Back to Journals » Clinical Ophthalmology » Volume 14
The Economic Value of MR-Imaging for Uveal Melanoma
Authors Grech Fonk L, Ferreira TA, Webb AG, Luyten GPM , Beenakker JWM
Received 13 November 2019
Accepted for publication 3 March 2020
Published 28 April 2020 Volume 2020:14 Pages 1135—1143
DOI https://doi.org/10.2147/OPTH.S238405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lorna Grech Fonk,1,2 Teresa A Ferreira,2 Andrew G Webb,2,3 Gregorius PM Luyten,1 Jan-Willem M Beenakker1,2
1Department of Ophthalmology, Leiden University Medical Centre, Leiden, the Netherlands; 2Department of Radiology, Leiden University Medical Centre, Leiden, the Netherlands; 3C.J. Gorter Centre for High Field Magnetic Resonance Imaging, Department of Radiology, Leiden University Medical Centre, Leiden, the Netherlands
Correspondence: Lorna Grech Fonk
Department of Ophthalmology, Leiden University Medical Centre, Leiden, the Netherlands
Email [email protected]
Objective: Uveal melanoma (UM) is the most common primary intra-ocular tumour. Treatment is determined by tumour size and location. Generally, smaller tumours are eligible for brachytherapy unless they are located close to posterior pole. Larger tumours are enucleated or undergo proton beam therapy (PBT), which is more expensive than brachytherapy and less available. Accuracy of tumour size determination is critical for accurate planning and delivery of treatment, particularly to ensure tumour coverage, critical structure sparing, and for the choice of treatment modality. This is particularly the case for tumour dimensions that are close to the cut-off point for a specific type of treatment: in the case of the brachytherapy protocol at our institution, 6– 8 mm. Ultrasound is conventionally used, but magnetic resonance imaging (MRI) has recently become an additional available tool. Although more expensive, it enables more accurate measurements and is particularly useful in combination with clinical fundus examination, fundus photography and ultrasound. Our aim in this paper was to determine the economic value of MRI for UM treatment.
Methods: We retrospectively analysed 60 patients’ MRI scans acquired as part of a study or for clinical care. For each patient, we assessed whether the extra cost of an MRI generated economic benefit or change in optimal treatment.
Results: MRI indicated a smaller tumour prominence than US in 10% of patients with intermediate tumour size, resulting in a change from PBT to brachytherapy. The costs of MRI, € 200–€ 1000, are significantly lower than the higher costs of PBT compared to brachytherapy, € 24,000 difference. In addition, the annual total economic burden of severe vision impairment associated with eye removal is € 10,000. Furthermore, for patients where ultrasound was impossible due to previous surgery, MRI enabled eye-preserving treatment.
Conclusion: An additional MRI for specific patients with UM improves economic value as it enables less expensive treatment in a sufficient percentage of patients to compensate for the MRI costs. Value is increased in terms of quality of care as it enables for some a treatment option which spares more vision.
Keywords: uveal melanoma, ocular tumour, MRI, radiotherapy, oncological imaging, ultrasound, eye diseases
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy with an annual incidence of 5 cases per million in the United States.1 The pathology can involve the iris, ciliary body or choroid.2,3 In UM, blurred vision and vision loss are the most common first symptoms. Depending on the size and location of the tumour(s), the treatment plan for UM patients is either radiotherapy or removal of the eye (enucleation). Local control is as high as 96%, and the 5-year survival rate for UM is only 86%,4,5 As clinical studies have shown, there is no significant difference in terms of patient survival rate after radiotherapy as compared to enucleation.6,7 As a result, the rationale for radiotherapy treatment is preservation of the eye and vision. Of different eye-preserving treatments, brachytherapy is currently the most common treatment8 and it involves the surgical placement of a radioactive ruthenium or iodine plaque onto the sclera, the outer layer of the eye. The radioactive plaque remains in place for up to 7 days, depending on the size of the tumour. Since the radioactive plaque is shielded on the back side, the radio-toxic side effects of this treatment are limited compared to other forms of radiotherapy9 and these side effects mainly involve cataract, radiation-optico neuropathy and retinopathy.10,11 A large study conducted by the federal government where information about vision and eye health was collected showed that 87% of patients are estimated to have gained a significant economic and social benefit by maining a degree of eyeshight.12 In approximately 4% of patients treated with Ruthenium106 brachytherapy,13 the eye is enucleated after more than 5 years post-treatment.13 Brachytherapy can be only used for small and medium-sized tumours, since in larger tumours the tumour apex is too distant from the radioactive shield to receive a sufficient dose of radiation.14 Furthermore, tumours near the optic disc are difficult to treat with plaques.15 For these and larger tumours, proton beam irradiation therapy (PBT) has become the preferred treatment to preserve the eye since it spares most of the patient’s vision and achieves tumour control in over 96% of the eyes.16 The side-effects of PBT are significant with a secondary enucleation rate of 5.4–14% at 5 years17 and other studies also show that PBT has a failure rate of 4.2%.18 PBT has been reported to be successful in sparing the eye in 89% of the patients 5 years after treatment.19,20 Proton beam facilities have become increasingly available worldwide; however, they are very costly to set up.21 As a result, economically, PBT is much more expensive than brachytherapy, with the complete costs of the treatment per patient being approximately €30,000 and €6,000, respectively.21 Stereotactic radiosurgery, specifically gamma knife together with eye fixation22 is also another option used in the treatment of UM; however, this is not included in our analyses because studies have shown that PBT gives better outcomes and therefore more and more centres shift from stereotactic radiosurgery to PBT.16
Since the exact size and geometry of the tumour is one of the main determinants of the optimal treatment modality, accurate imaging of the tumour is key to provide an optimal personalized treatment for the patient. Conventionally, the tumour dimensions are determined using high frequency (~10 MHz) ultrasound (US). However, both the quality of the US image and the estimated dimensions of the tumour have been shown to be very much operator-dependent due to their strong dependence on the orientation of the phased array transducer with respect to the complex three-dimensional geometry of the tumour, especially since only two-dimensional cross-sections are typically acquired and displayed.23 Magnetic Resonance Imaging (MRI) has several potential advantages as an imaging methodology over conventional US, including its ability to image the tumour in three-dimensions (3D) as well as to incorporate multiple tissue-contrasts based on proton density and MR relaxation times, as well as diffusion.24,25 Although Computed Tomography (CT) is also able to provide 3D volumetric data of the UM, MRI is the preferred imagining modality for ocular conditions as it provides a better soft tissue contrast, which can, for example, differentiate retinal detachment from UM.26 However, ocular MRI is challenging due to the absence of specialized commercial radiofrequency (RF) coils (often a very general single-channel “micro-coil” is used), and issues of eye motion during the scan due to blinking are common. Although UM was studied with MRI in the late 1980s,27 and different studies have shown promising steps towards the clinical application of MRI for UM, the advances towards higher field strengths have enabled the more routine clinical application of MRI for UM over the last decade.25,28-32 Our institution is the National Reference Centre for UM in The Netherlands, and so is a main centre for developing and assessing new imaging techniques for UM. Over the past 5 years, different hardware, software and data acquisition techniques for MRI of UM patients have been developed.25,28,33 During this time, our research group has shown how the addition of an MRI-scan to the conventional clinical workup of UM patients can have a positive effect on treatment planning. Beenakker et al, for example, found in one study of 10 patients that two had their treatment changed from enucleation (which would have been recommended based on the ultrasound scans) to eye-preserving treatment based on the higher quality three-dimensional MRI scans.25 Based on these results, an increasing number of UM patients have received an MR, either as part of their clinical care or in the context of scientific studies.
From a diagnostic point of view, the additional information available via MRI, in terms of dimensions, location and infiltration of the tumour, has proved very useful to the ophthalmologists.25,28 However, with every new type of treatment, there are also economic issues to be considered, and in this paper, we attempt to provide an economic view of the cost-effectiveness of an additional MRI scan for patients with UM, with the aim of providing a useful framework for its evaluation for other clinical imaging facilities dealing with such patients. In the last 5 years, we have scanned 60 patients, some for clinical care and others in the context of research studies and technique development. Although this group of patients is not completely representative of all UM-patients, it has provided us with sufficient experience to evaluate the potential added economic value of MRI for patients with UM.
Methods
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Approval of the protocol was obtained from the ethics institutional review board at the Leiden Medical Centre in The Netherlands. Written informed consent was obtained from all patients who were evaluated in the context of a clinical study before study participation.
Patient Population
We retrospectively analysed the data from all UM patients who have undergone an MRI scan between 2014 and 2019. Of a total 60 patients, 42 were included in the context of a scientific study, 16 were scanned due to doubts about the inconsistent US measurements, and 2 patients were scanned because the presence of a silicone oil tamponade (placed in a previous surgery) made US impossible. Table 1 summarizes the different patient groups and the measurements performed for each group.
 |
Table 1 Patient population. |
Summary of the MRI Scanning Protocol
One of the main challenges of ocular MRI is to prevent or minimize eye-motion-induced artefacts. To achieve this we use a “cued-blinking paradigm” for most of the scans. In this paradigm, the patient is given instructions to fix their gaze with the unaffected eye on a particular point and to blink at specific time intervals.28,34 To produce the fixation point within the magnet bore a projector is placed at one end of the bore of the magnet and an MRI-compatible mirror is placed on the RF coil a few centimetres from the eye to reflect the projected image to the patient, Figure 1. A simple cue is produced by a personal computer attached to the projector. In the case of patients with non-MRI-compatible glasses, a standard kit of MRI-compatible lenses is commercially available, and the appropriate ones can be chosen. In addition, a dedicated receive eye-coil is used, since the enhanced signal-to-noise compared to a head coil allows for shorter scanning times, increased patient comfort and reduced motion artefacts.24,28,35 For 3-Tesla MRI systems, there are a number of single loop “micro-coils” available with different diameters: for very high field systems such as 7 Tesla, it is relatively simple to design and construct such coils.28 For 3-Tesla scans, we use a coil with a diameter of 4.7 cm: the images have similar signal-to-noise at a slightly lower spatial resolution than at 7 Tesla.
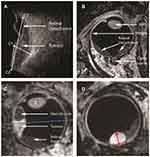
In order to limit image artefacts on the anterior side of the eye arising from magnetic susceptibility, the patients are instructed to close the eye with the tumour and a wet gauze is placed on their eye-lid.36 To assess the tumour dimensions, only a limited number of basic MRI scans are needed, namely T2-weighted and T1-weighted images before and post-injection of a gadolinium-based contrast agent. We have found that it is very important that these images have an isotropic spatial resolution, as this is required for 3D-reformatting of the images to assess the 3D shape of the tumour, as shown in Figure 2. As we aim to limit the time per scan to approximately 3 minutes, at 7 Tesla our images have an isotropic resolution of 0.65 mm3, while at 3 Tesla the resolution is 0.8 mm3. Although only a limited set of images are needed to determine the tumour dimensions, in practice, more advanced scan techniques, including diffusion-weighted imaging and dynamic contrast enhancement imaging are included as well, which aim to provide more specific information about the tumour.37,38
The total, basic scan protocol duration is less than 20 minutes, which makes it also feasible for patients who feel less at ease in an MR system. Figure 3 shows a series of images which form the basis required to determine the optimal therapy. The tumour boundaries are clearly visible on the T1-weighted images, while the T2-weighted scans are used to screen for scleral infiltration of the tumour. In addition, retinal detachment, a common complication of UM can easily be differentiated from tumour tissue on the post-contrast scan as a non-enhancing region.
Overall, therefore, from an economic point-of-view, in order to implement ocular MRI one requires an approximately 30-min scan, a dedicated receive coil, and minor investment in terms of a video projector and personal computer.
Results
Assessing the Additional Value of Ocular MRI Scans in UM Patients
In terms of general clinical assessment, the increased contrast between tissues and the 3D nature of the data have been determined by the ophthalmologists to be considerable advantages of MRI compared to the conventional US images, since with MRI you are certain that no oblique measurement is made, which would lead to an overestimation of the tumour size. In practice, depending on the type of brachytherapy provided, there is a strict cut-off in terms of tumour thickness which determines whether a patient is eligible for brachytherapy, or whether the more expensive PBT (if available) is the only remaining option for eye preservation: if PBT is not available or prescribed then enucleation is used.
For the ruthenium shields used at our institution, based on historical data this cut-off is set at 7 mm tumour prominence, where the dimension is defined as the distance between the top of the tumour and the outside of the sclera (other institutions may have different criteria).
Analysing the patient population that has undergone MRI scans over the past 5 years, these patients can be categorized into four well-defined groups, summarized in Table 2: (i) those for whom the ultrasound-derived tumour size was either very small or very large and therefore as expected the MRI scans did not affect the diagnostic outcome (43 patients), (ii) patients for whom the MRI provided confirmation of ultrasound-derived tumour size (and therefore treatment planning) when the dimensions were close to the cut-off point (11 patients), (iii) patients for whom the 3D delineation provided by MRI actually changed the treatment planning from enucleation to radioactive plaque (4 patients), and (iv) a small group of patients for whom US scanning is not possible (2 patients).
 |
Table 2 Tumours Placed in Different Categories Based on Size, Clinical or Study Groups and a Separate Group for Silicone Oil to Visualise if Treatment Had a Change or Not. |
As expected for patients with relatively small (<6 mm) or very large (>8.5 mm) tumour thickness, the MRI scans did not have any influence on the actual treatment plan chosen: these were plaque brachytherapy and enucleation, respectively. We found that for the small tumours the MRI often indicated a slightly smaller tumour dimension than the ultrasound scan.25 In theory (and perhaps in the future), the smaller potential tumour dimensions could allow for a slightly shorter radiation period, but would need clinical correlation to ensure sufficient dose to the tumour. Currently, the radiotherapy oncologists prefer to be on the conservative side and base their treatment protocol on the tumour size estimated by the US measurement as more research is needed before relying solely on MRI measurements for these patients is possible. For very large tumours, MRI also often showed slightly different dimensions compared to US: 2D US measurements generally over-estimate the tumour size and based on earlier studies the average difference between US and MRI was 1mm.25 Sixteen patients were scanned because the ophthalmologist had doubts regarding the accuracy of the US measurements. For most of these patients, the correct orientation of the US transducer was hindered by the surrounding anatomy, often the nose, resulting in an oblique 2D cross-section through the tumour which is known to overestimate the tumour thickness.25 For the majority of patients, the MRI provided the confirmation needed to prescribe the appropriate treatment. In other cases, the basal tumour diameter was too large to be visualised entirely in the field of view of the US. As the largest ruthenium shield is 20 mm in diameter, there was, therefore, some uncertainty as to whether the shield could cover the tumour completely. Without the MR-images this could only be verified peri-operatively, whereas with the additional MRI scans the complete tumour could be visualized. This resulted in some tumours being classified for as not being suitable for brachytherapy and these patients received PBT instead.
Importantly, in 4 patients, the MRI measurement changed the treatment modality from enucleation or proton beam therapy which would have within our institution been prescribed based on the ultrasound measurements alone, to radioactive plaque therapy. For one patient, for example, the tumour prominence on US was 7.5mm while on MRI 5.8mm. Although these observations are based on a relatively small number of patients, and a systematic study for a larger group of patients still has to be conducted, we estimate that for approximately one out of ten patients who have tumours which are close to the cut-off size; in this group, a similar change in treatment will be the case.
Finally, two patients received an MRI scan because US was impossible due to previous ocular surgery where silicone oil was used as a tamponade. These patients had been treated for retinal detachment, and as a result of their surgery, part of their eye was filled with a silicon oil. Since ultrasound waves are reflected at the silicon oil–water interface, US imaging of the tumour is highly problematic or not possible. Normally the eyes of these patients need to be enucleated as treatment planning and subsequent follow-up are impossible. With a modified MRI protocol the eyes of these patients could be imaged, which made them eligible for eye-preserving therapy.39
Discussion
Evaluating the Economic Ramifications for Including MRI Scans for UM Patients
In order to provide an analysis of the economic impact of including additional MRI scans for ocular patients, some indication of the total economic cost of vision loss must be made. A large systematic review on the economic burden of visual impairment and blindness showed that the total economic burden per year of severe vision impairment is approximately $13,000 (€10,000).40 In addition, one must estimate the cost of the additional MRI scan. This is highly variable depending upon which country one considers, and different health insurance schemes within an individual country. The USA generally represents the highest healthcare costs, with those in Europe, Asia and Australasia significantly lower. For the purposes of this article, we consider a broad range of €200–€1000.12
The first situation is the one in which MRI provides information which, actually changes the treatment planning as the tumour prominence is close to the maximum prominence for the available type of brachytherapy. For the radiation protocol used at our institute,13 this corresponds to patients for whom the US shows a tumour prominence between 6 and 8.5 mm. In all such cases, we have encountered so far the change in treatment entails substituting ruthenium plaque therapy for the (otherwise) prescribed enucleation or PBT, rather than vice versa. There are no hard guidelines between recommending enucleation and a treatment plan involving proton beam therapy. The choice is complicated also, of course, by the availability of a PBT facility, which has repercussions especially for older patients who may have to incur significant travel and hospitalization expenses and challenges. Based on the estimated cost of the loss of vision, over 5 years the costs of enucleation and PBT are roughly the same (on the order of €30,000 - €50,000). Therefore, it is clear that there is a significant economic benefit to adding an MRI scan for these patients, since the cost of plaque radiotherapy is on the order of €6,000. Furthermore, the patient burden of proton therapy, which involves the surgical placement of tantalum markers on the eye, a CT scan and the delivery of multiple radiation fractions, are much higher than plaque brachytherapy, and therefore the inconvenience of an MRI-scan is far outweighed by the relatively high chance of a less invasive treatment.
The second situation corresponds to patients for whom an US evaluation was impossible (for example, due to the presence of silicon oil). In this case, rather than the normal enucleation, MRI opened the route to possible eye-preserving brachytherapy. Again, in this case, the €6,000 price for brachytherapy is far less than the accumulated economic/societal cost over even a single year due to vision loss.
More difficult to assess quantitatively, but again representing a substantial improvement in patient outcome, are the situations in which PBT is the chosen therapy (either with or without an additional MRI scan). In this case, the MRI scan enables a verification of the estimation of the tumour geometry in all three-dimensions, and the additional safety margins that are built into the radiation planning can be reduced, resulting in a reduction in damage to the healthy part of the eye, as well as other potential side-effects. A similar argument applies for patients who receive brachytherapy: a more accurate delineation of the tumour may allow lower safety margins and a shorter radiation treatment.
For the patients with either a very small (<6mm) or large (>8.5mm) tumour, an MRI scan currently does not add any value in terms of treatment selection, and therefore would not be performed. However, as more advanced MRI techniques, including DWI (Diffusion weighted imaging) and PWI (perfusion weighted imaging), become available for ocular imaging,41 this may change in the future, as these more quantitative measurements might provide a non-invasive method to classify the tumour, for which currently an invasive intraocular biopsy is needed.
Accurate size measurements of the tumour are one of the main determinants of the optimal treatment modality for UM, although the location of the tumour can also limit the applicability of specific types of radiotherapy. Tumour size measurements are conventionally performed with 2D ultrasound imaging. Being a 3D imaging technique, MRI is able to provide a 3D delineation of the geometrical measurements of the tumour volume and offers, therefore, a more accurate description of the tumour dimensions than 2D US. If only the costs of treatment are considered, an additional MRI is cost-effective for patients where there is doubt on the accuracy of US measurements or if the tumour appears to be slightly too large for brachytherapy. Even if in only 5% of these patients, the MRI measurements resulted in a change from PBT to brachytherapy, the additional MRI scan would be economically cost-effective since PBT is much more expensive than brachytherapy. A similar evaluation is true for UM patients who cannot be evaluated with US. For these patients, the MRI results can prevent enucleation, which has an estimated economic burden of up to €10,000 per year due to severe visual impairment. This sum includes all treatment costs including clinic visits, once the treatment is concluded as well as indirect costs including loss of employment.40
Conclusion
We can conclude that an additional MRI for specific patients with UM has an added economic value, as it enables a less expensive treatment for a sufficient percentage of patients to more than compensate for the costs of the MRI. For these patients, the MRI also adds value in terms of quality of care, as it enables for some patients to be afforded a treatment modality that spares more of their vision. For UM patients where the optimal treatment modality is very clear on the US image, an additional MRI currently does not add any value selection.
Funding
This study was supported by the Landelijke Stichting Blinden en Slechtzienden, Utrecht, The Netherlands.
Disclosure
Dr Teresa A Ferreira and Dr. Jan-Willem M Beenakker report grants from Netherlands Organisation for Scientific Research (NWO, Protons4VISION, project number 14654), during the conduct of the study. Dr Jan-Willem M Beenakker reports non-financial support from Philips Healthcare during the conduct of the study. The authors report no other possible conflicts of interest in this work.
References
1. Chattopadhyay C, Kim DW, Gombos DS, et al. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122(15):2299–2312. doi:10.1002/cncr.29727
2. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6(7):1230–1244.
3. GPML. SS. Oxford Textbook of Oncology. United Kingdom Oxford University Press; 2013.
4. Mrazek AA, Chao C. Surviving cutaneous melanoma: a clinical review of follow-up practices, surveillance, and management of recurrence. Surg Clin North Am. 2014;94(5):
5. Aronow ME, Topham AK, Singh AD. Uveal Melanoma: 5-year update on incidence, treatment, and survival (SEER 1973–2013). Ocul Oncol Pathol. 2018;Apr(3):145–151. doi:10.1159/000480640
6. Suteu O, Blaga ML, Nicula F, et al. Incidence trends and survival of skin melanoma and squamous cell carcinoma in Cluj County, Romania. Eur J Cancer Prev. 2017;26 Joining:S176–82. doi:10.1097/CEJ.0000000000000382
7. Hawkins BS. Collaborative ocular melanoma study randomized trial of I-125 brachytherapy. Clin Trials. 2011;8(5):661–673. doi:10.1177/1740774511419684
8. Lin AJ, Rao YJ, Acharya S, Schwarz J, Rao PK, Grigsby P. Patterns of care and outcomes of proton and eye plaque brachytherapy for uveal melanoma: review of the National Cancer Database. Brachytherapy. 2017;16(6):1225–1231. doi:10.1016/j.brachy.2017.07.014
9. Peddada KV, Sangani R, Menon H, Verma V. Complications and adverse events of plaque brachytherapy for ocular melanoma. J Contemp Brachyther. 2019;11(4):392–397. doi:10.5114/jcb.2019.87407
10. Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Ophthalmology. 2020;127(4):S148–S157. doi:10.1001/archopht.124.12.1684
11. Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. 2018;10:1758834018757175. doi:10.1177/1758834018757175
12. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Welp A, Woodbury RB, McCoy MA, Teutsch SM, editors. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington (DC): National Academies Press (US); 2016.
13. Marinkovic M, Horeweg N, Fiocco M, et al. Ruthenium-106 brachytherapy for choroidal melanoma without transpupillary thermotherapy: similar efficacy with improved visual outcome. Eur J Cancer. 2016;68:106–113. doi:10.1016/j.ejca.2016.09.009
14. Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002;109(12):2197–2206. doi:10.1016/S0161-6420(02)01277-0
15. Simpson ER, Gallie B, Laperrierre N. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13(1):1–14. doi:10.1016/j.brachy.2013.11.008
16. Sikuade MJ, Salvi S, Rundle PA, Errington DG, Kacperek A, Rennie IG. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye (Lond). 2015;29(9):1194–1198. doi:10.1038/eye.2015.109
17. Damato B, Kacperek A, Errington D, Heimann H. Proton beam radiotherapy of uveal melanoma. Saudi J Ophthalmol. 2013;27(3):151–157. doi:10.1016/j.sjopt.2013.06.014
18. Chang MY, McCannel TA. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br J Ophthalmol. 2013;97(7):804–811. doi:10.1136/bjophthalmol-2012-302490
19. Wang Z, Nabhan M, Schild SE, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2013;86(1):18–26. doi:10.1016/j.ijrobp.2012.08.026
20. Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55(4):867–880. doi:10.1016/S0360-3016(02)04200-1
21. Moriarty JP, Borah BJ, Foote RL, Pulido JS, Shah ND. Cost-effectiveness of proton beam therapy for intraocular melanoma. PLoS One. 2015;10(5):e0127814. doi:10.1371/journal.pone.0127814
22. Ares WJ, Tonetti D, Yu JY, Monaco EA, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for uveal metastases: report of three cases and a review of the literature. Am J Ophthalmol. 2017;174:169–174. doi:10.1016/j.ajo.2016.11.009
23. Char DH, Kroll S, Stone RD, Harrie R, Kerman B. Ultrasonographic measurement of uveal melanoma thickness: interobserver variability. Br J Ophthalmol. 1990;74(3):183–185. doi:10.1136/bjo.74.3.183
24. de Graaf P, Goricke S, Rodjan F, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol. 2012;42(1):2–14. doi:10.1007/s00247-011-2201-5
25. Beenakker J-WM, Ferreira TA, Soemarwoto KP, et al. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. MAGMA. 2016;29(3):571–577. doi:10.1007/s10334-016-0529-4
26. Ferreira TA, Saraiva P, Genders SW, M V B, Luyten GPM, Beenakker J-W. CT and MR imaging of orbital inflammation. Neuroradiology. 2018;60(12):1253–1266. doi:10.1007/s00234-018-2103-4
27. de Keizer RJ, Vielvoye GJ, de Wolff-rouendaal D, Kakebeeke-Kemme HM. MRI in eye tumors. Doc Ophthalmol. 1989;73(1):93–100. doi:10.1007/BF00174130
28. Beenakker JWM, van Rijn GA, Luyten GPM, Webb AG. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 2013;26(12):1864–1869. doi:10.1002/nbm.v26.12
29. Lindner T, Langner S, Graessl A, et al. High spatial resolution in vivo magnetic resonance imaging of the human eye, orbit, nervus opticus and optic nerve sheath at 7.0 Tesla. Exp Eye Res. 2014;125:89–94. doi:10.1016/j.exer.2014.05.017
30. Wei W, Jia G, von Tengg-kobligk H, et al. Dynamic contrast-enhanced magnetic resonance imaging of ocular melanoma as a tool to predict metastatic potential. J Comput Assist Tomogr. 2017;41(5):823–827. doi:10.1097/RCT.0000000000000598
31. Daftari IK, Aghaian E, O’Brien JM, Dillon W, Phillips TL. 3D MRI-based tumor delineation of ocular melanoma and its comparison with conventional techniques. Med Phys. 2005;32(11):3355–3362. doi:10.1118/1.2068927
32. Paul K, Graessl A, Rieger J, et al. Diffusion-sensitized ophthalmic magnetic resonance imaging free of geometric distortion at 3.0 and 7.0 T: a feasibility study in healthy subjects and patients with intraocular masses. Invest Radiol. 2015;50(5):309–321. doi:10.1097/RLI.0000000000000129
33. Wezel J, Garpebring A, Webb AG, van Osch MJP, Beenakker J-WM. Automated eye blink detection and correction method for clinical MR eye imaging. Magn Reson Med. 2017;78(1):165–171. doi:10.1002/mrm.26355
34. Berkowitz BA, McDonald C, Ito Y, Tofts PS, Latif Z, Gross J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: eliminating blinking artifacts and demonstrating proof of concept. Magn Reson Med. 2001;46(2):412–416. doi:10.1002/(ISSN)1522-2594
35. Graessl A, Muhle M, Schwerter M, et al. Ophthalmic magnetic resonance imaging at 7 T using a 6-channel transceiver radiofrequency coil array in healthy subjects and patients with intraocular masses. Invest Radiol. 2014;49(5):260–270. doi:10.1097/RLI.0000000000000049
36. Richdale K, Wassenaar P, Teal Bluestein K, et al. 7 Tesla MR imaging of the human eye in vivo. J Magn Reson Imaging. 2009;30(5):924–932. doi:10.1002/jmri.v30:5
37. Kamrava M, Sepahdari AR, Leu K, et al. Quantitative multiparametric MRI in uveal melanoma: increased tumor permeability may predict monosomy 3. Neuroradiology. 2015;57(8):833–840. doi:10.1007/s00234-015-1546-0
38. Paul K, Huelnhagen T, Oberacker E, et al. Multiband diffusion-weighted MRI of the eye and orbit free of geometric distortions using a RARE-EPI hybrid. NMR Biomed. 2018;31(3):e3872. doi:10.1002/nbm.v31.3
39. Jaarsma-Coes MG, Goncalves Ferreira TA, van Haren GR, Marinkovic M, Beenakker J-WM. MRI enables accurate diagnosis and follow-up in uveal melanoma patients after vitrectomy. Melanoma Res. 2019;29(6):655–659. doi:10.1097/CMR.0000000000000568
40. Koberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471. doi:10.1136/bmjopen-2013-003471
41. Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Razek AAKA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35(1):170–175. doi:10.3174/ajnr.A3619
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.