Back to Journals » Cancer Management and Research » Volume 12
The Detection of Plasma Soluble Podoplanin of Patients with Breast Cancer and Its Clinical Signification
Authors Zhu X, Xu M, Zhao X, Shen F, Ruan C, Zhao Y
Received 13 September 2020
Accepted for publication 25 November 2020
Published 23 December 2020 Volume 2020:12 Pages 13207—13214
DOI https://doi.org/10.2147/CMAR.S281785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xinyi Zhu,1,2,* Mengqiao Xu,3,* Xingpeng Zhao,4,* Fei Shen,1,2 Changgeng Ruan,1,2 Yiming Zhao1,2
1Jiangsu Institute of Hematology, Key Laboratory of Thrombosis and Hemostasis of the Ministry of Health, The First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu, People’s Republic of China; 2Collaborative Innovation Center of Hematology, Soochow University, Suzhou 215006, Jiangsu, People’s Republic of China; 3Department of Laboratory Medicine, The Affiliated Municipal Hospital of Taizhou University, Taizhou 318000, Zhejiang, People’s Republic of China; 4Clinical Laboratory Center, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang 471000, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yiming Zhao
Jiangsu Institute of Hematology, Key Laboratory of Thrombosis and Hemostasis of the Ministry of Health, The First Affiliated Hospital of Soochow University, 188 Shizi Street, Suzhou 215006, Jiangsu, People’s Republic of China
Tel + 86-512-67781379
Fax + 86-512-65113556
Email [email protected]
Background: Podoplanin (PDPN) is a type-1 membrane sialoglycoprotein that is expressed in many cancer tumors including breast cancer; nonetheless, its roles in tumor occurrence, development, and metastasis are unclear. In this study, we aimed to investigate the clinical significance of plasma soluble PDPN (sPDPN) levels in patients with breast cancer and its significance in the diagnosis and metastasis.
Materials and Methods: Blood samples from healthy controls (CTL), patients with fibroadenomas of breast (FOB), and breast cancer (pathological type: invasive ductal carcinoma, IDC) were collected. sPDPN levels in the plasma of CTL and patients with FOB and IDC were measured by the ELISA.
Results: The plasma sPDPN levels in IDC patients (159 cases, 22.59± 3.70 ng/mL) were higher than those in FOB patients (50 cases, 8.29± 1.09 ng/mL; P< 0.05) and CTL (100 cases, 1.21± 0.12 ng/mL; P< 0.0001). The sPDPN levels in patients at stage III and stage IV (30.08± 4.66 ng/mL) were higher than in patients at stage I and stage II (11.84± 1.12 ng/mL; P=0.005). The sPDPN levels in patients with high-moderate and moderate differentiation (17.50± 3.02 ng/mL) were lower than those in patients with moderately low and low differentiation (35.73± 4.26 ng/mL; P=0.026). The sPDPN levels in patients with metastasis (30.60± 4.27 ng/mL) were much higher than those in patients without metastasis (13.02± 1.30 ng/mL; P=0.017).
Conclusion: Plasma sPDPN may be used as a new marker for the determination of the clinical stage, differentiation degree, and metastasis status of breast cancer.
Keywords: podoplanin, breast cancer, ELISA, monoclonal antibody
Introduction
Although its mortality has declined, breast cancer is the most common malignant tumor and the second leading cause of cancer-related death among women.1,2 According to available data, about two million new patients were diagnosed with and 0.6 million patients died of breast cancer in 2018. Breast cancer has early age onset and survival of patients is closely associated with their clinical stages, where earlier stage usually implies better outcome. Thus, early diagnosis and accurate determination of disease status are very critical for breast cancer patients. At present, clinical detection methods of breast cancer include primarily imaging, cytology, serum tumor marker detection and histopathology. Histopathology is currently the gold standard for diagnosis. However, it is highly traumatic and challenging to obtain biopsy tissue and small nodules are difficult to diagnose with. Therefore, the search for a reliable marker of breast cancer is of great clinical significance for patients.
Podoplanin (PDPN), a mucin-type transmembrane glycoprotein known as the marker of lymphatic endothelial cells,3 is highly expressed in many types of cancer tissue and cells,4 such as lung cancer,5,6 malignant melanoma,7 osteosarcoma8 and brain gliomas.9 PDPN is the only known endogenous ligand of the C-type lectin-like receptor 2 (CLEC-2) expressed on platelets.10 The binding of tumor cell PDPN to platelet CLEC-2 triggers platelet activation and aggregation.11,12 To date, many studies have shown that the expression of PDPN is related to the malignancy, invasiveness, and metastasis of tumor.7,13,14
Recently, we developed two monoclonal antibodies (mAb) against human PDPN, SZ-163, and SZ-168, and established an enzyme-linked immunosorbent assay (ELISA) to quantitate plasma soluble PDPN utilizing these two mAbs.15 In this study, we examined plasma sPDPN levels in controls (CTL, 100 cases), patients with fibroadenomas of breast (FOB, 50 cases), and breast cancer patients (pathological type: invasive ductal carcinoma, IDC, 159 cases) were measured with the newly established ELISA method to evaluate the correlation between sPDPN and tumor occurrence and metastasis status of breast cancer.
Materials and Methods
Patient Selection and Sample Collection
Patients (159 cases of IDC, 50 cases of FOB and 100 cases of CTL) were selected from the First Affiliated Hospital of Soochow University or Luoyang Central Hospital Affiliated to Zhengzhou University in China.
All the 159 IDC patients were female, aged 25–78 years, with a median age of 58.5 years. Inclusion criteria of IDC patients: all the patients were diagnosed as IDC of the breast by histopathology and had not received anti-tumor treatment before blood collection such as surgery, radiotherapy and chemotherapy. Breast cancer staging was determined according to the TNM staging standard published by the Union for International Cancer Control (UICC).16
All FOB patients were female, aged 23–68 with a median of 52.5. The control group were female with various ABO blood groups, aged 24–72, with a median of 57.
Exclusion criteria: Patients with viral infectious diseases such as hepatitis B, hepatitis C, acquired immune deficiency syndrome (AIDS) and syphilis, immune diseases such as rheumatoid and systemic lupus erythematosus, burn wounds and dysfunction of major organs such as liver and kidney were excluded.
Two milliliter of blood was collected from above patients during 2017–2019 by vein puncture and the blood was stored in tubes containing 3.6 mg of ethylene diaminetetraacetic acid. Plasma was prepared by centrifugation at 3000 rpm for 5 min and the supernatant was stored at −80°C.
Forty tissue samples of invasive breast cancer patients embedded in paraffin were collected in 2018 from the Department of Pathology of the First Affiliated Hospital of Soochow University, China. These patients were diagnosed according to UICC recommendations.16
Mice
Female Babl/c mice (4–6 weeks old) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All the animals were housed in an environment with a temperature of 22±1°C, relative humidity of 50±1%, and a light/dark cycle of 12/12 hr. The CO2 anesthesia was used when hybridoma cells were injected into the mice and ascites were collected through abdominal puncture. Compressed CO2 asphyxiation was used to sacrifice mice.
Antibodies
Hybridoma of SZ-163 and SZ-168, two mouse anti-human PDPN mAbs developed as described previously, were injected into mice sensitized by pristane.15 Ascites were collected 10 days later and were applied to Protein G affinity chromatography. IgG from the ascites was eluted with glycine hydrochloride, pH 2.8–3.0.
Detection of Plasma sPDPN by ELISA
A total of 100 μL of 5μg/mL SZ-163 IgG was coated into each well of a 96-well microtiter plate overnight at 4°C. After washing with PBS-0.05% Tween-20 (PBST) washing buffer, the wells were blocked with PBS containing 2% BSA (w/v) at 37°C for 2–3 hours. Then, 100μL of plasma samples or recombinant human PDPN-Fc (R&D Systems, Minneapolis, MN, USA) were added to the wells and incubated at 37°C for 2 hours. After washing, 100 μL of SZ-168-HRP was added and incubated at 37°C for 1 hour. To detecting the binding of SZ-168-HRP to PDPN, OPD substrate was used according to instruction and the signal was measured with a plate reader.
Any plasma sample was tested 20 times under the same conditions and at the same time (intra-assay), and was tested another 20 times at different times under the same conditions (inter-assay).
Detection of PDPN Expression in Breast Cancer Tissue by Immunohistochemistry (IHC) Staining
All paraffin-embedded tissue samples of 40 invasive ductal cancer patients were cut into 6-μm sections and stained with hematoxylin-eosin (HE) staining according to the kit’s procedure instructions (G1122, Solarbio, Beijing, China). 100μL of mAb against human PDPN, D2-40 (ab77854, Abcam, Cambridge, UK) was used to label podoplanin after dewaxing, hydration, and antigen retrieval. Then the sections were treated with Envision kit and 3,3-diaminobenzidine tetrahydrochloride, and finally, counterstained with hematoxylin. The results were determined by a professional pathologist.
Statistical Analysis
sPDPN levels are described as the mean ± SD. Mann–Whitney U-test and the nonparametric test were used to determine the statistical significance of the results in the levels of sPDPN. *P<0.05 was considered to be statistically significant. All statistical tests were two-sided.
Results
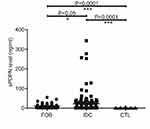
Plasma Levels of sPDPN in IDC, FOB Patients and CTL
As shown in Table 1 and Figure 1, there were significant differences in the sPDPN levels among CTL, FOB, and IDC (P<0.0001). The sPDPN levels in IDC patients were much higher than in FOB patients (22.59±3.70 ng/mL vs 8.29±1.09 ng/mL, P<0.05) and CTL (22.59±3.70 ng/mL vs 1.21±0.12 ng/mL, P<0.0001), and the sPDPN levels in FOB patients were much higher than in CTL (8.29±1.09 ng/mL vs 1.21±0.12 ng/mL, P<0.0001).
 |
Table 1 Plasma sPDPN Levels in FOB, IDC Patients and CTL |
In addition, in our ELISA method, we found that the high sPDPN level’s intra-assay coefficient of variation was 4.66% (0.5566/1.1935), and the inter-assay coefficient of variation was 6.50% (0.07788/1.1985); the low sPDPN level’s intra-assay coefficient of variation was 6.26% (1.37927/22.0475), and the inter-assay coefficient of variation was 9.68% (2.17894/22.513).
Relationship Between Plasma sPDPN Levels and Clinicopathological Parameters in IDC
One hundred fifty-nine cases of IDC were classified according to age, clinical stage, degree of differentiation, tumor size, and other parameters, and then sPDPN levels were statistically analyzed (Table 2).
 |
Table 2 Relationship Between Plasma sPDPN Levels and Clinicopathological Parameters in IDC Patients |
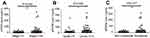
The results showed that the levels of plasma sPDPN in stage III and IV IDC patients (according to Tumor-Node-Metastasis classifications) were higher than those in stage I and II patients (30.08±4.66 ng/mL vs 11.84±1.12 ng/mL, P=0.005, Figure 2A). The levels of plasma sPDPN in IDC patients with high-moderately or moderately differentiated carcinoma were lower than those with moderately poorly or poorly differentiated (17.50±3.02 ng/mL vs 35.73±4.26 ng/mL, P=0.026, Figure 2B). The levels of plasma sPDPN in IDC patients with metastasis were significantly higher than those with non-metastasis (30.60±4.27 ng/mL vs 13.02±1.30 ng/mL, P=0.017, Figure 2C). The levels of plasma sPDPN in IDC patients with human epidermal growth factor receptor 2 (HER2) protein were lower than in those without HER2 protein (12.59±2.56 ng/mL vs 29.40±4.32 ng/mL, P=0.012).
The Diagnostic Value of Plasma sPDPN
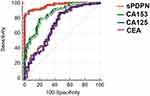
The sPDPN level, as a potential tumor marker, had the largest area under the curve (AUC) in Figure 3 compared with other tumor markers such as cancer antigen 125 (CA125), cancer antigen 153 (CA153), and carcinoembryonic antigen (CEA) in the diagnosis of breast cancer (0.961 vs 0.741, 0.860 and 0.716, respectively). Moreover, sPDPN had the highest sensitivity (88.98%) and specificity (96.00%) among all the markers evaluated (Table 3).
 |
Table 3 Comparison of the Diagnostic Value of sPDPN, CA125, CA153, and CEA for Breast Cancer |
On the other hand, the sPDPN level in the AUC provided no assistance for differentiating benign and malignant tumors nor for identifying benign FOB from the normal population.
The Value of Plasma sPDPN for Cancer Prediction
First, the single factor analysis was carried out using the values of the diagnosed breast cancer as the dependent variable, and age, sPDPN, CA125, CA153, and CEA as the independent variables. Next, multivariate analysis was performed to identify indicators with statistical significance (Table 4). The results of multivariate analysis showed that sPDPN and CA153 were independent predictors of breast cancer, especially if adjusted for other confounding factors, with sPDPN having the highest odds ratio (OR) value of 4.024 (P<0.001).
 |
Table 4 Logistic Regression Analysis of Independent/Related Indexes of Breast Cancer |
HE Staining and IHC Analysis by D2-40 of Breast Cancer Tissues
The tumor tissue was confirmed by HE staining (Figure 4A), indicated by large and deformed cell nucleus, proliferated interstitial fibrous tissue, and visible infiltration of inflammatory cells. PDPN expression in tumor tissues was confirmed by D2-40 staining (Figure 4B and C). Based on cell morphology, we suggested the positive tumor stroma was cancer-associated fibroblasts (CAFs).
Relationship Between D2-40 Staining in Breast Tumor Tissue and Clinicopathological Parameters
A total of 40 IDC patients were classified according to age, clinical stage, degree of differentiation, lymph node metastasis status and other parameters. Statistical analysis was then performed for the positive rate of D2-40 staining in cancer tissues. The results showed that the positive rate was much higher in groups of stage III and IV, lymph node metastasis, negative estrogen receptor and negative progesterone receptor than that of the counter group. The P values are: clinical stage, P=0.022; lymph node metastasis, P=0.042; estrogen receptor, P=0.010, progesterone receptor, P=0.037 (Table 5). The sPDPN levels in the positive D2-40 staining group were significantly higher than those in the negative staining group (23.74±2.71 ng/mL vs 21.09±1.89 ng/mL, P=0.003). The Ki-67 labeling rate in the positive D2-40 staining group was also higher than that in the counter group (57.14±12.54% vs 21.54±8.86%, P<0.001).
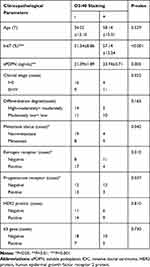 |
Table 5 Comparison of D2-40 Staining in Various IDC Patient Groups Classified with Clinical Parameters |
Discussion
Podoplanin, a mucin-type membrane glycoprotein rich in O-glycoside chains,17 is mainly expressed on the lymphatic endothelium,18 pneumocytes,19 and glomerular podocytes20 and plays essential roles in the development of lymphatic vessels,21,22 cerebrovascular system formation and maintenance of its integrity,23 as well as in the promotion of natural regulatory T cells.24 Besides normal tissues and cells, PDPN is also found in human inflammatory diseases25–27 and various cancer tissues28 indicted by D2-40 staining technique or flow cytometry analysis. Previous studies have suggested that PDPN is not only expressed in cancer cells,8 but also in cancer stroma,9 especially in fibroblasts.4 After the surface plasmon resonance imaging biosensor was applied for the detection of sPDPN in the serum sample,29 our lab established an efficient ELISA method with that we successfully and conveniently detected sPDPN in human plasma.15 In this study, we found that the sPDPN levels in IDC patients (22.59±3.70 ng/mL) were significantly higher than those in FOB patients (8.29±1.09 ng/mL; P<0.05) and CTL (1.21±0.12 ng/mL; P<0.0001). In addition, expression of PDPN may be correlated to metastasis,30,31 as we observed that patients of later clinical-stage, poor differentiation, lymph node metastasis, and negative HER2 expression had higher level of sPDPN. sPDPN may be proteolytically cleaved from the extracellular domain and enter the blood circulation. Alternatively, it can be secreted by tumor cells and stromal cells as a full-length protein attached to extracellular vesicles.32
Furthermore, compared with CA125, CA153, and CEA, sPDPN had the best diagnostic value in IDC patients (AUC=0.961, sensitivity=88.98%, specificity=96.00%) as well as was an independent predictor of breast cancer with OR = 4.024. CAF has been reported as a sign of breast cancer recurrence.33 Our IHC results are consistent with that finding suggesting that infiltration of positive PDPN CAFs can predict poor prognosis.34,35 The expression of PDPN (probably in tumor stroma) was detected in the surgical specimens of 15 out of 40 IDC patients, especially in the patients of late clinical stage who had lymph node metastasis, high expression of Ki67 and sPDPN, and were estrogen receptor- and progesterone receptor-negative. Recently, many studies have shown that the PDPN expressed on macrophages36 and CAFs37 could promote the metastasis of breast cancer in vivo and in vitro. We detected the elevated sPDPN in the plasma of breast cancer patients for the first time. We believe that sPDPN is a reliable indicator of breast cancer with relatively poor prognosis.
In the further, we will expand the number of specimens to explore the value of PDPN in differentiating benign and malignant tumors. Meanwhile, we will intend to establish a suitable animal breast cancer model to investigate the role of podoplanin in the occurrence, development and metastasis of breast cancer, so as to promotes PDPN as a breast cancer screening index. Furthermore, we will investigate whether SZ-168, the antibody against PDPN, could inhibit breast cancer progress and thus a potential therapeutic candidate for breast cancer treatment.
Conclusion
Plasma sPDPN levels in patients with breast cancer and PDPN expression in the tumor stroma have a certain evaluation predictive value for breast cancer patients’ general clinical status including clinical stage, degree of differentiation, and metastasis. Plasma sPDPN could be used as a new tumor marker for the diagnosis, clinical stage, degree of differentiation, and metastasis of breast cancer.
Ethics Approval and Consent to Participate
This study conformed to the ethical guidelines of the 2004 Declaration of Helsinki and was approved by the Institutional Ethics Committee at the First Affiliated Hospital of Soochow University, China (2017.11.01), and by the Institutional Ethics Committee at the Luoyang Central Hospital Affiliated to Zhengzhou University, China (2017.11.01). Informed consent was obtained from all healthy control individuals and patients.
The animal studies were also approved by the Animal Use and Ethics Committee of Soochow University (Suzhou, China) (2017.11.06). All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of Soochow University (Suzhou, China) institutional animal care and conducted according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Institutional Animal Care and Use Committee (IACUC) guidelines.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81873431) and Jiangsu province Natural Science Foundation (BK20181164).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Winters S, Martin C, Murphy D, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1–32.
3. Ugorski M, Dziegiel P, Suchanski J. Podoplanin – a small glycoprotein with many faces. Am J Cancer Res. 2016;6(2):370–386.
4. Quintanilla M, Montero-Montero L, Renart J, et al. Podoplanin in inflammation and cancer. Int J Mol Sci. 2019;20(3):707. doi:10.3390/ijms20030707
5. Miyata K, Takemoto A, Okumura S, et al. Podoplanin enhances lung cancer cell growth in vivo by inducing platelet aggregation. Sci Rep. 2017;7(1):4059. doi:10.1038/s41598-017-04324-1
6. Nakamura H, Sugano M, Miyashita T, et al. Organoid culture containing cancer cells and stromal cells reveals that podoplanin-positive cancer-associated fibroblasts enhance proliferation of lung cancer cells. Lung Cancer. 2019;134:100–107. doi:10.1016/j.lungcan.2019.04.007
7. Xu M, Wang X, Pan Y, et al. Blocking podoplanin suppresses growth and pulmonary metastasis of human malignant melanoma. BMC Cancer. 2019;19(1):599. doi:10.1186/s12885-019-5808-9
8. Kunita A, Kashima TG, Ohazama A, et al. Podoplanin is regulated by AP-1 and promotes platelet aggregation and cell migration in osteosarcoma. Am J Pathol. 2011;179(2):1041–1049. doi:10.1016/j.ajpath.2011.04.027
9. Eisemann T, Costa B, Peterziel H, et al. Podoplanin positive myeloid cells promote glioma development by immune suppression. Front Oncol. 2019;9:187. doi:10.3389/fonc.2019.00187
10. Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129(1):12–23. doi:10.1172/JCI122955
11. Mir Seyed Nazari P, Riedl J, Pabinger I, et al. The role of podoplanin in cancer-associated thrombosis. Thromb Res. 2018;164(Suppl 1):S34–S39. doi:10.1016/j.thromres.2018.01.020
12. Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res. 2012;129(Suppl 1):S30–S37. doi:10.1016/S0049-3848(12)70013-0
13. Krishnan H, Miller WT, Blanco FJ, et al. Src and podoplanin forge a path to destruction. Drug Discov Today. 2019;24(1):241–249. doi:10.1016/j.drudis.2018.07.009
14. Eisemann T, Costa B, Harter PN, et al. Podoplanin expression is a prognostic biomarker but may be dispensable for the malignancy of glioblastoma. Neuro Oncol. 2019;21(3):326–336. doi:10.1093/neuonc/noy184
15. Zhao X, Pan Y, Ren W, et al. Plasma soluble podoplanin is a novel marker for the diagnosis of tumor occurrence and metastasis. Cancer Sci. 2018;109(2):403–411. doi:10.1111/cas.13475
16. Amin MB, Edge SB, Green FL, et al. AJCC Cancer Staging Manual.
17. Fujita N, Takagi S. The impact of Aggrus/podoplanin on platelet aggregation and tumour metastasis. J Biochem. 2012;152(5):407–413. doi:10.1093/jb/mvs108
18. Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154(2):385–394. doi:10.1016/S0002-9440(10)65285-6
19. Ramirez MI, Millien G, Hinds A, et al. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256(1):61–72. doi:10.1016/S0012-1606(02)00098-2
20. Song K, Fu J, Song J, et al. Loss of mucin-type O-glycans impairs the integrity of the glomerular filtration barrier in the mouse kidney. J Biol Chem. 2017;292(40):16491–16497. doi:10.1074/jbc.M117.798512
21. Schacht V, Ramirez MI, Hong YK, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22(14):3546–3556. doi:10.1093/emboj/cdg342
22. Uhrin P, Zaujec J, Breuss JM, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115(19):3997–4005. doi:10.1182/blood-2009-04-216069
23. Lowe KL, Finney BA, Deppermann C, et al. Podoplanin and CLEC-2 drive cerebrovascular patterning and integrity during development. Blood. 2015;125(24):3769–3777. doi:10.1182/blood-2014-09-603803
24. Fuertbauer E, Zaujec J, Uhrin P, et al. Thymic medullar conduits-associated podoplanin promotes natural regulatory T cells. Immunol Lett. 2013;154(1–2):31–41. doi:10.1016/j.imlet.2013.07.007
25. Honma M, Minami-Hori M, Takahashi H, et al. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J Dermatol Sci. 2012;65(2):134–140. doi:10.1016/j.jdermsci.2011.11.011
26. Peters A, Pitcher LA, Sullivan JM, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. doi:10.1016/j.immuni.2011.10.015
27. Miyamoto Y, Uga H, Tanaka S, et al. Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol. 2013;54(2):199–207. doi:10.1016/j.molimm.2012.11.013
28. Krishnan H, Rayes J, Miyashita T, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 2018;109(5):1292–1299. doi:10.1111/cas.13580
29. Sankiewicz A, Guszcz T, Mena-Hortelano R, et al. Podoplanin serum and urine concentration in transitional bladder cancer. Cancer Biomark. 2016;16(3):343–350.
30. Matsuoka A, Mizumoto Y, Ono M, et al. Novel strategy of ovarian cancer implantation: pre-invasive growth of fibrin-anchored cells with neovascularization. Cancer Sci. 2019;110(8):2658–2666. doi:10.1111/cas.14098
31. Kim HM, Jung WH, Koo JS. Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: an immunohistochemical analysis. J Transl Med. 2015;13:222. doi:10.1186/s12967-015-0587-9
32. Martín-Villar E, Yurrita MM, Fernández-Muñoz B, et al. Regulation of podoplanin/PA2.26 antigen expression in tumour cells. Involvement of calpain-mediated proteolysis. Int J Biochem Cell Biol. 2009;41(6):1421–1429. doi:10.1016/j.biocel.2008.12.010
33. Li J, Yang C, Yang J, Zou L. Down-regulation of CCL17 in cancer-associated fibroblasts inhibits cell migration and invasion of breast cancer through ERK1/2 pathway. Cancer Manag Res. 2019;11:7439–7453. doi:10.2147/CMAR.S211651
34. Schoppmann SF, Berghoff A, Dinhof C, et al. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134(1):237–244. doi:10.1007/s10549-012-1984-x
35. Pula B, Jethon A, Piotrowska A, et al. Podoplanin expression by cancer-associated fibroblasts predicts poor outcome in invasive ductal breast carcinoma. Histopathology. 2011;59(6):1249–1260. doi:10.1111/j.1365-2559.2011.04060.x
36. Bieniasz-Krzywiec P, Martín-Pérez R, Ehling M, et al. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metab. 2019;30(5):917–936.e10. doi:10.1016/j.cmet.2019.07.015
37. Suchanski J, Tejchman A, Zacharski M, et al. Podoplanin increases the migration of human fibroblasts and affects the endothelial cell network formation: a possible role for cancer-associated fibroblasts in breast cancer progression. PLoS One. 2017;12(9):e0184970. doi:10.1371/journal.pone.0184970
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.