Inst. 2014;106(11). doi:10.1093/jnci/dju392
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.