Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 7
The deleterious effects of arteriovenous fistula-creation on the cardiovascular system: a longitudinal magnetic resonance imaging study
Authors Dundon B, Torpey K, Nelson A, Wong D, Duncan R, Meredith I, Faull R, Worthley S, Worthley M, Torpey D
Received 17 April 2014
Accepted for publication 15 May 2014
Published 16 September 2014 Volume 2014:7 Pages 337—345
DOI https://doi.org/10.2147/IJNRD.S66390
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Benjamin K Dundon,1–3 Kim Torpey,3 Adam J Nelson,1 Dennis TL Wong,1,2 Rae F Duncan,1 Ian T Meredith,2 Randall J Faull,1,3 Stephen G Worthley,1,4 Matthew I Worthley1,4
1Cardiology Department, Royal Adelaide Hospital, Central Adelaide Local Health Network, Discipline of Medicine, University of Adelaide, Adelaide, SA, Australia; 2Monash Cardiovascular Research Centre, MonashHEART, Monash Health, Melbourne, Vic, Australia; 3Central Northern Renal and Transplantation Service, Royal Adelaide Hospital, Central Adelaide Local Health Network, Adelaide, SA, Australia; 4South Australian Health and Medical Research Institute, Adelaide, SA, Australia
Aim: Arteriovenous fistula-formation remains critical for the provision of hemodialysis in end-stage renal failure patients. Its creation results in a significant increase in cardiac output, with resultant alterations in cardiac stroke volume, systemic blood flow, and vascular resistance. The impact of fistula-formation on cardiac and vascular structure and function has not yet been evaluated via "gold standard" imaging techniques in the modern era of end-stage renal failure care.
Methods: A total of 24 patients with stage 5 chronic kidney disease undergoing fistula-creation were studied in a single-arm pilot study. Cardiovascular magnetic resonance imaging was undertaken at baseline, and prior to and 6 months following fistula-creation. This gold standard imaging modality was used to evaluate, via standard brachial flow-mediated techniques, cardiac structure and function, aortic distensibility, and endothelial function.
Results: At follow up, left ventricular ejection fraction remained unchanged, while mean cardiac output increased by 25.0% (P<0.0001). Significant increases in left and right ventricular end-systolic volumes (21% [P=0.014] and 18% [P<0.01]), left and right atrial area (11% [P<0.01] and 9% [P<0.01]), and left ventricular mass were observed (12.7% increase) (P<0.01). Endothelial-dependent vasodilation was significantly decreased at follow up (9.0%±9% vs 3.0%±6%) (P=0.01). No significant change in aortic distensibility was identified.
Conclusion: In patients with end-stage renal failure, fistula-formation is associated with an increase in cardiac output, dilation of all cardiac chambers and deterioration in endothelial function.
Keywords: cardiac, cardiovascular disease, vascular biology
Introduction
Hemodialysis (HDx) remains the cornerstone of renal replacement therapy for end-stage renal failure (ESRF) patients worldwide. However its creation has a number of adverse hemodynamic consequences. Immediately following creation, arteriovenous fistula (AVF) is associated with an increase in cardiac output (CO), achieved predominantly through a reduction in systemic vascular resistance, increased myocardial contractility, and an increase in stroke volume (SV) and heart rate.1 Over the following week, circulating blood volume increases in conjunction with increases in atrial and brain natriuretic peptides.2,3 These alterations are associated with early increases in left ventricular (LV) filling pressure with the potential for resultant impact on atrial and ventricular chamber dimensions and function.2
To maintain sufficient systemic perfusion, CO must increase proportionately, resulting in longer-term implications for cardiac and vascular structure and function.3–5 Historically, AVF-creation has been associated with worsening of LV hypertrophy and resultant diastolic dysfunction and left atrial dilatation.2,6 In some patients, a giant AVF may lead to progressive high-output cardiac failure, despite preserved LV systolic function.7–9 To date, many of the studies evaluating the cardiovascular (CV) effects of AVF have used an echocardiographic methodology.
CV magnetic resonance (CMR) imaging has been recognized as the modern “gold standard” for the evaluation of cardiac structure and function. Assessing, not only cardiac, but also, vascular structure and function, CMR has substantially greater accuracy and reproducibility for the assessment of cardiac indices compared with alternative methodologies, such as echocardiography.10–16 Importantly, CMR can assess aortic stiffness, which has proven prognostic significance in patients with chronic kidney disease (CKD).14,17,18 Furthermore, this imaging modality can assess peripheral arterial diameter and flow – allowing the serial evaluation of alterations in brachial artery flow induced by distal AVF-formation, as well as evaluation of peripheral artery endothelial function – by a standard flow-mediated dilatation technique. Thus, CMR is able to provide accurate, reproducible, and comprehensive assessments of cardiac and vascular structure and function within a single investigation but has not been previously utilized for evaluating the impact of AVF-creation on cardiac and vascular function.
This study sought to evaluate the effects of AVF-creation on CV structure and function in the modern era of ESRF management, utilizing recent advances in noninvasive CMR imaging.
Methods
This study was approved by the Ethics of Human Research Committee of the Royal Adelaide Hospital. Written informed consent was obtained from all patients prior to study entry.
Patients aged >18 years with advanced stage 5 CKD were recruited prior to clinically driven, elective AVF-creation for anticipated HDx commencement in the subsequent 6–12months. Patients with previous AVF-creation, renal replacement therapy, or deteriorating renal dysfunction anticipated to require HDx within the following 6 months were excluded. Patients with significant preexisting valvular heart disease or contraindications to magnetic resonance imaging were also excluded.
Study investigations
Following informed consent, all subjects were scheduled for elective surgical AVF-formation, as per institutional clinical practice. Clinical history and study CMR evaluation of baseline CV structure and function were scheduled to occur 2 weeks prior to the elective surgical date. At 6 months following elective AVF-formation, repeat clinical history and the identical CMR protocol were performed, to evaluate alterations in CV structure and function following AVF-formation and maturation in the context of declining renal function.
CMR protocol
All CMR studies were performed utilizing a Siemens Magnetom® Avanto 1.5 Tesla magnetic resonance imaging scanner (Siemens AG, Erlangen, Germany).
Cardiac protocol
Cardiac structure and function studies were undertaken as previously described.19–21 In summary, retrospectively electrocardiogram (ECG)-gated true fast imaging with steady-state free precession (TrueFISP) CMR sequences were performed during expiratory breath holding, to assess atrial area and ventricular structure and function (25 phases per cardiac cycle; TR 2.7 ms, TE 1.4 ms, flip angle 60°). Long-axis reference views were used for positioning 8–12 perpendicular LV short-axis slices from the level of the mitral valve to the LV apex. The short axis section thickness was 6 mm, with short-axis intersection intervals of 4 mm.
Offline analysis was performed to determine diastolic and systolic cardiac chamber dimensions and diastolic LV mass, utilizing proprietary software (Argus Software, Houston, TX, USA) and standard methodology.19,20 For the right ventricular (RV) and LV data set, short-axis endocardial and epicardial contours were manually traced in end diastole (start of the R wave) and end systole. The LV and RV end-diastolic and end-systolic cavity surface areas were summed to obtain volumes, with a standard Simpson’s rule used to evaluate the ejection fraction, as previously described.21 The LV and RV end-diastolic volumes, end-systolic volumes, SVs, ejection fractions, CO, and LV mass, indexed to body surface area, were thus derived via standard equations. Minimal intra- and interobserver variability exists in these chamber measurements, as previously published by our group.21
Vascular protocol
Aortic distensibility
Immediately following completion of the cardiac protocol, ECG-gated TrueFISP cine sequences were acquired of the ascending aorta (AA), descending thoracic aorta, and proximal abdominal aorta, in a sagittal oblique orientation. Utilizing the midpoint of the right pulmonary artery as the reference level in each patient, a perpendicular cross-sectional TrueFISP cine image was then taken at the AA and the proximal descending aorta (PDA). A final TrueFISP cine image was then acquired at the level of the distal descending aorta (DDA), 5–10 cm distal to the diaphragm, as previously described.15
Brachial artery blood pressure was taken concurrently, using a magnetic resonance imaging–compatible automated noninvasive sphygmomanometer, with the results of three measurements averaged.
Aortic distensibility was then calculated using the following equation
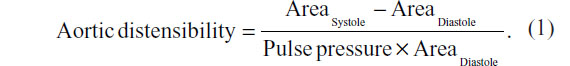
Measures of aortic distensibility were recorded at each location individually, and then the three measurements were averaged, for an indicative average aortic distensibility for each subject. Minimal intra- and interindividual variability exists in our laboratory for this measurement (1% and 2% respectively).15
Brachial artery blood flow – AVF arm
Brachial artery blood flow was then quantified in the AVF arm, utilizing a dedicated velocity-encoded sequence optimized for peak brachial artery flow velocity. Analysis of brachial artery flow was performed offline, utilizing proprietary software (Argus Software), at baseline and 6 months following AVF surgery.
Brachial artery flow-mediated dilatation
Brachial artery cross-sectional area and blood flow was then measured in the arm contralateral to the AVF. Utilizing validated techniques, endothelium-dependent endothelial function was assessed using a brachial artery flow-mediated vasodilation technique.22–24 Following a 10-minute period of rest, endothelium-independent function was assessed at the identical arterial location, following response to sublingual glyceryl trinitrate,24 and images analyzed offline.
Statistical methods
All measures were tested for normality using the Kolmogorov–Smirnov/Lilliefors Test. All normally distributed values were represented as mean ± standard deviation. Parameters that were deemed nonparametrically distributed were reported as median and interquartile range (IQR). Paired parameters were analyzed by two-tailed paired Student’s t-test. Where normally distributed, comparison of nonpaired measures was performed by unpaired t-test or one-way analysis of variance (ANOVA), as appropriate. Where parameters were not normally distributed, paired analyses were performed utilizing the Wilcoxon matched pairs test, and nonpaired analyses were performed using the Mann–Whitney U test. Correlation between parameters was performed, using a Pearson’s correlation for normally distributed samples, and Spearman’s correlation for nonparametric data. Comparison of binary test outcomes pre- and postsurgery was performed using Fisher’s exact test. Significance was predetermined as P<0.05.
Results
A total of 24 patients were evaluated at baseline and 6 months following successful AVF-creation (age 59±12 years, 58% male) (Table 1). The average time from surgery to follow-up scan was 198±32 days. No patients had commenced HDx at the time of follow up.
  | Table 1 Subject baseline characteristics |
Clinical and serological indices at 6 months were predominantly unchanged following AVF-creation (weight 80±19 kg vs 78±17 kg) [P=0.07]; systolic blood pressure 146±19 mmHg vs 146±17 mmHg [P=0.96]; Hb 115±12 g/dL vs 119±15 g/dL [P=0.26]). All subjects were undergoing treatment with erythropoietin (EPO)-analogues at baseline and at follow up. Notably, resting heart rate was significantly increased at follow up compared with baseline (71±12 bpm vs 76±11 bpm [P=0.008]). Serum creatinine increased significantly 6 months following study enrolment, as would be expected clinically (529±91 μmol/L vs 652±123 μmol/L [P=0.0006]).
Cardiac results
In the presence of advanced CKD, baseline imaging in our cohort revealed some interesting observations compared with previously published controls.25,26 Preserved LV systolic function (LV ejection fraction [LVEF] 72%±7%), with normal LV end-diastolic volumes (149±31 mL) and CO (7.5±1.5 L/min), was observed. Despite the high prevalence of hypertension, LV mass was within normal limits at baseline (132±33 g). The left atrial area was mildly increased across the cohort at baseline (27±6 cm2), with the right atrial area at the upper limits of the normal range (23±6 cm2).
Substantial alterations in cardiac structure and function were identified at follow up, as shown in Table 2 and both Figures 1 and 2.
Notably, 62.5% of subjects had CMR measures for LVEF, LV end-diastolic volume, LV end-systolic volume, LVSV, and LV mass index within normal limits prior to AVF-creation; however, this had reduced to 41.7% of subjects, at 6 months following AVF-creation (P=0.24).
Vascular results
Aortic distensibility
Aortic distensibility was not significantly altered by the creation of a surgical AVF at the 6-month follow-up scan (2.3±1.2 mmHg−1 vs 2.2±1.2 mmHg−1 [P=0.59]). AA, PDA, DDA, and average aortic distensibility ([AA + PDA + DDA]/3) were all numerically, but not statistically significantly, lower following AVF-creation.
Prior to AVF-creation, baseline aortic distensibility decreased progressively from the DDA to the AA, consistent with the known regional increase in aortic stiffness (reduced aortic compliance) in the more proximal aortic segments15 (AA 1.41±1.0 mmHg−1 vs PDA 2.20±1.3 mmHg−1 [P=0.023]; AA 1.41±1.0 mmHg−1 vs DDA 3.20±2.0 mmHg−1 [P=0.0003]; PDA 2.20±1.3 mmHg−1 vs DDA 3.20±2.0 mmHg−1 [P=0.046]). These findings remained similar following AVF-creation.
Brachial artery flow-mediated dilatation
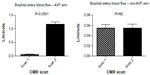
A total of 21 subjects completed the full endothelial function protocol at both scans, with a summary of these findings shown in Figure 3. In these subjects, brachial artery endothelium-dependent vasodilatation, as measured by flow-mediated dilatation, was markedly reduced at 6-month follow-up imaging following AVF-creation (9.0%±9.2% vs 3.0%±5.7% [P=0.012]). Endothelium-independent vasodilatation, in response to glyceryl trinitrate, was unaffected by AVF-creation in the subjects evaluated (13.3%±9.1% vs 12.2%±10.0% [P=0.74]). Notably, in a subpopulation of 17 subjects where this sequence was performed, there was no significant change in baseline brachial artery blood flow in the arm contralateral to the AVF (ie, endothelial function study arm) (0.054±0.03 L/minute vs 0.055±0.03 L/minute [P=0.97]) (Figure 4). The baseline cross-sectional brachial artery area was unchanged across the study cohort between baseline and follow-up scans (26.5±8 mm2 vs 27.7±7 mm2 [P=0.31]), negating alterations in baseline flow and brachial area characteristics as the cause of these endothelial function changes.27
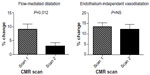  | Figure 3 Alterations in peripheral endothelial function following AVF-creation. |
There was no significant correlation between percent change in flow-mediated dilatation and percent change in AVF flow (r=0.17, P=0.52), percent change in LV CO (r=−0.29, P=0.24), or percent change in LVSV (r=−0.27, P=0.28).
Brachial artery blood flow – arteriovenous fistula arm
As expected, brachial artery blood flow on the side of the AVF was substantially increased 6-months following AVF-creation (0.057±0.04 L/minute vs 1.158±0.44 L/minute [P<0.0001]) (Figure 4). This represents a more than 20-fold increase in mean ipsilateral brachial artery blood flow 6 months following AVF-creation. Notably, there was no significant association between increases in the AVF-arm brachial artery blood flow and adaptive change in LV CO (r=0.09, P=0.71) or LVSV (r=0.13, P=0.59), implicating a complex systemic circulatory adaptation to AVF-creation.
Discussion
As demonstrated by this single-arm pilot study, elective AVF-creation is associated with substantial alterations in cardiac structure and function, necessary to accommodate the increase in CO sufficient to service the fistula, whilst maintaining a homeostatic systemic supply. These changes include biatrial and biventricular dilatation, increased ventricular SV and CO, as well as a clinically significant increase in LV mass. Our study has been the first to systemically evaluate right and left, ventricular and atrial parameters along with measures of vascular function following AVF-creation, via gold standard imaging assessment utilizing CMR. In our study, this adaptive cardiac remodeling, coupled with a >2,000% increase in brachial artery blood flow ipsilateral to the newly created AVF, was also associated with a marked reduction in remote peripheral endothelial function. Although necessary for the provision of HDx, it is striking that an elective, purposeful medical intervention could cause such widespread maladaptation within the CV system of already high-risk individuals.
Previous transthoracic echocardiogram studies have demonstrated the potential for increased LV mass and LV dilatation following AVF-creation.2,6 Parfrey et al have previously demonstrated the negative prognostic implications of concentric LV hypertrophy, LV dilatation, and LV dysfunction, as measured by echocardiography in ESRF patients commencing dialysis.4 Furthermore, London et al have previously demonstrated a marked prognostic advantage to therapeutic interventions in ESRF that successfully reduce LV mass by at least 10%.28 Such measures were associated with a 22% reduction in all-cause mortality (relative risk [RR] 0.78, 95% confidence interval [CI] 0.63–0.92, P=0.0012) and a 28% reduction in CV event-free survival (RR 0.72, 95% CI 0.51–0.90, P=0.0016).28
In the present study, we demonstrated a 17.2% increase in mean LV end-diastolic volume and a 12.7% increase in mean LV mass 6 months following AVF-creation. Although beyond the scope of this observational study, such cardiac adaptations may thus be postulated to impact deleteriously on CV morbidity and mortality. Furthermore, recognition of the impact of a 25% increase in mean CO at 6 months following AVF-creation may be an important factor in the clinical management of predialysis patients, despite this being rarely reported using conventional cardiac imaging modalities.
Likewise, RV structure and function have historically been neglected in the evaluation of cardiac structure and function in this cohort. Due to its unusual shape and anterior location, transthoracic echocardiography provides only a limited evaluation of RV size and systolic function. Utilizing CMR, we demonstrated substantial alterations in RV structure and function following AVF-creation. Although intuitive, such findings are important to appreciate, given the known association of AVF-creation and the development of clinically significant pulmonary hypertension in ESRF patients. The need for substantial increases in pulmonary blood flow related to increased CO, coupled with demonstrable increases in LV mass and left atrial dilatation, provides a mechanistic cardiac contribution to previous observations of elevated pulmonary arterial pressure in the ESRF context.29 Furthermore, it is conceivable that AVF-creation may be associated with endothelial dysfunction in the pulmonary vascular bed, as seen peripherally in our study. The combination of alterations in cardiac structure and function associated with AVF-creation demonstrated in this study and the possible contribution of pulmonary vascular endothelial dysfunction could contribute to the increase in pulmonary arterial pressure in ESRF, with resultant clinical implications. The role of subclinical RV remodeling and dysfunction in the exercise intolerance of ESRF remains to be determined, but the results from this study provide an important link in this line of enquiry.
Left atrial size is more commonly increased in ESRF in comparison with the general community.30 Left atrial dilatation has previously been identified as an independent risk factor for the development of atrial fibrillation in ESRF and may contribute to the poor CV prognosis of patients with CKD.31,32 Furthermore, whether through the predisposition to AF or as a marker of chronic LV diastolic impairment, recent evidence confirms an association between LA dilatation and increased mortality in ESRF patients with LV hypertrophy.32–34 Our study would support the conclusion that changes in atrial dimension following AVF-creation may play a significant role in the promotion of atrial arrhythmia within the ESRF milieu.
Endothelial dysfunction is known to be a critical precondition to the development of atherosclerosis and subsequent CV sequelae.35 CKD provides a particularly complex precipitant for endothelial dysfunction, resulting from multiple circulating factors injurious to the endothelium and promotional of the necessary proinflammatory setting for endothelial dysfunction and accelerated atherosclerosis.36 Notably, impairment in forearm flow-mediated dilatation has been shown to be associated with an increase in all-cause mortality in ESRF37 but has been shown to improve following transplantation, providing a valuable, modifiable marker of vascular structure and function in response to therapeutic interventions in CKD.
In this study, we found a clinically significant reduction in brachial artery endothelial function 6 months following AVF-creation. Such a finding strongly implicates a mechanistic trigger to remote endothelial dysfunction from the increased blood flow and vascular shear stress in the AVF arm, as well as that induced by the compensatory increase in systemic CO required to service the fistula. Furthermore, such findings occurred in the absence of detectable changes in baseline brachial artery blood flow or cross-sectional area in the studied arm. Such findings implicate AVF-creation in the promotion of the complex proatherogenic milieu of progressive CKD and ESRF, and such conduits may contribute significantly to the development of accelerated cardiovascular disease associated with this condition.
No significant alteration was seen in aortic distensibility at 6 months following AVF-creation, although a consistent numerical fall in distensibility was noted at each of the aortic levels assessed. Such a finding may of course be a chance observation but provides substrate for the hypothesis that 6 months is too short a follow-up period to identify chronic alterations in central vascular stiffness in response to AVF-creation in the context of chronic uremia.
Although similarities exist between our study design and that of other published studies evaluating the cardiac effects of AVF-creation,2,6 the most significant limitation of the current study is the absence of a control group. While supply of a control cohort in such a study, clinically requiring a fistula for dialysis, would raise a number of significant ethical issues, confirmation of these findings would need to be undertaken in a randomized control trial setting. While acknowledging this pilot study is hypothesis-generating, it could provide data to adequately power a randomized control study. Such a study would provide mechanistic impetus to undertake a clinical trial to evaluate CV outcomes in subjects who had early vs delayed AVF-creation in ESRF.
Additionally, although values for the majority of clinically relevant parameters evaluated remained stable between the study investigations, the progressive decline in renal function witnessed cannot be excluded as a potential contributor to the CV alterations demonstrated. Exploring further mechanistic contributors to our findings, ie, measures of oxidative stress to explain endothelial function results, would also add to the observational findings of the study.
This pilot study suggests that elective AVF-creation in predialysis CKD is associated with significant increases in left and right atrial and ventricular chamber volumes and LV mass, commensurate with the requirement for a 25% mean increase in CO. Furthermore, AVF-creation is associated with deterioration in systemic endothelial function. Such alterations in CV structure and function may contribute to the poor health outcomes observed in this cohort.
Acknowledgments
The authors would like to sincerely thank Ms Kerry Williams and Mr Ben Koschade for their tireless assistance in the performance of the CMR scans for this study and Mr Angelo Carbone for his consistent contribution to the research team.
Dr BK Dundon was supported by a National Health and Medical Research Council (NHMRC)/National Heart Foundation of Australia (NHFA) Post-graduate Research Scholarship and a Cardiac Society of Australia and New Zealand (CSANZ) Post-graduate Research Scholarship. Dr DTL Wong was supported by an NHMRC/NHFA Post-graduate Research Scholarship. Associate Professor MI Worthley is supported by a South Australia Health Practitioner Fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
Guyton AC, Sagawa K. Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. Am J Physiol. 1961;200:1157–1163. | |
Iwashima Y, Horio T, Takami Y, et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40(5):974–982. | |
Ori Y, Korzets A, Katz M, Perek Y, Zahavi I, Gafter U. Haemodialysis arteriovenous access – a prospective haemodynamic evaluation. Nephrol Dial Transplant. 1996;11(1):94–97. | |
Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11(7):1277–1285. | |
Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):282–287. | |
Ori Y, Korzets A, Katz M, et al. The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am J Kidney Dis. 2002;40(4):745–752. | |
Anderson CB, Codd JR, Graff RA, Groce MA, Harter HR, Newton WT. Cardiac failure and upper extremity arteriovenous dialysis fistulas. Case reports and a review of the literature. Arch Intern Med. 1976;136(3):292–297. | |
Ahearn DJ, Maher JF. Heart failure as a complication of hemodialysis arteriovenous fistula. Ann Intern Med. 1972;77(2):201–204. | |
MacRae JM, Pandeya S, Humen DP, Krivitski N, Lindsay RM. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis. 2004;43(5):e17–e22. | |
Adams JN, Brooks M, Redpath TW, et al. Aortic distensibility and stiffness index measured by magnetic resonance imaging in patients with Marfan’s syndrome. Br Heart J. 1995;73(3):265–269. | |
Edwards NC, Ferro CJ, Townend JN, Steeds RP. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart. 2008;94(8):1038–1043. | |
Fattori R, Bacchi Reggiani L, Pepe G, et al. Magnetic resonance imaging evaluation of aortic elastic properties as early expression of Marfan syndrome. J Cardiovasc Magn Reson. 2000;2(4):251–256. | |
Groenink M, de Roos A, Mulder BJ, Spaan JA, van der Wall EE. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82(2):203–208. | |
Mark PB, Doyle A, Blyth KG, et al. Vascular function assessed with cardiovascular magnetic resonance predicts survival in patients with advanced chronic kidney disease. J Cardiovasc Magn Reson. 2008;10:39. | |
Nelson AJ, Worthley SG, Cameron JD, et al. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens. 2009;27(3):535–542. | |
Wiesmann F, Petersen SE, Leeson PM, et al. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44(10):2056–2064. | |
Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease – a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216(2):446–451. | |
Zimmerli LU, Mark PB, Steedman T, et al. Vascular function in patients with end-stage renal disease and/or coronary artery disease: a cardiac magnetic resonance imaging study. Kidney Int. 2007;71(1):68–73. | |
Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7(5):775–782. | |
Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17(3):323–329. | |
Teo KS, Carbone A, Piantadosi C, et al. Cardiac MRI assessment of left and right ventricular parameters in healthy Australian normal volunteers. Heart Lung Circ. 2008;17(4):313–317. | |
Barac A, Campia U, Panza JA. Methods for evaluating endothelial function in humans. Hypertension. 2007;49:748–760. | |
Corretti MC, Anderson TJ, Benjamin EJ, et al; International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. | |
Deanfield J, Donald A, Ferri C, et al; Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. | |
Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8(3):417–426. | |
Anderson JL, Horne BD, Pennell DJ. Atrial dimensions in health and left ventricular disease using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2005;7(4):671–675. | |
Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–369. | |
London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. 2001;12(12):2759–2767. | |
Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61(4):612–622. | |
Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C. Left atrial volume in end-stage renal disease: a prospective cohort study. J Hypertens. 2006;24(6):1173–1180. | |
Atar I, Konas D, Açikel S, et al. Frequency of atrial fibrillation and factors related to its development in dialysis patients. Int J Cardiol. 2006;106(1):47–51. | |
Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;51(2):255–262. | |
Patel RK, Jardine AG, Mark PB, et al. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55(6):1088–1096. | |
Korantzopoulos P, Kokkoris S, Liu T, Protopsaltis I, Li G, Goudevenos JA. Atrial fibrillation in end-stage renal disease. Pacing Clin Electrophysiol. 2007;30(11):1391–1397. | |
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. | |
Stenvinkel P, Pecoits-Filho R, Lindholm B. Coronary artery disease in end-stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol. 2003;14(7):1927–1939. | |
London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65(2):700–704. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.