Back to Journals » Clinical Epidemiology » Volume 12
The DANish Comorbidity Index for Acute Myocardial Infarction (DANCAMI): Development, Validation and Comparison with Existing Comorbidity Indices
Authors Wellejus Albertsen L , Heide-Jørgensen U , Schmidt SAJ , Grey C, Jackson R , Sørensen HT , Schmidt M
Received 19 August 2020
Accepted for publication 8 October 2020
Published 20 November 2020 Volume 2020:12 Pages 1299—1311
DOI https://doi.org/10.2147/CLEP.S277325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eyal Cohen
Lisbeth Wellejus Albertsen,1 Uffe Heide-Jørgensen,1 Sigrun Alba Johannesdottir Schmidt,1 Corina Grey,2 Rod Jackson,2 Henrik Toft Sørensen,1 Morten Schmidt1,3,4
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 2Section of Epidemiology and Biostatistics, School of Population Health, University of Auckland, Auckland, New Zealand; 3Department of Cardiology, Regional Hospital West Jutland, Herning, Denmark; 4Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark
Correspondence: Morten Schmidt Email [email protected]
Objective: To develop and validate the DANish Comorbidity index for Acute Myocardial Infarction (DANCAMI) for adjustment of comorbidity burden in studies of myocardial infarction prognosis.
Methods: Using medical registries, we identified patients with first-time myocardial infarction in Denmark during 2000– 2013 (n=36,685). We developed comorbidity indices predicting 1-year all-cause mortality from all comorbidities (DANCAMI) and restricted to non-cardiovascular comorbidities (rDANCAMI). For variable selection, we eliminated comorbidities stepwise using hazard ratios from multivariable Cox models. We compared DANCAMI/rDANCAMI with Charlson and Elixhauser comorbidity indices using standard performance measures (Nagelkerke’s R2, Harrell’s C-statistic, the Integrated Discrimination Improvement, and the continuous Net Reclassification Index). We assessed the significance of the novel DANCAMI variables not included in the Charlson Comorbidity Index. External validation was performed in patients with myocardial infarction in New Zealand during 2007– 2016 (n=75,069).
Results: The DANCAMI included 24 comorbidities. The rDANCAMI included 17 non-cardiovascular comorbidities. In the Danish cohort, the DANCAMI indices outperformed both the Charlson and the Elixhauser comorbidity indices on all performance measures. The DANCAMI indices included multiple variables that were significant predictors of 1-year mortality even after controlling for all variables in the Charlson Comorbidity Index. These novel variables included valvular heart disease (hazard ratio for 1-year mortality=1.25, 95% CI: 1.14– 1.35), coagulopathy (1.13, 95% CI: 1.05– 1.22), alcohol and drug abuse (1.35, 95% CI: 1.15– 1.58), schizophrenia (1.60, 95% CI: 1.46– 1.76), affective disorder (1.29, 95% CI: 1.22– 1.36), epilepsy (1.26, 95% CI: 1.05– 1.50), neurodegenerative disorder (1.30, 95% CI: 1.10– 1.54) and chronic pancreatitis (1.71, 95% CI: 1.14– 2.56). The results were supported by the external validation in New Zealand.
Conclusion: DANCAMI assessed comorbidity burden of patients with first-time myocardial infarction, outperformed existing comorbidity indices, and was generalizable to patients outside Denmark. DANCAMI is recommended as a standard approach for comorbidity adjustment in studies of myocardial infarction prognosis.
Keywords: comorbidity, myocardial infarction, prognosis, risk score
Introduction
Comorbidity burden is a strong predictor of myocardial infarction (MI) mortality.1 Although declining, 30-day MI mortality risk remains around 15% overall and increases to almost 30% among patients with a Charlson Comorbidity Index (CCI) score of ≥3.1 Underlining its clinical and public health importance, the prevalence of a high comorbidity burden in patients with MI is increasing with the aging population.1 The need to better understand the effect of a comorbidity burden on MI prognosis is therefore compelling.
Comorbidity prediction models (indices) are widely used for this purpose. Comorbidity indices have been developed specifically for cardiac patients2–6 and for mixed populations with subsequent testing in cardiac patients.7–10 The CCI is one of the most commonly used comorbidity indices in research.7 It was developed in 1984 from 559 medical inpatients to predict 1-year mortality.7 It did not include psychiatric diagnoses although the need for exploring the coexistence of physical and mental illness has recently been highlighted.11 A more contemporary comorbidity index is the van Walraven-weighted version of the Elixhauser Comorbidity Index (ECI),8 developed from a mixed patient group to predict in-hospital mortality. Neither index seems ideal for assessing the predictive ability of comorbidity burden in contemporary MI patients. We therefore developed and validated the DANish Comorbidity index for Acute Myocardial Infarction (DANCAMI) for adjustment of comorbidity burden in research on MI patients.
Methods
Setting and Data Sources
The Danish National Health Service provides universal tax-supported health care, guaranteeing free access to general practitioners and hospitals in Denmark.12 All Danish residents are assigned a unique central personal registry (CPR) number at birth or upon immigration.13 Using the CPR number, we linked the Danish Civil Registration System (mortality and migration data),13 The Danish National Patient Registry (DNPR) (hospital discharge data),14 the Aarhus University Prescription Database (dispensed prescriptions),15 and the Clinical Laboratory Information System Research Database (laboratory data).16
Study Cohort and Outcome
We used the DNPR to identify all patients aged ≥15 years hospitalized with a first-time inpatient MI diagnosis in the Northern and Central Denmark Regions between 1 January 2000 and 31 December 2013. We excluded patients with any previous in- or outpatient MI diagnosis recorded in the DNPR. Follow-up continued through 2014. We defined the outcome as time to all-cause mortality within 1 year from MI admission.13
Potential Predictors
We assembled a list of comorbidities from previously constructed indices and clinical knowledge. For each MI patient, we identified comorbidities from all in- and outpatient diagnoses in the DNPR within the 5 years before MI hospitalization. This included diagnoses recorded during the index admission, except for potential complications of MI, antithrombotic treatment, or associated immobilization (angina pectoris, heart failure, venous thromboembolism, atrial fibrillation/flutter, heart block, ventricular tachycardia, valvular heart disease, and stroke).
Based on Danish 5-year mortality estimates, we categorized cancer as high-risk (survival <30%) or low-risk (survival ≥30%) cancer. High-risk cancers included cancers of the hypopharynx, esophagus, stomach, liver, gallbladder, pancreas, trachea and lung, as well as mesothelioma, acute myeloid leukemia, unspecified leukemia, and secondary cancer. All remaining types of cancer were considered low-risk cancers.
Diabetes, chronic pulmonary disease, and hypertension might be treated solely in general practice, and not be registered in the DNPR.14 We therefore also identified diabetes as a HbA1c level >48 mmol/L16 or from antidiabetic prescriptions.15 We supplemented diagnosis codes for chronic pulmonary disease with any prescription record for a drug used to treat obstructive airway disease.15 We defined hypertension as a hospital diagnosis, redemption of antihypertensive combination tablets, or redemption of at least two antihypertensive drugs within 90 days before MI admission. Finally, we supplemented diagnosis codes for schizophrenia and affective disorders with relevant prescriptions within 90 days.15
The final list of potential predictors included 41 individual comorbidities (eTable 1). In addition to developing a comorbidity index accounting for both cardiovascular and non-cardiovascular comorbidities (DANCAMI), we also developed an index restricted to non-cardiovascular comorbidities (rDANCAMI) to enable researchers to adjust for individual non-cardiovascular comorbidities. The potential predictors for rDANCAMI included the 24 non-cardiovascular comorbidities from the final list of 41 comorbidities.
Existing Comorbidity Indices
Table 1 provides an overview of the variables included in the DANCAMI, CCI and ECI. For each MI patient, we calculated the CCI7 and the ECI8 scores. We based the CCI score on 18 comorbidities (MI excluded) and categorized it in four groups (0, 1, 2, and ≥3).7 The ECI score was based on 30 comorbidities and categorized in four groups (≤0, 1–5, 6–13, and ≥14).8
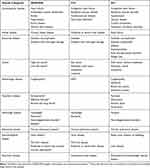 |
Table 1 Comorbidities included in the DANish Comorbidity index for Acute Myocardial Infarction (DANCAMI), Charlson Comorbidity Index (CCI), and Elixhauser Comorbidity Index (ECI) |
External Validation Cohort
We validated the performance of DANCAMI/rDANCAMI in patients with first-time MI in New Zealand between 1 January 2007 and 31 December 2016. We used the unique New Zealand National Health Index (NHI) number, assigned to patients at entry into the public health system (>98% of the population),17 to link the New Zealand National Minimum Dataset (hospital inpatient data),17 the National Mortality Collection (vital status),18 and the National Pharmaceutical Collection (dispensed prescriptions).19 The National Minimum Dataset includes nationwide information on all patients discharged from publicly funded hospitals, including admission dates, primary diagnoses, and secondary diagnoses.17 Except for HbA1c data (unavailable), the eligibility criteria, covariables, and outcome of the validation cohort were identical to those used for the Danish development cohort.
Statistical Analysis
Model Development
We used Cox regression to compute hazard ratios (HRs) with 95% confidence intervals (CIs) for the minimally (sex and age) adjusted association between each comorbidity and 1-year mortality. To select variables for the DANCAMI, we included in the Cox models all 41 comorbidities, sex and age, regardless of the results from the minimally adjusted analyses.20 Fractional polynomials supported a linear association between age and 1-year mortality. We then eliminated comorbidities with a HR <1.10 or a CI that overlapped 1. We fitted revised models with the remaining comorbidities, sex, and age. We repeated this approach until the models included only comorbidities with a HR ≥1.10. We tested the proportionality assumption using the global test based on scaled Schoenfeld residuals21 and with log-log plots for variables that appeared non-proportional. We assigned weights to each comorbidity in the final index by multiplying the beta coefficient from the multivariable models by ten and rounding to the nearest integer to yield the score components (Table 2). The final DANCAMI score was formed by adding the weights of each of the individual patient’s comorbidities.22,23 We repeated the above steps with the 24 non-cardiovascular comorbidities to develop rDANCAMI. In addition to continuous comorbidity scores, we categorized them into no (score=0), low (score=1–3), moderate (score=4–5) and severe comorbidity burden (score ≥6). The categorization cut-off values were based on the survival curves of the individual DANCAMI/rDANCAMI scores.
 |
Table 2 The Model Development of the DANish Comorbidity index for Acute Myocardial Infarction (DANCAMI) |
Model Performance
The focus of performance measurements was discriminatory ability because the DANCAMI was intended for research rather than clinical use. We evaluated the performance of the continuous and categorical comorbidity index scores using standard performance measures, including: (1) a modified version of Nagelkerke’s R2 to measure overall performance with explained variation; (2) Harrell’s C-statistic, which is equivalent to the area under the Receiver Operating Characteristic curve and indicates the proportion of all pairs of patients in which the patient who died first has higher predicted mortality;20 (3) the integrated discrimination improvement (IDI); and (4) the continuous Net Reclassification Index (NRI) performance measures. The IDI and NRI indicate how much a predictor adds to a model’s discriminatory power and are joint measures of a model’s comparative improvement in sensitivity and specificity.20 The NRI represents the net proportion of patients with a change in predicted risk in the correct direction when the comorbidity score is added to a baseline model containing age and sex.20 The IDI integrates the NRI over all possible cut-offs for the probability of an outcome and is the difference between predicted probabilities in those who do and those who do not experience the outcome. It corresponds to the difference in discrimination slopes of two models.20 A positive NRI or IDI indicates better prediction in the new model compared with the baseline model. We used resampling methods to calculate CIs for the performance measures.
Comparison with Existing Comorbidity Indices
We calculated Nagelkerke’s R2 and Harrell’s C-statistic for the (1) baseline model (age and sex) and the baseline model plus the (2) DANCAMI, (3) rDANCAMI, (4) CCI, and (5) ECI. The relative difference between performance of the baseline model and the four different comorbidity indices was assessed. IDI and NRI were assessed to compare the baseline model with each of the four comorbidity indices. Of note, these standard model performance measures have largely been developed for assessing the performance of dichotomous diagnostic tests. The application of these metrics to risk prediction scores are questionable because they are insensitive to the addition of important predictors.20
Improved performance is therefore better assessed by the HRs of the additional predictors in our index that were not included in the existing indices. As a key analysis, we therefore tested the significance of the novel DANCAMI variables not included in the CCI by including them in a model containing the CCI variables. In this model, significant HRs of the novel DANCAMI variables would support an improved ability of DANCAMI over the CCI in predicting 1-year all-cause mortality.
External Validation
For external validation, we estimated the ability to predict 1-year mortality in the New Zealand MI cohort by using the DANCAMI/rDANCAMI. We applied the same methods described above to assess performance and for comparison with existing comorbidity indices.
Sensitivity Analyses
We performed eight sensitivity analyses. To evaluate how the decisions made during model development affected the final indices, we (1) changed the HR cut-off from 1.10 to 1.20; (2) used the exact rather than rounded beta coefficients for score components; (3) used the HRs instead of beta coefficients for score components; (4) performed split-sample internal validation according to diagnosis year (temporal validation rather than randomly)20 by repeating the model development in the derivation cohort (2000–09) and assessed performance in the validation cohort (2010–13); (5) restricted to MI hospital survivors; and stratified by (6–7) age and sex and (8) ethnicity groups in New Zealand (European, Maori, Pacific, Indian, and Chinese/other Asian). All statistical analyses were conducted using Stata Version 14.2 (Stata Corp, College Station, Texas, USA).
Results
Model Development
The Danish cohort included 36,685 MI patients (61% men) with a median age of 72 years (interquartile range: 61–81 years). eTable 1 presents the prevalence of each comorbidity assessed and their associations with 1-year mortality (adjusted for age and sex). The most prevalent comorbidity in the population was hypertension (53%), followed by chronic pulmonary disease (22%), and stable angina pectoris (19%). We observed significant associations with 1–year mortality for most predictors, except deep vein thrombosis in a lower limb, pulmonary embolism, heart block, immune system disorders, human immunodeficiency virus infection, endocrine disorders (excluding diabetes), anxiety and behavioural disorders, and inflammatory bowel disease. Nonsignificant associations were due to a combination of small effect sizes and low prevalence. The model development resulted in the inclusion of 24 comorbidities in the DANCAMI and 17 in the rDANCAMI. Weights indicating the severity of each included variable are presented in Table 2.
The characteristics of the Danish and New Zealand cohorts are shown in Table 3. The 1-year mortality in the Danish MI cohort was 24%. Most of these deaths occurred during hospital admission with in-hospital mortality at 14%. The DANCAMI score categories showed that 29% had no, 41% had low, 11% had moderate, and 19% had severe comorbidity burden. The corresponding proportions for rDANCAMI were 57%, 26%, 5.2%, and 12%, respectively. Survival decreased with increasing comorbidity burden (Figure 1).
 |
Table 3 Characteristics of the Danish and New Zealand Myocardial Infarction Cohorts |
Model Performance
The explained variance (R2) was significantly higher in the prediction model including age, sex and DANCAMI (R2=0.33, 95% CI: 0.32–0.34) than in the baseline model containing only age and sex (R2=0.28, 95% CI: 0.27–0.29) (Table 4). Similarly, the discrimination of DANCAMI was better than the baseline model (C-statistic: 0.75 vs. 0.73). Adding the DANCAMI score to the baseline model improved discrimination (IDI=0.054) compared with the baseline model alone. Similarly, improved discrimination was observed by a total NRI of 0.52 where 77% of non-events and 49% of events had a better predicted probability of 1-year mortality (eTable 2). DANCAMI score categories performed almost as well in R2 and C-statistics as the continuous score (Table 4), but the IDI was lower (0.044 vs. 0.054) and the NRI higher (0.55 vs. 0.52).
Comparison with Existing Comorbidity Indices
Compared with the CCI and the ECI, both the continuous and the categorical DANCAMI scores performed better in the four standard performance measures (Table 4). Although the differences in performance measures were minor, the superiority of DANCAMI over the CCI was strongly supported by the result that each of the eight DANCAMI variables, not included in the CCI, predicted 1-year mortality despite adjustment for the CCI (Table 5). These novel variables included valvular heart disease (HR for 1-year mortality=1.25, 95% CI: 1.14–1.35), coagulopathy (HR=1.13, 95% CI: 1.05–1.22), alcohol and drug abuse (HR=1.35, 95% CI: 1.15–1.58), schizophrenia (HR=1.60, 95% CI: 1.46–1.76), affective disorder (HR=1.29, 95% CI: 1.22–1.36), epilepsy (HR=1.26, 95% CI: 1.05–1.50), neurodegenerative disorder (HR=1.30, 95% CI: 1.10–1.54) and chronic pancreatitis (HR=1.71, 95% CI: 1.14–2.56).
 |
Table 5 Adjusted Hazard Ratios for the Charlson Comorbidity Index and Additional DANCAMI Variables, Derived in the Danish and New Zealand Myocardial Infarction Cohorts |
External Validation
In the New Zealand MI cohort (n=75,069), the 1-year mortality was 20% (half of which occurred in-hospital), the male proportion was 59%, and the median age was 71 years (Table 3). The proportion of MI patients with at least one comorbidity (DANCAMI score >0) was slightly lower than in Denmark (67% vs. 71%), but higher in patients with at least one non-cardiovascular comorbidity (rDANCAMI >0) (47% vs. 43%). As in the Danish cohort, the two most prevalent comorbidities were hypertension (38%) and chronic pulmonary disease (17%), but here diabetes with end-organ failure came third (16%) (Table 3).
DANCAMI scores also added to the predictive performance compared with the baseline model. Discrimination was better than that of the baseline model (C-statistic 0.77 vs. 0.73) and R2 was higher (0.37 vs. 0.28). IDI was 0.079 and NRI was 0.682 with 78% of non-events and 56% of events obtaining a more correct predicted probability of 1-year mortality compared with the predictions of the baseline model (eTable 2). Performances of the CCI and the ECI were nearly identical to that of DANCAMI, except for NRI where the CCI performance was lower (Table 4). rDANCAMI performance was lower than the other three indices (Table 4). Similar to the Danish cohort, DANCAMI score categories performed almost as well in R2 and C-statistics as the continuous score, but with a lower IDI and a higher NRI (Table 4).
Sensitivity Analyses
The sensitivity analyses showed that (1) changing the inclusion threshold to 1.20 (ie, removing all comorbidities with a score of one) or (2) changing severity weights to precise beta-coefficients had limited effect on the model performance (eTable 3); (3) using HRs as severity weights worsened all performance measures (eTable 3); and (4) 1-year mortality was higher in the derivation cohort (26%) than in the validation split-sample cohort (21%), although baseline characteristics were similar (eTable 4a). The number of comorbidities obtaining different severity weights was 7 in the refitted DANCAMI and 5 in the refitted rDANCAMI. Still, both refitted models performed better than the CCI and the ECI when tested in the validation cohort (eTable 4b).
Among patient subgroups (eTables 5–7), the models performed best for (5) patients surviving the initial MI hospitalization (6) younger age groups <75 years: (7) males (mostly attributable to the baseline model and not the added comorbidity score); and (8), European ethnicity (while the ECI was best in the other ethnic groups).
Discussion
We developed and validated two comorbidity indices predicting 1-year mortality for Danish patients after their first hospital admission for MI based on any type of comorbidity (DANCAMI) or non-cardiovascular comorbidities (rDANCAMI). The new indices included multiple variables not included in the current comorbidity indices and outperformed both the CCI and the ECI. The DANCAMI/rDANCAMI score categories performed almost equally as well as the continuous scores.
Previous Literature
In contrast to previous studies, DANCAMI was developed in a contemporary cohort with contemporary comorbidities (eg, exclusion of AIDS as a comorbidity and inclusion of psychiatric disorders). The rDANCAMI is the first comorbidity index for MI patients to include only non-cardiovascular comorbidities. However, other comorbidity indices have been developed specifically for MI patients. A 1994 US study used Medicare data to develop a comorbidity index predicting 2-year MI mortality.2 However, the patients were diagnosed in 1987 and were all 30-day survivors; and therefore not generalizable to all MI patients. A Chinese comorbidity index was developed in 2016 to predict in-hospital mortality in MI patients admitted to a Beijing hospital during 2006–2010.3 The study aimed to develop a method to adjust for heterogeneity between Chinese hospitals. In contrast to DANCAMI, the Chinese index included potential complications of MI such as cardiac arrest and shock. In 2011, a Spanish comorbidity index was developed for patients hospitalized during 2002–2008 with non-ST-segment elevation MI.4 This index included only 1017 patients and may not generalize to all MI patients. A 2001 Canadian study developed two separate comorbidity indices predicting 30-day and 1-year mortality among MI patients with age group and sex included in the indices.5 Unfortunately, the authors only reported regression coefficients and odds ratios with 95% CIs and did not generate a simpler scoring system.5 Finally, a Canadian comorbidity index developed in 2019 included a study population of patients undergoing diagnostic and/or therapeutic cardiac catheterization.6 The difference between this and our MI study populations complicates comparison.
Unlike most other comorbidity indices, the DANCAMI and the rDANCAMI include multiple mental and behavioural health disorders such as alcohol/drug abuse, schizophrenia, and affective disorders, which were assigned relatively high weights of 3 to 5 in our study. In some indices, these disorders were not included or not considered for inclusion.3–7 The ECI8 and the 1994 US study2 both included psychiatric diagnoses. In the ECI, drug abuse and depression scored less than zero, while alcohol abuse and psychoses scored zero. In the US study, the prevalence of these disorders was very low compared with our Danish cohort. This discrepancy could be due to use of different definitions of these diagnoses. The high prevalence and significant HRs of the psychiatric diagnoses in DANCAMI and rDANCAMI indicate that these variables are important predictors of mortality. This assumption is supported by our analysis showing that all novel variables in DANCAMI, including the mental and behavioural disorders, where significant predictors of mortality in the Danish cohort after adjusting for the CCI variables. The novel variables were also significant predictors of mortality in the New Zealand MI cohort. These results indicate that the inclusion of these additional predictors in the comorbidity indices are important for accurate outcome prediction. The value of these added predictors is not clearly reflected in the standard performance measures (eg, R2 and C-statistic), which was not surprising as these are global measures that are relatively insensitive to the addition of new variables.20
Both DANCAMI and rDANCAMI showed higher discrimination in the New Zealand validation cohort than in the Danish development cohort, which was unexpected. However, the CCI and the ECI also showed higher discrimination in the New Zealand cohort. These findings likely reflect different case mixes in the two nationwide cohorts, eg, a more ethnically diverse population in New Zealand than in Denmark. DANCAMI was slightly superior in the subgroup of the New Zealand cohort with European ethnicity that is likely to be more comparable to the Danish population.
The CCI and the ECI have previously been validated in MI patient populations. In studies performed in the US,24 Taiwan,25 and five different European countries,26 the ECI outperformed the CCI in predicting in-hospital24–26 and 1-year mortality.25 These studies differ from our study as they included comorbidities as separate variables in their performance analyses instead of using a summary score. A Japanese study compared the performance of the CCI and the ECI using individual comorbidities vs. a summary score, and found that the ECI performed better with individual comorbidities.27 However, the CCI and the ECI performed similar when the summary score was applied. This finding demonstrates that performance of individual comorbidity indices can vary depending on their application. In our performance analyses of the summary scores, the CCI showed better performance than the ECI in the Danish cohort. In contrast, the ECI performed marginally better than the CCI in the New Zealand cohort.
Despite the concerns of information loss that may arise with the simplification of summary scores,20 they can be a useful tool to adjust for comorbidity burden in observational studies in which multiple variables often are included in regression analyses. The same applies when categorizing summary scores. The categorized groups are a simple and easily accessible method to illustrate and adjust for comorbidity burden. However, they provide further simplification of the original prediction model since they assign the same predicted risk to patients with different comorbidity scores.20 Moreover, there is no clear consensus on the method behind the categorization of summary scores.20 We created four categories by examining the survival curves of the individual DANCAMI and rDANCAMI scores. In our performance analyses, the DANCAMI score categories performed almost as good as the continuous scores. Similar results were found for the ECI where the continuous scores and categories performed similar in C-statistics.8
Strength and Limitations
Study strengths include the nationwide population-based design (reducing selection bias) and large sample size (reducing random error). Furthermore, rDANCAMI allows researchers to study the effect of individual cardiovascular diseases separately while adjusting for non-cardiovascular comorbidity burden. We used recommended methods to generate summary scores in our final indices20 and considered a variety of variables for both indices, including psychiatric diagnoses, which have previously been overlooked.
Although we used a five-year look-back period to identify comorbidities and defined variables from algorithms including both diagnoses, prescriptions, and laboratory data, misclassification of some conditions is unavoidable.28 However, the positive predictive values have been reported to be high for both cardiac14,29 and CCI comorbidities (98% overall).30 Like previous studies,2,8 we found several comorbidities that were associated with a decreased 1-year mortality (eg, stable angina pectoris and anxiety) in our multivariable model. These seemingly protective comorbidities could result from a bias in coding in which severity of overall patient illness may inversely affect the coding of chronic and nonfatal comorbidities.8 We therefore excluded these comorbidities from our final indices.
We lacked detailed clinical information, eg, electrocardiogram results and cardiac biochemical markers, which may be important predictors particularly of short-term mortality. This is evident in clinical risk prediction models, such as the Global Registry of Acute Coronary Events (GRACE) risk score,31 and may explain the superior performance of our indices among patients surviving hospital admission. However, detailed clinical information is often not available in routine secondary care data which makes it less useful as predictors in this setting.
Our robust external validation indicated that both DANCAMI and rDANCAMI generalize well outside the Danish cohort. Still, it should be noted that DANCAMI was developed and validated in patients with MI. Thus, its performance in patients with other cardiovascular diseases remains to be examined.
Conclusions
Comorbidity burden was a strong predictor of mortality in MI patients and must be controlled for accurately when studying outcomes in MI patients. We developed two separate comorbidity indices with (DANCAMI) and without (rDANCAMI) cardiovascular comorbidities to predict 1-year mortality following first-time MI. The indices were based on comorbidities in contemporary MI patients. DANCAMI performed better than the previous most commonly used comorbidity indices and included novel comorbidities with incremental ability to predict mortality. Both indices can be used to control for comorbidity burden in MI patients either by applying the continuous or the categorized summary score. The indices are likely generalizable to MI patients in other Western countries similar to Denmark and New Zealand. We therefore recommend DANCAMI as a standard approach for comorbidity adjustment in studies of MI prognosis.
Data Permission
The study was approved by the Danish Data Protection Agency (record number 2013-41-1924). The use of New Zealand data was approved by the Northern Ethics Committee Y in 2003 (AKY/03/12/314), with annual approval by the National Multi-Region Ethics Committee since 2007 (MEC07/19/EXP).
Ethics Committee Approval
No ethical committee approval was needed.
Patient Involvement Statement
No patient involvement.
Transparency Declaration
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Author Contributions
MS and HTS conceived the study idea and designed the study together with co-authors. LWA and MS assembled the list of potential comorbidities from review of existing indices and medical conditions included in the ICD-10. MS, UHJ, RJ and LWA directed the analyses, which were carried out by LWA. LWA and MS organized the writing and wrote the initial draft, reply letter, and revised manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. LWA attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MS is the guarantor.
Funding
The study was supported by the Danish Heart Association, Department of Clinical Epidemiology’s Research Foundation, Handelsgartner Ove William Buhl Olesen og ægtefælde fru Edith Buhl Olesens mindelegat, Politimester J.P.N. Colind og hustru Colinds Mindelegat and Danske lægers forsikring under SEB pension. MS was supported by the Novo Nordisk Foundation (grant NNF19OC0054908). The funding sources had no role in the design, conduct, analysis, or reporting of the study.
Disclosure
Lisbeth Wellejus Albertsen reports grants from The Danish Heart Association, Department of Clinical Epidemiology’s Research Foundation, Handelsgartner Ove William Buhl Olesen og ægtefælde fru Edith Buhl Olesens mindelegat, Politimester J.P.N. Colind og hustru Colinds Mindelegat, and Danske lægers forsikring under SEB pension, during the conduct of the study. Corina Grey is supported by a grant from Heart Foundation of New Zealand, during the conduct of the study. Morten Schmidt is supported by the Novo Nordisk Foundation (grant NNF19OC0054908). The authors report no other potential conflicts of interest for this work.
References
1. Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long-term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344(jan25 2):e356. doi:10.1136/bmj.e356
2. Normand SL, Morris CN, Fung KS, McNeil BJ, Epstein AM. Development and validation of a claims based index for adjusting for risk of mortality: the case of acute myocardial infarction. J Clin Epidemiol. 1995;48(2):229–243. doi:10.1016/0895-4356(94)00126-B
3. Qu Z, Zhao LP, Ma X, Zhan S. Building a patient-specific risk score with a large database of discharge summary reports. Med Sci Monit. 2016;22:2097–2104. doi:10.12659/MSM.899262
4. Sanchis J, Nunez J, Bodi V, et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation acute coronary syndrome. Mayo Clin Proc. 2011;86(4):291–296. doi:10.4065/mcp.2010.0702
5. Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37(4):992–997. doi:10.1016/S0735-1097(01)01109-3
6. Azzalini L, Chabot-Blanchet M, Southern DA, et al. A disease-specific comorbidity index for predicting mortality in patients admitted to hospital with a cardiac condition. CMAJ. 2019;191(11):E299–E307. doi:10.1503/cmaj.181186
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
8. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi:10.1097/MLR.0b013e31819432e5
9. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. doi:10.1186/1472-6963-10-245
10. Holman CD, Preen DB, Baynham NJ, Finn JC, Semmens JB. A multipurpose comorbidity scoring system performed better than the Charlson index. J Clin Epidemiol. 2005;58(10):1006–1014. doi:10.1016/j.jclinepi.2005.01.020
11. Stirland LE, Gonzalez-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ. 2020;368:m160. doi:10.1136/bmj.m160
12. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S179083
13. Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
14. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
15. Ehrenstein V, Antonsen S, Pedersen L. Existing data sources for clinical epidemiology: Aarhus University Prescription Database. Clin Epidemiol. 2010;2:273–279. doi:10.2147/CLEP.S13458
16. Grann AF, Erichsen R, Nielsen AG, Froslev T, Thomsen RW. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138. doi:10.2147/CLEP.S17901
17. National Health Board. National Minimum Dataset (Hospital Events) Data Dictionary. Ministry of Health Wellington; 2014.
18. Ministry of Health. Mortality Collection Data Dictionary. Ministry of Health Wellington; 2009.
19. Ministry of Health. Pharmaceutical Claims Data Mart Data Dictionary. Ministry of Health Wellington, 2017.
20. Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–W73. doi:10.7326/M14-0698
21. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi:10.1093/biomet/69.1.239
22. Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–28. doi:10.1016/j.jclinepi.2016.03.031
23. Knottnerus JA, Tugwell P, Wells G. Editorial comment: ratios should be multiplied, not added. J Clin Epidemiol. 2016;79:30. doi:10.1016/j.jclinepi.2016.11.007
24. Stukenborg GJ, Wagner DP, Connors AF
25. Chu Y, Ng Y, Wu S. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv Res. 2010;10(1). doi:10.1186/1472-6963-10-140
26. Gutacker N, Bloor K, Cookson R. Comparing the performance of the Charlson/Deyo and Elixhauser comorbidity measures across five European countries and three conditions. Eur J Public Health. 2015;25(Suppl 1):15–20. doi:10.1093/eurpub/cku221
27. Yamana H, Matsui H, Sasabuchi Y, Fushimi K, Yasunaga H. Categorized diagnoses and procedure records in an administrative database improved mortality prediction. J Clin Epidemiol. 2015;68(9):1028–1035. doi:10.1016/j.jclinepi.2014.12.004
28. Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol a Biol Sci Med Sci. 2007;62(3):281–285.
29. Sundboll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi:10.1136/bmjopen-2016-012832
30. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of patients. BMC Med Res Methodol. 2011;11:83. doi:10.1186/1471-2288-11-83
31. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi:10.1001/archinte.163.19.2345
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


