Back to Journals » Clinical Epidemiology » Volume 7
The Danish National Prescription Registry in studies of a biological pharmaceutical: palivizumab – validation against two external data sources
Authors Haerskjold A, Henriksen L, Way S, Malham M, Hallas J , Pedersen L, Stensballe LG
Received 27 August 2014
Accepted for publication 26 November 2014
Published 8 May 2015 Volume 2015:7 Pages 305—312
DOI https://doi.org/10.2147/CLEP.S73355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Henrik Sørensen
Ann Haerskjold,1,2 Lonny Henriksen,2 Susanne Way,1 Mikkel Malham,3 Jesper Hallas,4 Lars Pedersen,5 Lone Graff Stensballe1
1The Child and Adolescent Clinic 4072, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark; 2The Research Unit Women's and Children's Health, The Juliane Marie Centre for Women, Children and Reproduction, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark; 3Department of Pediatrics, Hvidovre University Hospital, Hvidovre, Denmark; 4Department of Clinical Pharmacology, Institute of Public Health, University of Southern Denmark, Denmark; 5Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus N, Denmark
Background: National prescription databases are important tools in pharmacoepidemiological studies investigating potential long-term adverse events after drug use. Palivizumab is a biological pharmaceutical used as passive prophylaxis against severe infection with respiratory syncytial virus in high-risk children.
Objective: To assess the registration of palivizumab in the Danish National Prescription Registry (DNPR) and to examine if palivizumab reimbursement data obtained from the Danish Health and Medicines Authority could serve as a supplement to data from the DNPR.
Methods: Registration of palivizumab exposure in the DNPR between 1999 and 2010 was compared to two external data sources: registration of palivizumab exposure in medical records, and palivizumab reimbursement data.
Results: During the study period, 182 children with palivizumab exposure were registered in the DNPR. A total of 207 children were registered for palivizumab reimbursement. The sensitivity of palivizumab registration in the DNPR was 26% (20%–34%), and the specificity of no palivizumab registration in the DNPR was 97% (94%–99%), with data from the medical record as the reference. Palivizumab registration sensitivity in reimbursement data was 29% (22%–36%), and the specificity of no palivizumab registration in the DNPR was 97% (94%–99%), with data from the medical record as the reference.
Conclusion: Exposure to palivizumab was underestimated in the DNPR. Reimbursement data are a readily accessible data supplement, which only slightly increased the sensitivity of palivizumab registration in the DNPR. Our findings underline the need to improve DNPR information concerning drugs administered in hospitals.
Keywords: drug; health register; medical records; respiratory syncytial virus; validation
Introduction
Scandinavia has a long tradition of registry-based epidemiological research,1,2 and this research has been facilitated by a long history of national health care registries. The tracking of individual disease and prescription data over time is made feasible by linking individual information using a unique person identifier known as the Central Personal Registration (CPR) number.
Since 1994, the Danish Registry of Medical Product Statistics has maintained prescription drug records for Danish inhabitants. Medicinal products are classified according to the World Health Organization (WHO) Anatomical Therapeutic Classification System (ATC) coding system. Data have been available to researchers in an irreversible encrypted version since 2003 through the Danish National Prescription Registry (DNPR), which is available through the governmental institution, Statistics Denmark. The DNPR contains information by CPR number on all drugs prescribed in Danish outpatient pharmacies.3,4 However, drugs administered in-hospital are not recorded by CPR number, which challenges individual studies of drugs that are administered in hospital.
Palivizumab (Synagis®) is a monoclonal antibody used as a passive prophylaxis against severe respiratory syncytial virus (RSV) infection among high risk children, ie, preterm children, children with bronchopulmonary dysplasia (BPD), and children with hemodynamically significant heart disease (HSHD).5 Palivizumab is administered by monthly intramuscular injections (15 mg/kg) throughout the RSV season (November to March).
Drug registers may have high public health relevance in facilitating studies of long-term drug use consequences. Population-based databases like DNPR are unique tools in pharmacoepidemiological research given that the registration of drug usage is valid, ie, have high measures of sensitivity and specificity. Misclassification of drug exposure based on registry data will impede studies. We conducted this validation study with the overall aim to assess the validity of DNPR as data source in studies of palivizumab exposure. The primary study objective was to assess the sensitivity and specificity of palivizumab exposure in the DNPR using medical records data as the reference. Furthermore, we assessed if palivizumab reimbursement data could serve as a supplement to DNPR data.
Methods
Hypotheses
Two hypotheses were tested. Both hypotheses were based on the Danish clinical recommendations for palivizumab prescriptions:6–8
- our primary hypothesis was that palivizumab exposure among Danish children would be registered in the DNPR;
- secondly, we hypothesized that inclusion of reimbursement data on palivizumab from Danish Health and Medicines Authority (DHMA) might serve as a DNPR supplement, partly also including in-hospital administration of palivizumab.
In Denmark, the parents of children prescribed palivizumab usually receive a prescription from their hospital health care provider. Subsequently, parents purchase palivizumab at a community pharmacy and return to the hospital outpatient clinic, or the family physician for their child to receive their palivizumab injection. Based on this procedure and because palivizumab is administered as monthly injections throughout the RSV season we established the first hypothesis. However, a child who received palivizumab injection(s) only during hospitalization with no subsequent dose(s) after discharge would be misclassified as unexposed to palivizumab according to DNPR data. Clinicians prescribing palivizumab apply to the DHMA for reimbursement. Reimbursement data are registered by the patient’s CPR number in an administrative system, together with the diagnosis of the child and the name of the physician who applied for the reimbursement.9 Because palivizumab is costly, we assumed most physicians would apply for reimbursement and that the registration of reimbursement may also include palivizumab administered in-hospital without registration in DNPR.
Data sources
The CPR number was used to link the following registries: The Danish Medical Birth Registry,10 DNPR,4 The Danish National Patient Registry,11 The Prescription Database of Northern Jutland (PDNJ), The Odense University Pharmacoepidemiological Database (OPED),12,13 and palivizumab reimbursement data.
Research with DNPR data is facilitated by access to anonymized in-house data via a safe on-line data portal at Statistics Denmark. This policy precludes validation studies requiring re-identification of patient’s CPR number. The OPED and the PDNJ are two regional sub-copies of the DNPR covering 40% of the Danish population, and these databases permit person-identifiable information for research purposes. While DNPR contains data on all prescriptions for Danish inhabitants, the OPED and PDNJ contain information on all computerized prescription refunds from the Country of Funen and Country of Northern Jutland. For registration of palivizumab the database contents are similar, including information on CPR number, drug (ATC code, name), dose(s), and quantity.
OPED and the PDNJ data were used to identify the CPR number of a subset of children exposed to palivizumab to sample and scrutinize the medical records. Data from medical records on palivizumab exposure were exported to Statistics Denmark, linked to the in-house data, and anonymized for comparison analysis with DNPR.
Study population and period
Using the Medical Birth Registry, we identified a study base of 778,011 children born between 1997 and 2010 and surviving day one after birth. Based on palivizumab recommendations6,8 we identified three cohorts of children for whom palivizumab prophylaxis would be recommended, but without a registration of palivizumab exposure in the DNPR (Figure 1):
- children born before 26 weeks of gestation were identified using information on gestational age from The Medical Birth Registry;
- children with BPD were identified in The National Patient Registry by a diagnosis of BPD (International Classification of Diseases 10th Revision: P27);
- children with HSHD were identified by an algorithm combining data on HSHD-specific hospitalizations and HSHD-specific prescribed drugs adding criteria for age at hospitalization and repeated medication. The algorithm to identify children with HSHD was developed by a specialist in pediatric cardiology.14
The goal was to scrutinize 10% of each of the three groups of children, which is generally considered sufficient for a validation procedure.15 Because we expected that not all medical journals in the hospitals would be identified, we randomly oversampled 150 children in each of the three groups of palivizumab unexposed children, ie, we sampled a larger number than needed. When the goal of identifying approximately 10% of the medical records from each group of children was achieved medical records collection was stopped. An equal fraction of children was included from each study year.
Data from the OPED and the PDNJ were furthermore collected to identify CPR number in a cohort of children exposed to palivizumab (ATC code J06BB16) according to DNPR (Figure 1).
Medical record review
Medical records were reviewed at the three large tertiary hospitals in Denmark (Skejby University Hospital in the North of Denmark, Odense University Hospital of Southern Denmark and the University Hospital of Copenhagen, Rigshospitalet) where children who may be considered for palivizumab prophylaxis were expected to be followed. Medical records were reviewed until the child’s second birthday or until the first notification of palivizumab treatment. Palivizumab exposure status was registered using a standardized registration form. If a child was followed in another hospital, or only part of the medical record was accessible, the child was excluded from the present study. Reimbursement data were obtained from the DHMA.
Statistical analyses
The sensitivity and specificity of palivizumab registration in the DNPR were estimated and presented with 95% confidence intervals (CIs). In a post hoc analysis, the distribution of baseline factors in children who had a registration of palivizumab exposure only in the medical records, were compared using chi-square tests with children who had a registration of palivizumab in the DNPR and/or reimbursement data. Data analyses were performed using Stata 13.
Ethical approval
No ethical approval is required for Danish registry-based studies. The study was approved by the Danish Data Protection Board (J no 2010. 41-5166). The Danish National Board of Health approved the access to the medical records (J no 7-604-04-2/312/KWH). The DHMA approved access to reimbursement data for this study (J no 2012121945).
Results
Figure 1 shows the data collection of medical records for palivizumab exposed and unexposed children according to DNPR. One child recorded with palivizumab exposure in PDNJ and OPED was not registered in the DNPR and therefore not included in the analyses.
Palivizumab registration in the DNPR with medical records as the reference
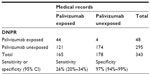
Of the 56 children identified through OPED and PDNJ with palivizumab registration, only 48 medical records were included in the analysis. The remaining eight cases were excluded due to incomplete medical record data or missing medical records at the hospital. Of the 48 children with a registration of palivizumab exposure in the DNPR, 44 children had a registration of palivizumab in the medical records (Table 1).
A total of 295 children’s medical records without palivizumab registration in the DNPR were identified and scrutinized for palivizumab exposure. These consisted of: n=93 medical records among children born <26 weeks of gestation, n=106 medical records among children with BPD, and n=96 medical records among children with HSHD. Among the 295 children, 121 (41%; n=60 immature, n=59 with BPD, and 2 children with HSHD) had a registration of palivizumab in medical records, yielding a sensitivity of 26% (95% CI 20%–34%) and a specificity of 97% (94%–99%; Table 1).
Palivizumab reimbursement registration with medical records as the reference
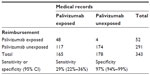
A total of 207 children were recorded with reimbursement for palivizumab of which 165 were also recorded in the DNPR. For 117 (71%) no registration of reimbursement was found. The reimbursement data sensitivity when compared to medical records data was 29% (95% CI 22%–36%) and the specificity was 97% (94%–99%; Table 2).
Palivizumab registration in the DNPR with reimbursement data as the reference
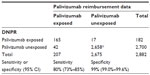
The comparison of DNPR with data on palivizumab reimbursement as reference yielded a sensitivity of 80% (73%–85%) and a specificity of 99% (99%–100%, Table 3).
Post hoc analysis comparing background factors in children with registration of palivizumab exposure in the medical records only with children with registration of palivizumab exposure in the DNPR/reimbursement data
Significantly different distributions of background factors between children with palivizumab registration in the DNPR and/or reimbursement and palivizumab registration in medical records were found for gestational age, birth weight, fetal growth, and underlying medical conditions of the child (Table 4). Children with palivizumab registration only in medical records had low birth weight, low gestational age, were accurate for gestational age, and had more often BPD.
Discussion
Palivizumab is a biological pharmaceutical given as a passive prophylaxis to vulnerable children. Long-term follow-up studies are important for monitoring the safety of palivizumab. This validation study determined that palivizumab exposure was underestimated in the DNPR and was also underestimated in reimbursement data.
Medical records were used to access if palivizumab was administered in hospitals without a DNPR registration. We found the sensitivity of palivizumab registration in the DNPR to be low and our primary hypothesis that palivizumab exposure would be registered in the DNPR was not confirmed. The underestimation of palivizumab usage was in line with other validation studies of palivizumab data.16,17
Palivizumab is costly, which may be an incentive to secure reimbursement. We hypothesized that palivizumab, administered in-hospital and therefore not registered in the DNPR may be registered by reimbursement data. However, the sensitivity of reimbursement data with medical records as reference was only slightly better, because no reimbursement was sought for the majority of children exposed to palivizumab.
The good accordance observed between data in the DNPR and reimbursements was probably explained by discharge procedure logistics. In most cases, physicians discharge the child, write prescriptions, and apply for reimbursement to the family.
Low sensitivity of palivizumab registration in the DNPR will cause low or conservative estimates in long-term follow-up studies of palivizumab. It may also lead to bias if children with palivizumab registration in data sources used in epidemiological studies do not represent the overall population of palivizumab-exposed children. A post hoc analysis determined that more children with low gestational age, low birth weight, accurate for gestational age, and BPD were found among children with registration of palivizumab in medical records. In addition, the fact that the DNPR and/or reimbursement data failed to identify those children, likely reflects the fact that these children may have very long hospitalization periods from birth, which increases the probability of in-hospital palivizumab exposure.
Data validity for research is important. The present study is the first to validate palivizumab registration in the DNPR using medical record information and palivizumab reimbursement data as references. Numerous prior validation studies referenced medical records data.16–18 However, the registration of drug use in medical records may also have limitations. It may be difficult to identify the source of data accepted as the “gold standard”. Reimbursement data are accessible by DHMA application. In contrast, review of medical records to capture the registration of drugs administered in-hospital is resource-intensive and time consuming. Nonetheless, the inclusion of reimbursement data did not increase the study sensitivity.
The present study underlines the need to improve the DNPR in terms of registering drugs administered in-hospital by CPR number. Governmental initiatives in both Norway and Sweden have been established with the aim of creating national databases, which include individual information on drugs administered in-hospital.19 The same attention is warranted in Denmark with the goal of improving DNPR for research purposes. The regional database, Odense University Hospital Pharmaco-epidemiological Database, has already been established and contains data on in-hospital medication by CPR.20 Such information will clearly improve future registry-based drug studies and are needed on a national basis.
Our validation process was subject to limitations. First, no “gold standard” data source on palivizumab exposure was identified. We used the two data sources available, medical records, and reimbursement data. Data collection in medical records was performed only in the three tertiary university hospitals, because we hypothesized that children with severe diseases were referred to and followed-up at these highly specialized units. Thus, a child may have received palivizumab in a non-university hospital after being discharged from the university hospital, and this was most notable for children with HSHD where only two (out of 121) children were registered with palivizumab exposure in medical records. The unavailability of medical records to confirm palivizumab exposure outside a university hospital and also the number of medical records not available at the departments is a limitation, which could cause bias in the present study. However, the potential bias introduced by the selection procedure of medical records was accounted for by the additional comparison with national un-selected reimbursement data as the reference.
Our access to DNPR was provided by Statistics Denmark. Statistics Denmark irreversibly encrypts CPR numbers and allows data access only through their own servers. These policies preclude studies requiring re-identification of patients, and hampers medical records comparison. Thus, validation procedures in this study were made possible using regional sub-databases covering 40% of Denmark.
Pharmacoepidemiological studies with long-term follow-up are of interest to global public health. The use of the CPR number, in combination with a long history of health registries in Scandinavian countries offers unique possibilities to carry out valuable pharmacoepidemiological studies that may otherwise be considered unethical or too expensive. However, the quality of such studies depends on the quality of the registration in the drug databases. In conclusion, our findings underline the need to improve the information in the DNPR concerning drugs administered in-hospital.
Funding
This study was funded by AbbVie.
Acknowledgments
The authors would like to acknowledge Gunnar Bergman, MD PhD, Department of Pediatric, Cardiology, Astrid Lindgren Children’s Hospital, Karolina University Hospital, Sweden and Marie Linder MSc PhD, Centre for Pharmacoepidemiology, Karolinska Institutet, Stockholm, Sweden for their unique work to define children with hemodynamically significant heart disease.
Disclosure
LGS received a grant from AbbVie, which funded the present study and the wages of AH and LH, SW, MM, JH and LP. The authors report no other conflicts of interest in this study.
References
Frank L. Epidemiology. The epidemiologist’s dream: Denmark. Science. 2003;301(5630):163. | |
Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. | |
Johannesdottir SA, Horvath-Puho E, Ehrenstein V, Schmidt M, Pedersen L, Sorensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303–313. | |
Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. | |
Sommer C, Resch B, Simoes EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–154. | |
RSV forebyggelse hos børn med medfødt hjerte sygdom [RSV prophylaxis in children with congenital heart disease]. Danish Committee on Pediatric cardiology. 2004. Available at: http://www.paediatri.dk/images/pdf_filer/dps_vejl/001kar.pdf. Accessed January 30, 2014. | |
RSV forebyggelse hos præmature med palivizumab (Synagis) 2002. RSV prophylaxis in preterm infants with palivizumab (synagis). Available at: http://www.paediatri.dk/images/pdf_filer/dps_vejl/neo/013neo.pdf. Accessed January 30, 2104. | |
Skejby University Hospital. Synagis – procedure ved behandling [Synagis – procedure of treatment]. 2013 Available at: http://e-dok.rm.dk/edok/Admin/GUI.nsf/Desktop.html?open&openlink=http://e-dok.rm.dk/edok/enduser/portal.nsf/Main.html?open&unid=X64EC06F3EBDBF669C12578610032AFA7&dbpath=/edok/editor/AAUHBO.nsf/&windowwidth=1100&windowheight=600&windowtitle=S%F8g. Danish. | |
Tilskud til medicin [homepage on the Internet] [Reimbursement to medication. Danish National Board of Health]. Sundhedsstyrelsen. Available from: https://sundhedsstyrelsen.dk/da/medicin/tilskud. Accessed January 4, 2015. | |
Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323. | |
Mosbech J, Jorgensen J, Madsen M, Rostgaard K, Thornberg K, Poulsen TD. Patientregisteret [The national patient registry]. Evaluation of data quality. Ugeskr Laeger. 1995;157(26):3741–3745. Danish. | |
Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44(4):445–448. | |
Nielsen GL, Sorensen HT, Zhou W, Steffensen FH, Olsen J. The Pharmacoepidemiologic Prescription Database of North Jutland – a valid tool in pharmacoepidemiological research. Int J Risk Saf Med. 1997;10(3):203–205. | |
Bergman G,Hærskjold A,Stensballe LG,Kieler H,Linder M. Children with hemodynamically significant congenital heart disease can be identified through population-based registers. Clinical Epidemiology. 2015;7:199–127. | |
surveymonkey.com [homepage on the Internet]. Available from:http://blog.surveymonkey.com/blog/2011/09/15/how-many-people-do-i-need-to-take-my-survey/. Accessed January 4, 2015. | |
Jacobson VJ, Feaganes J, Wegner S. Reliability of medicaid claims versus medical record data: in a cost analysis of palivizumab. Pharmacoeconomics. 2007;25(9):793–800. | |
Linder M, Byströn C, Bergman G, Kieler H, Haerskjold A. Use of palivizumab is underestimated in the Swedisg Prescribed drug register. Clinical Epidemiology. Epub 2014. | |
Vestberg K, Thulstrup AM, Sorensen HT, Ottesen P, Sabroe S, Vilstrup H. Data quality of administratively collected hospital discharge data for liver cirrhosis epidemiology. J Med Syst. 1997;21(1):11–20. | |
Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. | |
Larsen MD, Cars T, Hallas J. A MiniReview of the use of hospital-based databases in observational inpatient studies of drugs. Basic Clin Pharmacol Toxicol. 2013;112(1):13–18. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.