Back to Journals » Cancer Management and Research » Volume 13
The Correlation of Age with Prognosis of Atypia of Undetermined Significance and Follicular Lesion of Undetermined Significance in Thyroid Nodules
Authors Kaliszewski K , Diakowska D , Rzeszutko M, Wojtczak B, Rudnicki J
Received 31 January 2021
Accepted for publication 10 March 2021
Published 8 April 2021 Volume 2021:13 Pages 3101—3111
DOI https://doi.org/10.2147/CMAR.S304686
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Krzysztof Kaliszewski,1 Dorota Diakowska,2 Marta Rzeszutko,3 Beata Wojtczak,1 Jerzy Rudnicki1
1Department of General, Minimally Invasive and Endocrine Surgery, Wroclaw Medical University, Wroclaw 50-556, Poland; 2Department of Nervous System Diseases, Faculty of Health Science, Wroclaw Medical University, Wroclaw 51-618, Poland; 3Department of Pathomorphology, Wroclaw Medical University, Wroclaw 50-368, Poland
Correspondence: Krzysztof Kaliszewski
Department of General, Minimally Invasive and Endocrine Surgery, Wroclaw Medical University, Borowska Street 213, Wroclaw 50-556, Poland
Email [email protected]
Purpose: Although some prognostic variables and risk factors for thyroid cancer (TC) are age-related, the association between age and the risk of TC in patients with thyroid nodules (TNs) assigned to atypia of undetermined significance (AUS) and follicular lesion of undetermined significance (FLUS) is poorly estimated. The aim of this study was to assess the histopathology of AUS/FLUS and the risk of TC according to the age of the patients at the time of AUS/FLUS diagnosis.
Patients and Methods: Among 5021 individuals treated for TNs at one institution from 2008 to 2018, 161 (3.2%) patients with 161 TNs assigned to the AUS/FLUS category (1 nodule per patient) were selected and stratified by age at initial diagnosis: < 55 years, 55– 75 years and > 75 years. Logistic regression analysis was used to estimate the association of age with the risk of TC diagnosis.
Results: Ninety-one (56.52%) patients < 55 years old, 58 (36.02%) patients 55– 75 years old, and 12 (7.45%) individuals > 75 years old were identified. There were 130 (80.7%) females and 31 (19.3%) males with a mean age of 50.6 ± 16.12 years. Among the evaluated TNs, 142 (88.2%) were ultimately diagnosed as benign, and 19 (11.8%) were diagnosed as malignant. Younger age in patients was significantly related to malignancy outcome (p=0.024 for age < 55 years). Patients aged 55– 75 years had a significantly lower risk of TC than the other age categories (p=0.040). The risks of high vascularity and fast tumor growth were significantly higher in the youngest category than in the other categories (age < 55 years old: p=0.045 and p=0.002, respectively).
Conclusion: Although patients with TNs classified as AUS/FLUS by ultrasound-guided fine needle aspiration biopsy (UG-FNAB) are not typically qualified for surgery, it is worth noting that younger patients with an AUS/FLUS diagnosis might be at a higher risk of TC.
Keywords: age, risk factors, AUS/FLUS, thyroid cancer, surgery
Introduction
The total incidence of thyroid cancer (TC) is rapidly increasing worldwide, mainly due to incidental thyroid nodules (TNs) found on ultrasound examinations. In our earlier study, we described this phenomenon as “cancer screening activity”.1 Cibas et al2 estimated that TNs might be observed in 50% of patients aged 50 years, with a low overall malignancy risk of 5 to 7%.
The most commonly employed preoperative diagnostic tool for TN evaluation remains the ultrasound-guided fine needle aspiration biopsy (UG-FNAB) procedure.1,2 On the basis of this screening test, The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was introduced in 20092 and subsequently modified in 2017.3,4 It consists of six categories that enhance and standardize TN management. Each category has an estimated TC risk and recommended guidelines. However, among these six categories of TBSRTC, further management is still complicated for the third category.5,6 This category contains heterogeneous lesions assigned to two subgroups: atypia of undetermined significance (AUS) and follicular lesion of undetermined significance (FLUS).3–6 The prevalence of malignancy within this category ranges from 5% to 37%.7,8 Some other studies report an even higher malignancy rate, up to 55%, suggesting diagnostic and simultaneous therapeutic lobectomy in cases of AUS/FLUS diagnosis.9,10 The implied risk of malignancy of AUS/FLUS assessed and presented in 2009 in TBSRTC was 5–15%.2 In the recent edition of this classification, it has been changed to 10–30% if noninvasive follicular neoplasms with papillary-like features (NIFTPs) are considered malignant tumors and 6–18% if NIFTPs are excluded from malignancy.3,4 However, in opinion of many authors, the risk of malignancy in category III TBSRTC is found to differ widely depending on the type of atypia observed in the specimen.3,4 According to some studies, it was found to be higher in cellular atypia and lower in follicular architectural atypia.11,12 In our opinion, the accurate prevalence is found to vary among many institutions and observation times. In our previous study, we estimated a 10.2% incidence of TC in the AUS/FLUS category.13 According to others, the cytological features of AUS/FLUS seem not sufficiently exposed to categorize TNs as benign, malignant or even suspicious for malignancy.6 Pathologists use it as the last resort, with only 7% of UG-FNAB results, which receive this diagnosis.2 Others describe this category as a “gray zone” between benignity and malignancy.6 Some recent studies have shown the prevalence of AUS/FLUS diagnosis to be as high as 10–12%.11,14,15 More interestingly, category III of TBSRTC is highly variable, with an approximate reproducibility of only 50%, even among the most experienced pathologists.16 This category is further divided into six subcategories: architectural atypia, Hürthle cell aspirates, cytologic and architectural atypia, cytologic atypia, atypical lymphoid cells, and atypia not otherwise specified (NOS).10 The variability and higher rates of malignancy caused by these changes highlight both the uncertainty and the importance of further management.
Age is recognized as one of the prognostic factors for well-differentiated thyroid cancer (WDTC) and one of the most important parameters in the 8th Edition of the American Joint Committee on Cancer (AJCC) TNM classification system for TCs.17 However, little attention was made in previous studies to its influence on the risk of malignancy, especially in patients with TNs assigned to the AUS/FLUS category. Therefore, the aim of this study was to assess the influence of age on the histopathology and prognosis of AUS/FLUS TN categories.
Patients and Methods
We performed retrospective chart reviews of 5021 patients with TNs who were admitted and surgically treated at the Department of General, Gastroenterological and Endocrine Surgery of Wroclaw Medical University (Poland) between 2008 and 2018. All of the patients before surgery had UG-FNAB performed at least once, and all specimens were reported using TBSRTC classification.18
After implementing exclusion criteria (family history of TC, previous radiation on the neck area, change in cytology category from AUS/FLUS to a lower or higher category of the Bethesda classification during subsequent UG-FNAB procedures when needed), 161 (3.2%) patients with TNs assigned to the AUS/FLUS category were included.
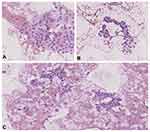
The patients qualified for surgery on the basis of cytology results (Figure 1) and the presence of the clinical and ultrasound features of malignancy (Figure 2) or the presence of pressure symptoms in the neck. Suspicious ultrasound features13,19,20 of TNs assigned to the AUS/FLUS category included microcalcifications, irregular margins, hypoechogenicity (less than surrounding strap muscles), taller-than-wide configuration, and high vascularity (intranodular flow with multiple vascular poles chaotically arranged). The presence of many suspicious features often correlates with category 4 or 5 of the Thyroid Imaging Reporting and Data System (TI-RADS) classification.20
A total of 121 (75.15%) patients underwent surgery after the first AUS/FLUS diagnosis. Twenty-seven (16.77%) individuals had two consecutive AUS/FLUS diagnoses, and 13 (8.08%) patients had 3 or more consecutive AUS/FLUS results. The steps for patient selection are presented in Figure 3.
 |
Figure 3 Flowchart of patient selection with individuals finally included in the study. AUS/FLUS: atypia of undetermined significance/follicular lesion of undetermined significance. |
All of the patients underwent diagnostic/therapeutic surgery: 48 (29.82%) lobectomies and 113 (70.18%) thyroidectomies. The surgical tissue specimens were fixed in 10% buffered formalin and diagnosed histopathologically. Representative blocks were selected. A minimum of 5–8 blocks were taken from each lesion. Serial sectioning and careful cutting of the representative tissue sample were performed. A routine method of specimen processing was performed. The sections were cut into 4-µm-thick sections, from which conventional hematoxylin and eosin (H&E) staining sections were prepared. The H&E sections were evaluated by two experienced thyroid lesion pathologists to confirm the diagnosis, features of the tumor and extent of the malignant process. The risk of malignancy (ROM) for the AUS/FLUS category was calculated after grouping cases by age.
Statistical Analysis
Descriptive data are presented as the number of observations and percent or mean, standard deviation (±SD) and range (min.-max). Categorical variables were analyzed using the χ2 test and Fisher’s exact test. Logistic regression analysis was performed to calculate the odds ratios for the risk of cancer presence, high vascularity and fast growth (≥1mm/year) of thyroidal nodules according to age ranges: <55, 55–75 and >75 years old. A 2-tailed p-value of p<0.05 was considered statistically significant. Statistical analyses were performed using Statistica v.13.3 software (Tibco Software Inc. CA, USA).
Results
A total of 5021 patients with TNs underwent UG-FNAB and subsequent surgery. Among them, 161 (3.2%) cases were classified into the AUS/FLUS category using TBSRTC. Thus, of these cases, our overall category III TBSRTC utilization rate was 3.2%, which is significantly lower than the recommended 7% utilization for the AUS/FLUS category.21 The demographic and clinical data and ultrasound features of the overall study group are presented in Table 1.
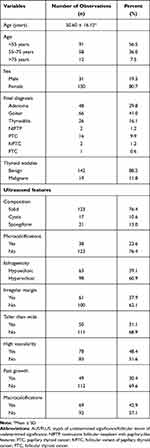 |
Table 1 Demographic and Clinical Parameters and Ultrasound Features of the 161 Patients with Bethesda Category III (AUS/FLUS) |
There were 130 (80.7%) females and 31 (19.3%) males with a mean age of 50.6 ± 16.12 years old. Average nodule size ± SD was 19 ± 15 mm. To confirm the final postsurgical diagnosis, all histopathological specimens of all patients were additionally analyzed. Two (1.2%) PTCs were reclassified as NIFTPs. Among TNs, 142 (88.2%) were finally diagnosed as benign, and 19 (11.8%) were diagnosed as malignant. The malignant histopathological diagnoses were as follows: 16 (9.9%) classical variant of papillary thyroid cancer (cvPTC), 2 (1.2%) follicular variant of PTC (fvPTC), and 1 (0.6%) FTC. Among benign TNs, the final histopathology diagnoses were 48 (29.8%) follicular adenomas, 66 (41.0%) goiters, 26 (16.1%) thyroiditis, and 2 (1.2%) NIFTPs. We compared the demographic, clinical and ultrasound factors in three subgroups of patients with Bethesda category III according to age (Table 2). Next, we performed logistic regression analysis of ultrasound variables (hypoechogenicity, microcalcifications, high vascularity, fast growth, irregular margins, taller than wide shape and macrocalcifications) as risk factors of cancer presence in total group of patients with AUS/FLUS category (Table 3).
 |
Table 3 Logistic Regression Analysis of Ultrasound Variables as Risk Factors of Cancer Presence in Total Group of Patients with Bethesda III Category. Results Were Calculated by Chi-Square Wald Test |
Patients below 55 years old demonstrated significantly higher rates of malignant outcome (p=0.021), presence of tumor vascularization (p=0.006) and fast growth of nodules –nodule enlargement ≥1 mm/6 months (p=0.004). Logistic regression analysis was performed to confirm the association of age ranges with the risk of cancer presence, high vascularity and fast growth of tumors. The results presented in Table 4 show that younger age of patients is related to malignancy outcome (p=0.024 for age <55 years).
Patients aged 55–75 years had a significantly lower risk of cancer presence (p=0.040). The risk of high vascularity and fast growth of tumors significantly increased in patients aged below 55 years (p=0.045 and p=0.002, respectively), as shown in Table 4.
Patients aged above 75 years had a significantly lower risk of higher vascularity (p=0.019, Table 4), and patients aged 55–75 years demonstrated a significantly lower risk of fast nodule growth (p=0.004, Table 4).
Discussion
The clinical management of the AUS/FLUS diagnostic category has represented an ongoing challenge. In our study, the overall AUS/FLUS utilization rate was 3.2% (161/5021), which is under the recommended 7% utilization for this diagnostic category.21 However, we must emphasize that we analyzed only those patients who underwent surgery. Therefore, the evaluation of the rate of malignancy in patients with TNs assigned to AUS/FLUS was subjected to bias. It is obvious that many patients with category III TBSRTC estimated on UG-FNAB did not undergo surgery, and we cannot assume that all of these cases were benign. If we want to estimate the histology of all TNs assigned to the AUS/FLUS category, we cannot assess only operated cases. On the other hand, we cannot obtain all histopathology diagnoses from all individuals with TNs assigned to the AUS/FLUS category because not all of them need surgery. In our study of 161 TNs assigned to the AUS/FLUS category from the same number of patients (n=161), 66 (41.0%) nodules could be assigned to category II, which were histopathologically diagnosed as multinodular goiter and 16 (9.9%) nodules could be assigned to category V or IV. These 16 patients were histopathologically diagnosed as PTC. Because all of the specimens were additionally analyzed for the purpose of this study (besides its retrospective design), two cases previously diagnosed as papillary thyroid microcarcinoma (PTMC) were reclassified as NIFTP. In the last group of patients with nonmalignant tumors, 26 (16.1%) cases were diagnosed as thyroiditis. On the basis of our clinical observations (26 individuals, 16.1% of all, third group of histopathology diagnosis in AUS/FLUS category) and with accordance to some other studies’ results, we confirmed that these autoimmunological inflammatory entities are well recognized for cytomorphological pitfalls.22,23 Straccia et al,24 after a review of 4,475 surgically treated patients with TBSRTC category III, estimated that 27% of cases were malignant. Despite assessing the risk of malignancy as 10–30%, the current risk seems ambiguous, and there are some arguments to assess some accurate risk factors to specify their use.25 However, others suggest that removing the AUS/FLUS category will decrease the sensitivity of the whole TBSRTC classification.26 In our analysis, the risk of malignancy for all individuals was estimated to be 11.8%. This percentage of cases is in agreement with the original risk of malignancy estimated and proposed in the TBSRTC classification system.2–4,21 However, as we said, this is the number of patients who underwent surgery. Interestingly, in patients with two and three consecutive AUS/FLUS diagnoses, we estimated 18.51% and 38.46% risks of malignancy, respectively. In our study group, we had twenty-two cases in which after the first UG-FNAB, we obtained the AUS/FLUS category, but in the second procedure, we obtained a high suspicion of malignancy (category V). However, due to the aim of this study, these individuals were excluded from our analysis. We can add that in nodules with a cytological AUS/FLUS classification and subsequently high suspicion of malignancy, TC was diagnosed in 100% of these lesions. Sullivan et al15 obtained similar results. Others suggest that although many neoplastic and non neoplastic TNs present AUS/FLUS features, high-grade malignancies are rarely assigned to this category.27–29
Some authors have assessed several characteristic features of TNs, in which cytopathology results were AUS/FLUS diagnosis, which can be found as risk factors for malignancy.30–33 Features such as taller than wide shape, microcalcifications and fast growth were estimated as independent predictive factors for TC. In our previous study, we demonstrated that microcalcifications and fast growth of TNs could be used as predictive factors for the development of TC in patients with AUS/FLUS diagnosis.13 However, we did not evaluate it according to the patient’s age.
While many cases diagnosed as category III TBSRTC undergo repeated UG-FNAB, a number of studies have also been carried out that have focused on the use of ancillary molecular tests to facilitate appropriate clinical management. Such strategies may increase the cost of treatment, however their value is significantly high. Age seems to be an easily accessible clinical feature of patients that has received little attention in the literature regarding its effect on ROM in terms of TBSRTC diagnostic results. Because AJCC in the 8th edition of the TNM classification17 recently recommended 55 years of age as an ideal cutoff threshold for TC staging, we divided the enrolled patients into three groups (<55 years old, from 55 to 75 years old, and >75 years old) and performed our analysis. The AJCC suggests that TC in individuals aged 55 years and older is associated with a more aggressive clinical course and worse prognosis.34 It was supported by our results.35 Studies have assessed that a higher age cutoff rather than a previously estimated 45 years old improves the accuracy of the prognostic system and therefore should decrease the overtreatment rate of patients.35 In the present study, we found that younger age of the patient with AUS/FLUS diagnosis was an independent predictor for malignancy. We observed a statistically significant difference in the ROM between the patients below 55 years old and those 55 years old and older. The OR in the younger group was 3.72 compared to 0.18 in the older group. This means that despite TNs being more often diagnosed and having a worse clinical course in older patients when diagnosed with malignancy, in the case of category III TBSRTC, TNs have a higher ROM in younger patients. Therefore, age should be taken into consideration when using TBSRTC to facilitate a more accurate estimation of their actual ROM. Williams et al36 found that patient age is predictive of malignancy in patients less than 65 years of age in conjunction with other clinical parameters, such as nodule size and ultrasound features.
Hong et al37 assessed the independent effect of risk factors such as age, sex, nodule size, atypical descriptors and ultrasound features for malignancy in 129 patients with UG-FNAB results categorized as AUS/FLUS. They did not identify that age as a single parameter was significantly related to the malignancy of TNs assigned to the AUS/FLUS category. However, they confirmed it in the presence of speculated margins, nuclear grooving and irregular nuclei. On the basis of their analysis, they recommend surgical resection of TNs in patients with AUS/FLUS showing these histopathological findings.37
In our study, we estimated that the prevalence of AUS/FLUS diagnosis in analyzed patients was relatively high in TNs composed with thyroiditis. In 161 patients with AUS/FLUS diagnosis, 26 (16.1%) presented thyroiditis as an existing finding in histopathological diagnosis. Some authors state that thyroiditis increases cytological atypia on UG-FNAB, so the rate of malignancy for AUS/FLUS in TNs is lower with coexisting thyroiditis.38 They also concluded that cytological atypia promoted by thyroiditis may increase the number of AUS/FLUS diagnoses in TNs, which may lead to overestimation of malignancy rates in patients with autoimmunological inflammatory changes.38
Because of many discrepancies, some authors suggest more accurate division of the AUS/FLUS category into two-tiered subclassifications consisting of low cellularity with predominant microfollicular architectures with absence of colloid and nuclear atypia (such as the features in malignant tumors) assigned to the group with a higher risk of malignancy.39–41 However, Seo et al42 said that at this point, genetic diagnostic methods had played a basic role in predicting malignancy risk in AUS/FLUS nodules. The authors even suggest their routine daily use.42 At present, none of the molecular tests are available at our center. Generally, we can conclude that many of AUS/FLUS nodules harbor malignancy; however, in a tertiary referral center such as ours, this can be observed due to possible selection bias.
To enhance diagnostic accuracy and accelerate proper management with TNs assigned to the AUS/FLUS category, the American Thyroid Association (ATA) working group proposes a combination of clinical and ultrasound features, UG-FNAB repetition and molecular tests.43,44 They enumerated some features, such as TN diameter higher than 4 cm, family history of TC, radiation therapy on the neck area, hypoechogenicity, microcalcifications, irregular margins, taller-than-wide shape and extrathyroidal extension.43,44 In 2018, we added our own observations that in addition to microcalcifications, fast growth of TNs could be used as a predictive factor for the development of TC in patients with TNs classified into the AUS/FLUS category.13
After analysis of all patients with TNs designated to the AUS/FLUS category, we confirmed that this diagnosis is a “gray zone”, which does not give clinicians a definitive and clear answer regarding further management. Additionally, category III TBSRTC may delay the management of patients. In our study, we estimated that some clinical information plays a supportive role in decision making. Despite the many ultrasound features of TNs assigned to the AUS/FLUS category, some demographic characteristics might be helpful. In our analysis, we found that sex does not influence histology; however, in younger patients, the risk of TC in the AUS/FLUS category may be higher. The AUS/FLUS category requires strict cooperation between clinicians, cytopathologists, radiologists and surgeons.
Our study has some limitations that must be noted. The main, there was a small number of cases. We analyzed only these individuals, who were designated the AUS/FLUS category. All cases in which category III TBSRTC was changed to another (higher or lower) were excluded from the study. The study included selection bias because we evaluated only patients with AUS/FLUS categories who underwent surgery. However, it did not bring us to a higher ROM than that recommended by the TBSRTC classification.2–4,13 According to the ATA guidelines, surgically resected TNs assigned to the AUS/FLUS category in UG-FNAB have been reported to have an ROM that ranges from 6% to 48%.21
Conclusion
Although patients with TNs diagnosed with AUS and FLUS after UG-FNAB are not typically qualified for surgery, it is worth noting that younger patients with AUS/FLUS diagnoses might be at a higher risk of TC.
Abbreviations
TC, thyroid cancer; TN, thyroid nodule; AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; UG-FNAB, ultrasound-guided fine needle aspiration biopsy; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology; NIFTP, noninvasive follicular neoplasms with papillary-like feature; WDTC, well-differentiated thyroid cancer; AJCC, American Joint Committee on Cancer; TNM, Tumor, Nodes, Metastases; TI-RADS, Thyroid Imaging Reporting and Data System; H&E, hematoxylin and eosin; ROM, risk of malignancy; SD, standard deviation; PTC, papillary thyroid cancer; fvPTC, follicular variant of papillary thyroid cancer; FTC, follicular thyroid cancer; ATA, American Thyroid Association.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
All procedures were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Our study protocol was approved by the Bioethics Committee of Wroclaw Medical University, Poland (Signature number: KB-783/2017). We obtained verbal consent from the participants instead of written consent, and this procedure was approved by the Bioethics Committee. The data were analyzed retrospectively and anonymously from established medical records. The authors did not have access to identifying patient information or direct access to the study participants.
Acknowledgment
The authors are grateful to all the staff at the study center who contributed to this work.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Internal Grant for Science Development of Wroclaw Medical University in Poland (Grant Number: SUB.B110.21.056).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaliszewski K, Diakowska D, Wojtczak B, Rudnicki J, Schmitt F. Cancer screening activity results in overdiagnosis and overtreatment of papillary thyroid cancer: a 10-year experience at a single institution. PLoS One. 2020;15(7):e0236257. doi:10.1371/journal.pone.0236257
2. Cibas ES, Ali SZ. The bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665.
3. Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2017;6(6):217–222.
4. Krane JF, Nayar R, Renshaw AA. Atypia of undetermined significance/follicular lesion of undetermined significance. In: Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes.
5. Huhtamella R, Kholová I. Thyroid bethesda category AUS/FLUS in our microscopes: three-year-experience and cyto-histological correlation. Cancers (Basel). 2019;11(11):1670.
6. Almahari SA, Harb Z, Alshaikh S. Evaluation of thyroid nodules classified as Bethesda category III on cytology and their malignancy rate: an institutional experience. Cytojournal. 2019;16:18.
7. Pasha HA, Dhanani R, Mughal A, Ahmed KS, Suhail A. Malignancy rate in thyroid nodules with atypia or follicular lesion of undetermined significance. Int Arch Otorhinolaryngol. 2020;24(2):e221–e226.
8. Seagrove-Guffey MA, Hatic H, Peng H, Bates KC, Odugbesan AO. Malignancy rate of atypia of undetermined significance/follicular lesion of undetermined significance in thyroid nodules undergoing FNA in a suburban endocrinology practice: a retrospective cohort analysis. Cancer Cytopathol. 2018;126(10):881–888.
9. Vázquez YL, Penín Álvarez M, San Miguel Fraile P, Barragáns Pérez M. Riesgo de malignidad de los nodulos tiroideos con atypia de significado incierto. Endocrinol Nutr. 2015;62(10):507–510.
10. Ling J, Li W, Lalwani N. Atypia of undetermined significance/follicular lesions of undetermined significance: what radiologists need to know. Neuroradiol J. 2020. doi:10.1177/1971400920983566
11. Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346.
12. Valderrabano P, Khazai L, Thompson ZJ, et al. Cancer risk associated with nuclear atypia in cytologically indeterminate thyroid nodules: a systematic review and meta-analysis. Thyroid. 2018;28(2):210–219.
13. Kaliszewski K, Diakowska D, Wojtczak B, Forkasiewicz Z. Evaluation of selected ultrasound features of thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance for the Bethesda reporting system for thyroid cytology. Cancer Manag Res. 2018;10:2223–2229.
14. Garg S, Naik LP, Kothari KS, Fernandes GC, Agnihotri MA, Gokhale JC. Evaluation of thyroid nodules classified as Bethesda category III on FNAC. J Cytol. 2017;34(1):5–9.
15. Sullivan PS, Hirschowitz SL, Fung PC, Apple SK. The impact of atypia/follicular lesion of undetermined significance and repeat fine-needle aspiration: 5 years before and after implementation of the Bethesda System. Cancer Cytopathol. 2014;122(12):866–872.
16. Ho AS, Sarti EE, Jain KS, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid. 2014;24(5):832–839.
17. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018;68(1):55–63.
18. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. Springer: Cham, Switzerland; 2018.
19. Kumbhar SS, O’Malley RB, Robinson TJ, et al. Why thyroid surgeons are frustrated with radiologists: lessons learned from pre- and postoperative US. Radiographics. 2016;36(7):2141–2153.
20. Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14(5):587–595.
21. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
22. Jing X, Michael CW. Potential pitfalls for false suspicion of papillary thyroid carcinoma: a cytohistologic review of 22 cases. Diagn Cytopathol. 2012;40(Suppl 1):E74–E79.
23. Baloch ZW, LiVolsi VA. Cytologic and architectural mimics of papillary thyroid carcinoma. Diagnostic challenges in fine-needle aspiration and surgical pathology specimens. Am J Clin Pathol. 2006;125(Suppl):S135–S144.
24. Straccia P, Rossi ED, Bizzarro T, et al. A meta-analytic review of the bethesda system for reporting thyroid cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015;123(12):713–722.
25. Somma J, Schlecht NF, Fink D, Khader SN, Smith RV, Cajigas A. Thyroid fine needle aspiration cytology: follicular lesions and the gray zone. Acta Cytol. 2010;54(2):123–131.
26. Shi Y, Ding X, Klein M, et al. Thyroid fine‐needle aspiration with atypia of undetermined significance: a necessary or optional category? Cancer Cytopathol. 2009;117(5):298–304.
27. Liu X, Medici M, Kwong N, et al. Bethesda categorization of thyroid nodule cytology and prediction of thyroid cancer type and prognosis. Thyroid. 2016;26(2):256–261.
28. Evranos B, Polat SB, Baser H, et al. Bethesda classification is a valuable guide for fine needle aspiration reports and highly predictive especially for diagnosing aggressive variants of papillary thyroid carcinoma. Cytopathology. 2017;28(4):259–267.
29. Guleria P, Agarwal S, Iyer VK, Jain D, Mathur SR, Yadav D. Subcategorisation of AUS/FLUS thyroid lesions as per the 2017 Bethesda System for Reporting Thyroid Cytopathology: a retrospective study from a tertiary care centre analysing risk of malignancy (ROM) of the different subcategories. J Clin Pathol. 2019;72(11):771–777.
30. Gweon HM, Son EJ, Youk JH, Kim JA. Thyroid nodules with Bethesda system III cytology: can ultrasonography guide the next step? Ann Surg Oncol. 2013;20(9):3083–3088.
31. Yoo WS, Choi HS, Cho SW, et al. The role of ultrasound findings in the management of thyroid nodules with atypia or follicular lesions of undetermined significance. Clin Endocrinol (Oxf). 2014;80(5):735–742.
32. Kuru B, Atmaca A, Tarim IA, et al. Risk factors associated with malignancy and with triage to surgery in thyroid nodules classified as Bethesda category III (AUS/FLUS). Eur J Surg Oncol. 2016;42(1):87–93.
33. Lee S, Shin JH, Oh YL, Hahn SY. Subcategorization of Bethesda system category III by Ultrasonography. Thyroid. 2016;26(6):836–842.
34. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
35. Kaliszewski K, Diakowska D, Nowak Ł, Wojtczak B, Rudnicki J. The age threshold of the 8th edition AJCC classification is useful for indicating patients with aggressive papillary thyroid cancer in clinical practice. BMC Cancer. 2020;20(1):1166.
36. Williams BA, Bullock MJ, Trites JR, Taylor SM, Hart RD. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42(1):61.
37. Hong IK, Kim JH, Cho YU, Park SY, Kim SJ. Clinicopathological factors increased the risk of malignancy in thyroid nodules with atypical or follicular lesions of undetermined significance (AUS/FLUS) risk factor of malignancy in thyroid nodule with AUS/FLUS. Ann Surg Treat Res. 2016;90(4):201–206.
38. Mulder MB, Khazeni KC, Sussman MS, Lew JI, Farrá JC. Chronic lymphocytic thyroiditis may lower accuracy of AUS/FLUS cytopathology in surgical patients. J Surg Res. 2020;245:244–248.
39. Luu MH, Fischer AH, Stockl TJ, Pisharodi L, Owens CL. Atypical follicular cells with equivocal features of papillary thyroid carcinoma is not a low-risk cytologic diagnosis. Acta Cytol. 2011;55(6):526–530.
40. Kim SJ, Roh J, Baek JH, et al. Risk of malignancy according to sub-classification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda system for reporting thyroid cytopathology. Cytopathology. 2017;28(1):65–73.
41. Eisa N, Khan A, Akhter M, et al. Both ultrasound features and nuclear atypia are associated with malignancy in thyroid nodules with atypia of undetermined significance. Ann Surg Oncol. 2018;25(13):3913–3918.
42. Seo JW, Jang AL, Suh SH, Park HS, Kang MK, Hong JC. Atypia of undetermined significance on thyroid fine needle aspiration – risk factors for malignancy. Clin Otolaryngol. 2017;42(2):234–238.
43. Estrada Muñoz L, Díaz Del Arco C, Ortega Medina L, Fernández Aceñero MJ. Thyroid atypia/follicular lesion of undetermined significance: attitudes towards the diagnosis of bethesda system III nodules. Acta Cytol. 2017;61(1):21–26.
44. Steward D, Carty S, Sippel R, Yang P, Sosa J, Sipos J. Clinical validation of ThyroSeq v3 performance in thyroid nodules with indeterminate cytology: a prospective blinded multi-institutional validation study. In:
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.