Back to Journals » International Journal of General Medicine » Volume 14
The Correlation Between Whole Blood Copper (Cu), Zinc (Zn) Levels and Cu/Zn Ratio and Sepsis-Induced Left Ventricular Systolic Dysfunction (SILVSD) in Patients with Septic Shock: A Single-Center Prospective Observational Study
Authors Meng JB, Hu MH, Zhang M, Hu GP, Zhang W, Hu SJ
Received 21 August 2021
Accepted for publication 7 October 2021
Published 27 October 2021 Volume 2021:14 Pages 7219—7234
DOI https://doi.org/10.2147/IJGM.S335348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jian-Biao Meng,1,2 Ma-Hong Hu,2 Ming Zhang,3 Gong-Pai Hu,4 Wei Zhang,5 Shen-Jiang Hu1
1Department of Cardiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, 310003, People’s Republic of China; 2Intensive Care Unit, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang Province, 310012, People’s Republic of China; 3Intensive Care Unit, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People’s Republic of China; 4Department of Ultrasonography, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang Province, 310012, People’s Republic of China; 5Department of Cardiology, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang Province, 310012, People’s Republic of China
Correspondence: Shen-Jiang Hu
Department of Cardiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, 310003, People’s Republic of China
Email [email protected]
Purpose: This study aimed to explore relationships between whole blood copper (Cu), zinc (Zn) and Cu/Zn ratio and cardiac dysfunction in patients with septic shock.
Subjects and Methods: Between April 2018 and March 2020, septic shock patients with sepsis-induced left ventricular systolic dysfunction (SILVSD, left ventricular ejection fraction, LVEF< 50%) and with no sepsis-induced myocardial dysfunction (non-SIMD, septic shock alone and LVEF> 50%) and controls were prospectively enrolled. Whole blood Cu and Zn levels were measured using flame atomic absorption spectrophotometry.
Results: Eighty-six patients with septic shock including both 41 SILVSD and 45 non-SIMD and 25 controls were studied. Whole blood Cu levels and Cu/Zn ratio were significantly higher and Zn levels were lower in SILVSD compared with non-SIMD and controls (Cu, p=0.009, < 0.001; Zn, p=0.029, < 0.001; Cu/Zn ratio, p=0.003, < 0.001). Both increased whole blood Cu and Cu/Zn ratio and reduced Zn were associated with lower LVEF (all p< 0.001) and higher amino-terminal pro-B-type natriuretic peptide (NT-proBNP) (Cu, p=0.002; Zn, p< 0.001; Cu/Zn ratio, p< 0.001) and had predictive values for SILVSD (Cu, AUC=0.666, p=0.005; Zn, AUC=0.625, p=0.039; Cu/Zn ratio, AUC=0.674, p=0.029). Whole blood Cu levels and Cu/Zn ratio were increased but Zn levels were reduced in non-survivors compared with survivors (Cu, p< 0.001; Zn, p< 0.001; Cu/Zn ratio, p< 0.001). Whole blood Cu and Zn displayed the value of predicting 28-day mortality (Cu, AUC = 0.802, p< 0.001; Zn, AUC=0.869, p< 0.001; Cu/Zn ratio, AUC=0.902, p< 0.001).
Conclusion: Findings of the study suggest that whole blood Cu levels and Cu/Zn ratio are increased in SILVSD patients and positively correlated with cardiac dysfunction, while whole blood Zn levels are reduced and negatively associated with cardiac dysfunction. Moreover, both whole blood Cu, Zn and Cu/Zn ratio might distinguish between SILVSD and non-SIMD in septic shock patients and predict 28-day mortality.
Trial Registration: Registered at http://www.chictr.org.cn/ChiCTR1800015709.
Keywords: copper, zinc, Cu/Zn ratio, septic shock, sepsis-induced left ventricular systolic dysfunction, sepsis-induced myocardial dysfunction, diagnosis, prognosis
Background
Sepsis, one of the most frequent causes of death in intensive care units (ICUs), is characterized as life-threatening organ dysfunction caused by dysregulated host response to infection and occurs when the body's immune response to infection is impaired.1,2 Septic shock is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality.3 Low systemic vascular resistance is one of the most obvious characteristics in patients with septic shock, and the cardiac output (CO) is usually reduced in hypodynamic septic shock.4 Myocardial dysfunction can be seen in up to 30–80% septic shock patients whose cardiac function is not abnormal prior to septic shock.5
Sepsis-induced myocardial dysfunction (SIMD) was demonstrated in the study of 20 patients conducted by Parker et al for the first time.6 SIMD has become one of the major factors of mortality in patients with sepsis,7 and can be divided into left ventricular (LV) systolic/diastolic dysfunction or abnormalities of global/regional wall motion (WMA).8 Sepsis-induced LV systolic dysfunction (SILVSD) manifests reduced systolic function, cardiomegaly and lower ejection fraction (EF) to adapt to low systemic vascular resistance caused by vasoplegia.7,9 SILVSD is characterized by a left ventricular ejection fraction (LVEF) below 50% without LV diastolic dysfunction (LVDD) manifesting E/e’ ratios above 15 by transthoracic echocardiography (TTE).8 Alike B-type natriuretic peptide (BNP), an indirect indicator of heart function, which is released while wall stress is enhanced in sepsis and septic shock, amino-terminal pro-BNP (NT-proBNP) is also increased and elevated NT-proBNP is related to poor prognosis in sepsis and septic shock.10
Cardiac specific injury biomarkers such as troponin (cTNI, cTNT) and heart-type fatty acid binding protein (HFABP) have been studied in patients with septic shock and elevated TNI and HFABP are related to poor prognosis.11,12 However, the results of these studies exploring the impact of TNI levels on SIMD showed differences. Although Klouche et al did not discover an association between TNI and SIMD on echocardiography,13 others found that elevated TNI could be a prognostic factor in patients with SIMD.14,15 Hence, it is vital to look for other more reliable biomarkers with less controversy as prognostic factors for patients with SIMD.
Two of the most significant trace elements are copper (Cu) and zinc (Zn), which play a major role in many important biological functions. Cu and Zn act as cofactors for several enzymes to mediate important biochemical reaction and must be present in the body in an appropriate amount.16 Abnormal immune and inflammatory responses can alter the distribution of Cu and Zn in the body.17 Serum Zn level is decreased during infection, inflammation and sepsis. Studies in human patients and animal models showed that lower level of serum Zn was associated with severe pneumonia and sepsis and could increase sensitivity for infection and sepsis.18–20 Cu level is elevated in patients with sepsis. People with high levels of serum Cu are liable to suffer from infection, sepsis, myocardial infarction (MI), hypertension, cancer and chronic obstructive pulmonary disease (COPD).21,22 Cu and Zn levels also change in patients with heart failure (HF). Alexanian et al found that the high level of serum Cu in patients with acute heart failure (AHF) or chronic heart failure (CHF) was associated with LV systolic and diastolic dysfunction; on the contrary, a reduction of serum Zn level in CHF and AHF was correlated with LVDD.23 Apart from the changes of Cu and Zn levels, imbalance of Cu/Zn ratio also plays an important biological role in infection and inflammation.24 Lower level of Zn and higher Cu/Zn ratio in patients with extensive purulent soft tissue wounds are correlated with increased C-reactive protein (CRP) values and may be an independent predictor of patients with severe systemic inflammatory response.25 Increased serum Cu and Cu/Zn ratio are associated with the high risk of incident infections.26 However, there are no studies evaluating the change of levels of whole blood Cu, Zn and Cu/Zn ratio in SILVSD patients with septic shock. The current prospective clinical study was planned to explore the change of whole blood Cu, Zn and Cu/Zn ratio of SILVSD patients with septic shock and the association with prognosis of SILVSD patients with septic shock.
Subjects and Methods
In this prospective, non-interventional observational, single-center study, eligible patients and controls were opted as the study participants and referred to Tongde Hospital of Zhejiang Province. The study has been carried out between April 2018 and March 2020.
Ethical Statement
This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (approval no. [2018]016) and followed the ethical guidelines of the 1964 Helsinki Declaration and its later amendments. Written informed consents were obtained from controls and relatives of all enrolled patients who always suffered from varying degrees of consciousness disorder and even were sedated because of intubation and mechanical ventilation in ICU.
Subjects
According to the diagnosis criteria of septic shock and SILVSD, 41 SILVSD patients with septic shock and 45 non-SIMD patients with septic shock were enrolled. Diagnosis criteria: sepsis is defined as Sequential Organ Failure Assessment (SOFA) score ≥2 points consequent to the infection, and septic shock is identified using the clinical criteria of hypotension requiring vasopressor therapy to maintain mean arterial pressure (MAP) 65 mmHg or greater and having a serum lactate level greater than 2 mmol/L after adequate fluid resuscitation.1 SILVSD is diagnosed as a LVEF below 50% without LV diastolic dysfunction manifesting E/e’ ratios above 15 by TTE and abnormalities of global/regional wall motion.8 All patients suffered from septic shock with or without SILVSD were asked to participate in the study. Moreover, the exclusion criteria for the study were: (1) age <18 years or >80 years; (2) pre-existing cardiomyopathy, valvular heart disease, atrial fibrillation, myocarditis, ventricular outflow tract obstruction, AHF and CHF; (3) acute coronary syndrome within recent two weeks; (4) either LVDD or abnormalities of wall motion alone on TTE; (5) chronic inflammatory disease; (6) pregnancy; and (7) continuous blood purification (CBP) within recent two weeks. Furthermore, a group of 25 volunteers free of symptoms, signs and objective evidence of infection, sepsis, septic shock, AHF and CHF served as controls.
Data and Samples Collection
At the enrolment, demographic data, medical history, clinical finding and laboratory parameters were recorded for each subject. At admission, three 5-mL tubes of venous blood samples drawn from all patients with septic shock were collected in heparinized tubes with lithium heparin, and one 2-mL venous blood sample was collected in a tube with EDTA-K2. Two 5-mL tubes of venous blood samples and the 2-mL tube of venous blood sample were sent to the Department of Biochemistry at Tongde Hospital of Zhejiang Province (Hangzhou, China): a 5-mL tube of venous blood sample was used for measuring plasma levels of TNT, NT-proBNP, HFABP and PCT, the other for blood urea nitrogen (BUN), creatinine (Cr), total bilirubin (TB), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate, and the 2-mL tube of venous blood sample for measuring the counts of blood leukocyte and hs-CRP. The third 5-mL tube of venous blood sample was sent to DIAN DIAGNOSTICS (Hangzhou, China) for detection of the levels of Cu and Zn in whole blood. In order to avoid contamination of blood samples and tubes for measuring trace elements, rubber gloves were kept away from samples, tubes and sites for collecting samples during the period of collecting samples because there are metal elements containing in rubber gloves (manufacturer’s instruction: approval and revised date: June 12, 2017, Beijing Bohui Innovation Biotechnology Co., Ltd, Beijing, China).
HFABP concentrations were determined by immunochromatographic assay using the Diagnostic Kit for the Quantitative Determination of Human Heart-type Fatty Acid-Binding Protein Rapid Test (Finecare, Guangzhou, China) in the FS-201 Automatic Immunofluorescence Quantitative Analyzer (Finecare, Guangzhou, China). Plasma concentrations of TNT, NT-proBNP and PCT were determined by electrochemiluminescence (ECL) assay using the Elecsys Troponin T hs Assay Kit, Elecsys proBNP Immunoassay Kit and Elecsys BRAHMS PCT Assay Kit (Roche) in the cobas e411 Analyzer for immunoassay tests (Roche Diagnostics, North America). Complete blood cell counts and hs-CRP were determined using BC-6900 Automatic Hematology Analyzer and original matching reagents (Mindray, Shenzhen, China).
Trace Elements (Cu and Zn) Determination in Whole Blood
Samples that were sent to DIAN DIAGNOSTICS for detection of trace elements were stored in a refrigerator (2–6 °C) and then measured by flame atomic absorption spectrophotometry (FAAS) using the Bohui Human Multi-element Detection kit for Whole Blood or Serum (improved) (Reagents: 1.20 mL×50 pieces and Calibration solutions: 30 mL×4 bottles, Beijing Bohui Innovation Biotechnology Co., Ltd, Beijing, China) in the BH5100T Atomic Absorption Spectrometer (Bohui, Beijing, China) in 48 hours. Major components of the reagent are triton (<0.05%), lanthanum chloride (<1.2%), bovine serum and purified water and the calibration solution contains Cu, Zn calcium (Ca), magnesium (Mg), ferrum (Fe), potassium (K) and sodium (Na). Whole blood Cu and Zn were determined according to the manufacturer’s instruction (approval and revised date: June 12, 2017, Beijing Bohui Innovation Biotechnology Co., Ltd, Beijing, China.). Then, 40 µL of blood was added into the reagent and kept at room temperature (25 °C) for 30 minutes, and then the processed sample was injected into the FAAS. The reference values of whole blood Cu and Zn are in the range of 7.12 to 33.80 μmol/L and 67.72 to 111.30 μmol/L respectively in adults, which were based on the values of whole blood trace elements including Cu and Zn in 101,600 healthy individuals from China, and the reference range was determined by percentile.27–30
Standard Echocardiographic Examination
TTE was performed in the next morning of admission using M9 Ultrasound System (Mindray, Shenzhen, China) by an experienced echocardiographer in the critically ill who was not involved in patient care to evaluate LVEF (modified Simpson’s rule), abnormalities of global/regional wall motion and E/e’ ratios (tissue Doppler imaging).
Statistical Analysis
Continuous variables with normal distribution were described as means±standard deviations (SDs) and analyzed using the independent-samples t-test or one-way ANOVA, followed by the Scheffe test for multiple groups. Continuous variables with skewed distribution were described as medians [interquartile ranges (IQRs)] and analyzed using the Mann–Whitney U-test or the Kruskal–Wallis one-way ANOVA. Categorical variables were provided as proportions (%) and tested with the chi-square test. The correlations between whole blood Cu, Zn and Cu/Zn ratio and Acute Physiology and Chronic Health Evaluation (APACHE)-II score, SOFA score, LVEF, plasma TNT, NT-proBNP, HFABP and PCT were analyzed with partial correlation as controlling for fluid balance prior to ICU and hematocrit (HCT). Receiver operating characteristic (ROC) and area under the ROC curves (AUC) were used to evaluate the predictive and prognostic values of whole blood Cu, Zn concentrations and Cu/Zn ratio. p<0.05 would be recognized as statistically significant. SPSS software (version 23.0, IBM Corporation, Armonk, NY, USA), GraphPad Software (Prism 8.0.2, GraphPad Software) and MedCalc statistical software (version 11.4.2, MedCalc Software Bvba, Ostend) were used to conduct the statistical analysis.
Results
Demographic and Clinical Data
Between April 2018 and March 2020, 236 patients were diagnosed with septic shock. 129 patients were excluded because of 24 patients with age >80 years old, 11 patients with cardiomyopathy, 5 patients with valvular heart disease, 34 patients with acute coronary syndrome and 55 patients with chronic heart failure before enrolment. Then, 107 patients with septic shock who met the inclusion criteria were divided into non-SIMD group (without SIMD, n=45) and SIMD group (n=62) in terms of the diagnostic criteria on TTE. In order to investigate the changes of whole blood Cu and Zn in SILVSD patients with septic shock, 8 patients with LVDD alone and 13 patients with WMA alone were also excluded from the SIMD group. After exclusion, 41 SILVSD patients with septic shock, 45 non-SIMD patients with septic shock and 25 controls were included in the study. Therefore, data of 111 participants were analyzed (Figure 1).
The basic characteristics of the participants are shown in Table 1. As can be seen in Table 1, there are no significant differences in age and percentage of males among the three groups (Control vs non-SIMD vs SILVSD): 66.08±12.12 vs 65.53±13.17 vs 69.20±10.34 years old (age; p=0.335); 15 (60.0%) vs 29 (64.4%) vs 27 (65.9%) (males; p=0.888). The sources of infection included lung, abdomen, bloodstream, urinary tract and others. No significant differences in the sources of infection were revealed between SILVSD and non-SIMD patients (p=0.724), but the major etiologies were pneumonia in all patients. Compared with non-SIMD patients at enrollment, SILVSD patients had significantly higher APACHE-II score and SOFA score and higher TB, AST, ALT, BUN, Cr, TNT, NT-proBNP, HFABP and PCT levels (all p<0.05), but lower LVEF (p<0.05). Furthermore, SILVSD patients were administered more dosage of norepinephrine (NE) to maintain MAP than non-SIMD at admission (p=0.043). No significant difference in patients’ underlying diseases including hypertension, diabetes mellitus, COPD, anemia and stroke was shown between SILVSD and non-SIMD patients (p=0.867). With respect to the differences in fluid balance prior to ICU and HCT, there were no significant differences between SILVSD and non-SIMD (fluid balance prior to ICU: p=0.237; HCT: p=0.518).
 |
Table 1 Basic Characteristics |
Increased Whole Blood Cu and Cu/Zn Ratio and Reduced Zn and the Correlation with LVEF and NT-proBNP in SILVSD Patients
Whole blood Cu and Zn concentrations and Cu/Zn ratio and levels of LVEF and plasma NT-proBNP at admission were analyzed in 41 patients with SILVSD, 45 non-SIMD patients and 25 controls. Whole blood Cu concentrations in patients with SILVSD were higher than those in non-SIMD patients and controls (16.34±1.93 µmol/L vs 15.23±2.07 µmol/L, p=0.009; 16.34±1.93 µmol/L vs 14.02±1.65 µmol/L, p<0.001), and non-SIMD patients also showed increased levels of Cu compared with controls (15.23±2.07 µmol/L vs 14.02±1.65 µmol/L, p=0.013) (Figure 2A). Whole blood Zn levels in patients with SILVSD were lower than those in non-SIMD patients and controls (78.45±12.18 µmol/L vs 85.07±14.80 µmol/L, p=0.029; 78.45±12.18 µmol/L vs 94.90±14.78 µmol/L, p<0.001), and non-SIMD patients also showed reduced levels of Zn compared with controls (85.07±14.80 µmol/L vs 94.90±14.78 µmol/L, p=0.005) (Figure 2B). Cu/Zn ratio in patients with SILVSD was higher than those in non-SIMD patients and controls (0.216±0.053 vs 0.184±0.046, p = 0.003; 0.216±0.053 vs 0.151±0.029, p<0.001], and non-SIMD patients also showed increased Cu/Zn ratio compared with controls (0.184±0.046 vs 0.151±0.029, p=0.017) (Figure 2C).
As controlling for confounders of sensible fluid balance prior to ICU and HCT, partial correlation analysis showed that whole blood Cu was negatively correlated with LVEF (r=−0.551, p<0.001) (Figure 2D), but it was positively correlated with plasma NT-proBNP (r=0.489, p=0.002) (Figure 2E). While whole blood Zn was positively correlated with LVEF (r=0.626, p<0.001) (Figure 2F), it was negatively correlated with plasma NT-proBNP (r=−0.569, p<0.001) (Figure 2G). Cu/Zn ratio was negatively correlated with LVEF (r=−0.665, p<0.001) (Figure 2H), but it was positively correlated with plasma NT-proBNP (r=0.616, p<0.001) (Figure 2I).
Whole Blood Cu and Zn Levels and Cu/Zn Ratio in Relation to APACHE-II Score, SOFA Score, Plasma TNT, HFABP and PCT in SILVSD Patients
As controlling for confounders of sensible fluid balance prior to ICU and HCT, partial correlation analysis showed that whole blood Cu was positively correlated with APACHE-II score and SOFA score (APACHE-II: r=0.384, p=0.016; SOFA: r=0.353, p=0.028) (Figures 3A and B) and plasma TNT, HFABP and PCT (TNT: r=0.368, p=0.021; HFABP: r=0.389, p=0.014; PCT: r=0.424, p=0.007) (Figure 3C–E). On the contrary, whole blood Zn was negatively correlated with APACHE-II score and SOFA score (APACHE-II: r=−0.405, p=0.049; SOFA: r=−0.389, p=0.014) (Figures 3F and G) and plasma TNT, HFABP and PCT (TNT: r=−0.721, p<0.001; HFABP: r=−0.451, p=0.004; PCT: r=−0.482, p=0.002) (Figure 3H–J). Cu/Zn ratio was positively correlated with APACHE-II score and SOFA score (APACHE-II: r=0.364, p=0.023; SOFA: r=0.467, p=0.003) (Figures 3K and L) and plasma TNT, HFABP and PCT (TNT: r=0.633, p<0.001; HFABP: r=0.491, p=0.002; PCT: r=0.523, p=0.001) (Figure 3M-O).
Predictive Values of Whole Blood Cu, Zn and Cu/Zn Ratio for SILVSD
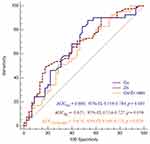
ROC curve analysis was applied to compare the predictive value of whole blood Cu, Zn and Cu/Zn ratio for SILVSD. The AUC for Cu, Zn and Cu/Zn ratio [Cu: (AUC=0.666; 95% CI, 0.556–0.764; p=0.005), Zn (AUC=0.625; 95% CI, 0.514–0.727; p=0.039), Cu/Zn ratio: (AUC=0.674; 95% CI, 0.564–0.771; p=0.029)] suggested that whole Cu, Zn and Cu/Zn ratio could predict SILVSD in patients with septic shock (Figure 4). When the cut-off points for whole blood Cu, Zn and Cu/Zn ratio were set at 14.2 µmol/L, 84.11 µmol/L and 0.204, they had sensitivity of 87.80%, 73.13% and 48.78% and specificity of 46.67%, 53.33% and 82.22% respectively.
Predictive Values of Whole Blood Cu, Zn and Cu/Zn Ratio for 28-Day Mortality
A follow-up of 28 days after enrollment or until death was conducted in each patient. Whole blood Cu levels at admission were significantly higher in non-survivors (n=30) than survivors (n=56) (17.20±2.25 µmol/L vs 14.99±1.49 µmol/L, p<0.001) (Figure 5A), but Zn concentrations were significantly lower in non-survivors than survivors (71.17±11.98 µmol/L vs 87.67±11.30 µmol/L, p<0.001) (Figure 5B). Cu/Zn ratio was significantly higher in non-survivors than in survivors (0.250±0.055 vs 0.173±0.026; p<0.001) (Figure 5C). The performance of Cu, Zn and Cu/Zn ratio for predicting 28-day mortality was evaluated by ROC curve analysis between hospital deaths and survivors for all enrolled patients. Whole blood Cu, Zn and Cu/Zn ratio displayed the values of predicting 28-day mortality [Cu: (AUC=0.802; 95% CI, 0.702–0.880; p<0.001); Zn: (AUC=0.869; 95% CI, 0.779–0.932; p<0.001); Cu/Zn ratio: (AUC=0.902; 95% CI, 0.891–0.956; p<0.001)] (Figure 5D). When the cut-off points for whole blood Cu, Zn and Cu/Zn ratio were set at 16.45 µmol/L, 79.15 µmol/L and 0.202, they had sensitivity of 73.33%, 83.33% and 86.67% and specificity of 85.71%, 83.93% and 91.07% respectively.
Discussion
The current study showed that whole blood Cu levels and Cu/Zn ratio were significantly higher in SILVSD patients with septic shock compared with non-SIMD patients with septic shock independently of age, sex, fluid balance prior to ICU, HCT, sources of infection and underlying diseases such as hypertension, diabetes mellitus, COPD, anemia and stroke, whereas whole blood Zn were significantly lower in SILVSD patients with septic shock compared with non-SIMD patients with septic shock. In SILVSD patients, as controlling for confounders of fluid balance prior to ICU and HCT, whole blood Cu and Cu/Zn ratio were negatively correlated with LVEF, but were positively correlated with plasma NT-proBNP, and whole blood Zn was positively correlated with LVEF, but was negatively correlated with plasma NT-proBNP. Moreover, whole blood Cu levels and Cu/Zn ratio were positively correlated with APACHE-II score, SOFA score and plasma levels of TNT, HFABP and PCT, but whole blood Zn levels were negatively correlated with APACHE-II score, SOFA score and plasma levels of TNT, HFABP and PCT. Although there was no significant difference in 28-day mortality between SILVSD and non-SIMD patients, the whole blood Cu levels and Cu/Zn ratio were higher in non-survivors compared with survivors, but whole blood Zn levels were lower in non-survivors patients compared with survivors; furthermore, whole blood Cu, Zn and Cu/Zn ratio were predictive of 28-day mortality.
A study performed by Ayoglu et al17 revealed that serum Cu and Zn levels were in the normal range in patients with sepsis and systemic inflammatory response syndrome (SIRS), which is almost consistent with the discovery that the levels of whole blood Cu and Zn were in the normal range in patients with septic shock in our research. However, in our study, the levels of Cu in SILVSD patients with septic shock were higher than non-SIMD patients with septic shock while the levels of whole blood Zn were lower than non-SIMD patients with septic shock. LVSD could be contribute to the differences of the whole Cu and Zn levels between SILVSD and non-SIMD group according to the previous study of Alexanian et al.23 In their research, compared with healthy volunteers, patients with either AHF or CHF had higher serum Cu and lower serum Zn that were correlated with LVDD; moreover, further analysis indicated that increased serum Cu and decreased serum Zn were significantly correlated with LVSD in patients with AHF, which might support the finding in our study.
Compared with researches that investigated the correlation between serum Zn and sepsis or septic shock,31,32 studies on the relationship between Cu and sepsis or septic shock are fewer but have shown that serum Cu levels are elevated in patients with sepsis.17 Akkaş et al and Martinez-Peinadoet al found that serum Cu concentrations were elevated in septic patients.33,34 Cu is vital for synthesis of collagen, oxidative injury, antioxidant response, iron transportation, acts as a cofactor for oxidative metalloenzymes and can result in anemia, leukopenia and pancytopenia,35,36 but the potential mechanisms of higher serum Cu in sepsis remain unclear absolutely. On the contrary, studies have shown that Zn is related to nutritional immunity and involved in the synthesis of acute phase proteins and acts as a hepatoprotective agent, or a differentiation signal for innate immune cells. Inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and IL-6 enhance the expression of zinc import proteins (mainly ZIP14) and MT in the liver, which redistribute Zn from blood into the liver during sepsis.31 Finally, septic shock is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality,1 so we assume that the whole blood Cu concentrations of patients with septic shock are higher than those of healthy volunteers but the whole blood Zn concentrations are lower than those in this study.
As far as the difference in whole blood Cu concentrations between the non-SIMD group and the SILVSD group is concerned, LVSD (LVEF<50%) may play an important role and is correlated with the change of whole blood Cu concentrations. Firstly, elevated whole blood Cu in SILVSD patients with septic shock probably represents a significant elevation of ceruloplasmin (Cp), because Cp binds about 95% of the circulating blood Cu.37 Cp can not only delivery Cu to cells, but oxidize iron (Fe2+ to Fe3+) that is incorporated into transferrin (TRF). The TRF-Fe3+ compound can scavenge ROS because it exerts an antioxidant glutathione–peroxidase activity.37 It is reported that inflammatory cytokines such as IL-1 and IL-6 can upregulate the synthesis of Cp in the liver,38 which may support our finding that a significant positive correlation exists between whole blood Cu and NT-proBNP, TNT and HFABP that reflects cardiac function and myocardial injury in septic shock.11 Secondly, hypoxia can stimulate the raised release of hypoxia-inducible factor (HIF) that promotes elevated synthesis of Cp in the liver,39 and consequently result in elevated whole blood Cu levels under hypoxia condition. Finally, although serum Cu can reflect Cp metabolism, there are up to 40% additional Cu that loosely bind and dissociate from Cp under excessive oxidative stress because the structure and function of Cp can be modulated by ROS.40 The free Cu can deteriorate the cardiac systolic and predict worse prognosis in SILVSD patients because it participates the ROS production by it pro-oxidant activity.41,42 This is consistent with our finding that the raised whole blood Cu levels were negatively correlated with cardiac systolic function (lower LVEF). Therefore, raised whole blood Cu concentrations in SILVSD may be a fraction of systemic mechanism induced by inflammation and hypoxia. This mechanism that is activated by cardiac systolic dysfunction can deteriorate cardiac systolic function that has been impaired by septic shock.
A finding of the study that whole blood Zn concentrations were lower in patients with LVSD is supported by some of the previous researches investigating the role of Zn in cardiac systolic dysfunction.43,44 The present study demonstrated that whole blood Zn was significantly lower in SILVSD than non-SIMD patients and positively correlated with LVEF. There are several possible mechanisms to explain this correlation. Firstly, during sepsis or septic shock, hypozincemia is one of the characteristics of acute phase reaction (APR), and as mentioned above, one of main reasons of hypozincemia is redistribution of Zn from blood to liver pro facilitated by raised ZIP 14 and MT which bind Zn from plasma and tissues leading to lower Zn bioavailability.45 Secondly, raised production of inflammation in LVSD further results in MT and consequently reduced Zn levels, which is in line with our finding that a significant negative correlation exists between whole blood Zn and NT-proBNP, TNT and HFABP that reflects cardiac function and myocardial injury in septic shock. Finally, besides inflammatory response and sepsis or septic shock, cardiac systolic dysfunction also aggravates hypozincemia in sepsis. In SILVSD patients, lower CO due to LVSD and hypoperfusion usually coexist, which can reduce renal perfusion and further lead to a hyperadrenergic state along with increased concentrations of cortisol, angiotensin II and aldosterone.46 Zn can be redistributed to the damaged sites selectively under a hyperadrenergic state,46 which might support our finding that the reduced whole blood Zn concentrations were positively correlated with cardiac systolic dysfunction (lower LVEF). Furthermore, studies have found that coupled intracellular and intramitochondrial Ca2+ accumulation in cardiac dysfunction, intracellular Zn2+ was raised because of increased Zn2+ entry and release of inactive Zn from MT-1 induced by nitric oxide under excessive catecholamine state. Hence, there was a significant reduction of whole blood Zn in SILVSD with septic shock.
There are some previous studies that investigated the relationship between serum Cu and Zn levels and prognosis in sepsis or septic shock and demonstrated that the higher mortality was correlated with higher serum Cu and lower serum Zn,19,33 but this correlation was not found in the research of Ayoglu et al in Turkey.17 In contrast, although the 28-day mortality was not significantly different between non-SIMD and SILVSD, compared with survivors with septic shock, we discovered that the increased levels of whole blood Cu and the reduced levels of whole blood Zn existed in non-survivors with septic shock, which is consistent with previous studies.19,33 After analysis of the correlations between whole blood Cu and Zn and APACHE-II score and SOFA score by partial correlation analysis, we found whole blood Cu was positively correlated with APACHE-II score and SOFA score and whole blood Zn was negatively correlated with APACHE-II score and SOFA score. Therefore, in this research, higher Cu concentration and lower Zn concentration may be associated with severity of multi-organ dysfunction syndrome (MODS) at admission in patients with septic shock.
As mentioned above, SILVSD patients also manifested higher levels of whole blood Cu that were positively associated with APACHE-II score and SOFA score and lower levels of whole blood Zn that were negatively correlated with APACHE-II score and SOFA score than non-SIMD patients. Cu and Zn could also predict SILVSD in this study subjects (Cu: AUC=0.666; 95% CI, 0.556–0.764; p=0.005, and Zn: AUC=0.625; 95% CI, 0.514–0.727; p=0.039). It is reported that multiple indicators including LVEF, TNT and NT-proBNP have been applied to provide improved predictive accuracy to myocardial dysfunction in septic shock.47–49 Concomitant research of echocardiographic indicators (LVEF) and biomarkers (NT-proBNP or TNT) has not provided convincing results.50,51 Therefore, the combination of whole Cu and Zn with traditional biomarkers including NT-proBNP and LVEF could be performed for the accurate prediction of SILVSD.
We also investigated the prognostic value of Cu and Zn. A significant increase in Cu levels at admission and a decrease in Zn levels were found in non-survivors compared with survivors. A cut-off level of 16.45 μmol/L and 79.15 μmol/L of Cu and Zn had a sensitivity of 73.33% and 83.33% and a specificity of 85.71% and 83.93% for prediction of 28-day mortality with an AUC of 0.802 and 0.869 respectively. These results were in agreement with previous researches.41,44 Excessive Cu in the human organism has been recognized in cell damage in sepsis and HF because it is involved in production of ROS and results in deterioration of the inflammation and cardiac systolic dysfunction. Previous studies showed that hypozincemia was associated with lymphopenia, thymic atrophy and dysfunction of cell- and antibody-mediated immunity,52,53 which could decrease lots of pre-B and pre-T cells and lead to the immune paralysis in both sepsis and cardiac dysfunction.54,55 Studies revealed that elevated TNT was related to higher short-term or long-term mortality in patients with sepsis or septic shock.56,57 So, excessive Cu and hypozincemia have been indicators of poor prognosis in critically ill patients. It is reported that HFABP may predict the prognosis of sepsis and septic shock, but the AUC of HFABP for evaluation of the outcome of sepsis is less than 0.7.58 Webb et al indicated that elevated serum PCT levels were correlated with in-hospital mortality in septic patients.59 Therefore, the combination of whole Cu and Zn with traditional biomarkers including TNT, PCT and HFABP may be performed for more prognostic value of SILVSD.
A report conducted by Osredkar and Suskar indicated that clinical significance of Cu/Zn ratio could be greater than either Cu or Zn.24 Increased whole blood Cu/Zn ratio shows an antioxidant defense,60 and is positively correlated with the rise of blood systolic pressure and incidence of cardiovascular disease.61 In this study, the sensitivity of whole blood Cu/Zn ratio in predicting SILVSD was less than Cu or Zn, but its specificity was more than Cu or Zn obviously. Furthermore, both the sensitivity and specificity of whole blood Cu/Zn ratio in predicting 28-day mortality in patients with septic shock was higher than either Cu or Zn. Laine et al reported that higher plasma Cu/Zn ratio level was correlated with increased risk of getting an infection in middle-aged and older men (CI=1.07–1.69, p=0.005).62 Similarly, Cu/Zn ratio is an important indicator to reflect the inflammatory status of cystic fibrosis patients.63 And, in infected neonates, higher Cu/Zn ratio is correlated with CRP levels on day 1 and day 3, which indicates the Cu/Zn ratio could be regarded as a disease biomarker to recognize infected newborns.64
The whole blood Cu and Zn concentrations were employed in this study, which is not consistent with previous studies that adopted serum or plasma Cu or Zn levels to the investigation.19,25,33,65 But they have been applied in most hospitals in our area for several years and are reliable indicators for clinical staff.29,30,66 Besides, several studies have shown that there is a good relationship between levels of trace elements in whole blood and those in serum.67–69 In China, Li Hai-Tao and Wei Lan-Fen reported that levels of Cu and Zn in whole blood were positively associated with those in serum (rCu=0.49, rZn=0.37, all p<0.001) in 1994 through analyzing the relationship between trace elements in whole blood and serum and indicated that serum trace elements levels could be replaced with those in whole blood.70 Compared with the process of determining levels of trace elements in serum or plasma, analysis of whole blood trace elements needs less volume of a blood sample and some necessary steps such as centrifugation and isolation of blood samples are omitted, through which hemolysis during the period of centrifugation and isolation and errors induced by hemolysis can be avoided.70 Therefore, analysis of trace elements in whole blood is convenient, accurate and economical, so it was conducted in this study.
However, this study has several limits. First, in the current study, although the statistics differences in the of levels whole blood Cu and Zn and Cu/Zn ratio were significant between the SILVSD group and the non-SIMD group, there is a little regret without a control group of heart dysfunction alone. Secondly, the sample size of this study is small so the current findings need confirming in future researches. Since the disease in this study has a prolonged course and complex cellular/metabolic mechanisms, studying a single time point at admission also limits our findings. Finally, because this is an observational study, it is necessary to design a randomized control trial to explore the influence of Zn supplement or Cu chelator to patients with SILVSD in the future.
Conclusion
In conclusion, in the present study, whole blood Cu, Zn concentrations and Cu/Zn ratio were altered more significantly in SILVSD patients with septic shock than non-SIMD patients, and were of predictive and prognostic values for SILVSD patients with septic shock. To the best of our knowledge, the current study is the first presenting the alterations in whole blood Cu, Zn levels and Cu/Zn ratio in SILVSD patients with septic shock.
Data Sharing Statement
We declare that all relevant raw data and materials described in the manuscript will be freely available through corresponding author to any scientist, researcher and reader wishing to use them for non-commercial purposes, without breaching participant confidentiality. This study maintained patient data confidentiality in accordance with the ethical standards of the Helsinki Declaration.
Compliance with Ethical Standards
Written informed consent was provided by controls and relatives of all enrolled patients who always suffered from varying degrees of consciousness disorder and even were sedated because of intubation and mechanical ventilation in ICU. This study was conducted in accordance to the Declaration of Helsinki and approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (Approval No. [2018]016).
Acknowledgments
Thanks to Dr Xiao-Gang Guo for analyzing some data and reviewing and revising the manuscript.
Funding
This study was funded by Zhejiang Provincial Natural Science Foundation of China, China (No.LGF18H150010) and the Zhejiang Provincial Administration of Traditional Chinese Medicine, China (No. 2018ZB034).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301–308. doi:10.1001/jama.2016.20329
3. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi:10.1097/ccm.0000000000002255
4. Beesley SJ, Weber G, Sarge T, et al. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625–634. doi:10.1097/ccm.0000000000002851
5. Werdan K, Schmidt H, Ebelt H, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87(4):266–274. doi:10.1139/y09-012
6. Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi:10.7326/0003-4819-100-4-483
7. Suzuki T, Suzuki Y, Okuda J, et al. Sepsis-induced cardiac dysfunction and β-adrenergic blockade therapy for sepsis. J Intensive Care. 2017;5:22. doi:10.1186/s40560-017-0215-2
8. Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. doi:10.1016/j.jcrc.2014.03.007
9. Song MJ, Lee SH, Leem AY, et al. Predictors and outcomes of sepsis-induced cardiomyopathy in critically ill patients. Acute Crit Care. 2020;35(2):67–76. doi:10.4266/acc.2020.00024
10. Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettila V, Group FS. Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35(5):1277–1283. doi:10.1097/01.CCM.0000261893.72811.0F
11. Meng JB, Hu MH, Lai ZZ, et al. Levosimendan versus dobutamine in myocardial injury patients with septic shock: a randomized controlled trial. Med Sci Monit. 2016;22:1486–1496. doi:10.12659/msm.898457
12. Bessière F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39(7):1181–1189. doi:10.1007/s00134-013-2902-3
13. Klouche K, Pommet S, Amigues L, et al. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: relationships with systolic myocardial dysfunction and intensive care unit mortality. J Intensive Care Med. 2014;29(4):229–237. doi:10.1177/0885066612471621
14. Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart Lung. 2015;44(1):75–81. doi:10.1016/j.hrtlng.2014.10.002
15. Lee YJ, Lee H, Park JS, et al. Cardiac troponin I as a prognostic factor in critically ill pneumonia patients in the absence of acute coronary syndrome. J Crit Care. 2015;30(2):390–394. doi:10.1016/j.jcrc.2014.12.001
16. Chitturi R, Baddam VR, Prasad L, Prashanth L, Kattapagari K. A review on role of essential trace elements in health and disease. J Dr NTR Univ Health Sci. 2015;4(2):75. doi:10.4103/2277-8632.158577
17. Ayoglu H, Sezer U, Akin M, et al. Selenium, copper, zinc, iron levels and mortality in patients with sepsis and systemic inflammatory response syndrome in Western Black Sea Region, Turkey. J Pak Med Assoc. 2016;66(4):447–452.
18. Gibson RS. Zinc deficiency and human health: etiology, health consequences, and future solutions. Plant Soil. 2012;361(1–2):291–299. doi:10.1186/s40560-017-0215-2
19. Saleh NY, Abo El Fotoh WMM. Low serum zinc level: the relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract. 2018;72(6):e13211. doi:10.1111/ijcp.13211
20. Shimamori T, Tsukano K, Sera K, Noda J, Suzuki K. Sequential changes in serum zinc concentrations in calves with experimentally induced endotoxin shock measured by the particle-induced X-ray emission method. J Veter Med Sci. 2019;81(2):165–168. doi:10.1292/jvms.18-0527
21. Salonen JT, Salonen R, Korpela H, Suntioinen S, Tuomilehto J. Serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland. Am J Epidemiol. 1991;134(3):268–276. doi:10.1093/oxfordjournals.aje.a116080
22. Tanrikulu AC, Abakay A, Evliyaoglu O, Palanci Y. Coenzyme Q10, copper, zinc, and lipid peroxidation levels in serum of patients with chronic obstructive pulmonary disease. Biol Trace Elem Res. 2011;143(2):659–667. doi:10.1007/s12011-010-8897-5
23. Alexanian I, Parissis J, Farmakis D, et al. Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin Res Cardiol. 2014;103(11):938–949. doi:10.1007/s00392-014-0735-x
24. Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clin Toxicol. 2017;S3:1. doi:10.4172/2161-0494.S3-001
25. Alieva TU, Fedorov SV, Sviridov SV. Blood plasma zinc and copper concentrations in patients with purulent soft tissue wounds. Anesteziol Reanimatol. 2010;31(3):8–12.
26. Malavolta M, Giacconi R, Piacenza F, et al. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology. 2009;11(3):309–319. doi:10.1007/s10522-009-9251-1
27. WS/T402-2012. Define and Determine the Reference Intervals in Clinical Laboratory. Vols. WS/T402-2012. Beijing: Ministry of health of the People`s Republic of China. Standards Press of China; 2012.
28. Shang H, Wang YS, Shen ZY. National Guide to Clinical Laboratory Procedures (The Fourth Edition). Beijing: People`s Medical Publishing House; 2014.
29. Bai XM, Li YL, Yu HY, Bi XY, Zhao R, Qi N. Investigation on the levels of trace elements in whole blood and plasma of adults in Beijing area (in Chinese). J Modern Labor Med. 2010;25(5):133–135. doi:10.3969/j.issn.1671-7414.2010.05.057
30. Deng JS, Pu ZY, Li XK. Analysis of total blood zinc content in different age groups (in Chinese). Med Inform. 2018;31(23):128–130. doi:10.3969/j.issn.1006-1959.2018.23.036
31. Alker W, Haase H. Zinc and sepsis. Nutrients. 2018;10(8):976. doi:10.3390/nu10080976
32. Souffriau J, Libert C. Mechanistic insights into the protective impact of zinc on sepsis. Cytokine Growth Factor Rev. 2018;39:92–101. doi:10.1016/j.cytogfr.2017.12.002
33. Akkaş İ, Ince N, Sungur MA. Serum trace element and heavy metal levels in patients with sepsis. Aging Male. 2020;1–5. doi:10.1080/13685538.2020.1740200
34. Martinez-Peinado M, Rueda-Robles A, Nogueras-Lopez F, Villalon-Mir M, Oliveras-Lopez MJ, Navarro-Alarcon M. Serum zinc and copper concentrations and ratios in cirrhotic patients: correlation with severity index. Nutricionhospitalaria. 2018;35(3):627–632. doi:10.20960/nh.1579
35. Robinson SD, Cooper B, Leday TV. Copper deficiency (hypocupremia) and pancytopenia late after gastric bypass surgery. Baylor Univ Med Center Proc. 2013;26(4):382–386. doi:10.1080/08998280.2013.11929011
36. Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26(4–5):268–298. doi:10.1016/j.mam.2005.07.015
37. Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother. 2003;57(9):386–398. doi:10.1016/s0753-3322(03)00012-x
38. Fox PL, Mazumder B, Ehrenwald E, Mukhopadhyay CK. Ceruloplasmin and cardiovascular disease. Free Radic Biol Med. 2000;28(12):1735–1744. doi:10.1016/s0891-5849(00)00231-8
39. Chepelev NL, Willmore WG. Regulation of iron pathways in response to hypoxia. Free Radic Biol Med. 2011;50(6):645–666. doi:10.1016/j.freeradbiomed.2010.12.023
40. Shukla N, Maher J, Masters J, Angelini GD, Jeremy JY. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor? Atherosclerosis. 2006;187(2):238–250. doi:10.1016/j.atherosclerosis.2005.11.035
41. Dadu RT, Dodge R, Nambi V, et al. Ceruloplasmin and heart failure in the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2013;6(5):936–943. doi:10.1161/circheartfailure.113.000270
42. Málek F, Dvorák J, Jiresová E, Spacek R. Difference of baseline serum copper levels between groups of patients with different one year mortality and morbidity and chronic heart failure. Cent Eur J Public Health. 2003;11(4):198–201.
43. Topuzoglu G, Erbay AR, Karul AB, Yensel N. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biol Trace Elem Res. 2003;95(1):11–17. doi:10.1385/bter:95:1:11
44. Arroyo M, Laguardia SP, Bhattacharya SK, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148(6):301–308. doi:10.1016/j.trsl.2006.08.003
45. Mocchegiani E, Muzzioli M, Cipriano C, Giacconi R. Zinc, T-cell pathways, aging: role of metallothioneins. Mech Ageing Dev. 1998;106(1–2):183–204. doi:10.1016/s0047-6374(98)00115-8
46. Whitted AD, Stanifer JW, Dube P, et al. A dyshomeostasis of electrolytes and trace elements in acute stressor states: impact on the heart. Am J Med Sci. 2010;340(1):48–53. doi:10.1097/MAJ.0b013e3181e5945b
47. Kim JS, Kim M, Kim YJ, et al. Troponin testing for assessing sepsis-induced myocardial dysfunction in patients with septic shock. J Clin Med. 2019;8(2):239. doi:10.3390/jcm8020239
48. Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: a comparative retrospective study. Medicine. 2018;97(13):e0263. doi:10.1097/md.0000000000010263
49. Røsjø H, Masson S, Caironi P, et al. Prognostic value of secretoneurin in patients with severe sepsis and septic shock: data from the albumin Italian outcome sepsis study. Crit Care Med. 2018;46(5):e404–e410. doi:10.1097/ccm.0000000000003050
50. Sturgess DJ, Marwick TH, Joyce C, et al. Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Crit Care. 2010;14(2):R44. doi:10.1186/cc8931
51. Rolando G, Espinoza ED, Avid E, et al. Prognostic value of ventricular diastolic dysfunction in patients with severe sepsis and septic shock. Revista Brasileira De Terapia Intensiva. 2015;27(4):333–339. doi:10.5935/0103-507X.20150057
52. Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4(1):82–91. doi:10.3945/an.112.003038
53. Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5–6):353–357. doi:10.2119/2008-00033.Prasad
54. Negm FF, Soliman DR, Ahmed ES, Elmasry RA. Assessment of serum zinc, selenium, and prolactin concentrations in critically ill children. Pediatr Health Med Therap. 2016;7:17–23. doi:10.2147/phmt.s99191
55. Wang G, Feng X, Yu X, et al. Prognostic value of blood zinc, iron, and copper levels in critically ill children with pediatric risk of mortality score III. Biol Trace Elem Res. 2013;152(3):300–304. doi:10.1007/s12011-013-9623-x
56. Vallabhajosyula S, Sakhuja A, Geske JB, et al. Role of admission troponin-T and serial troponin-T testing in predicting outcomes in severe sepsis and septic shock. J Am Heart Assoc. 2017;6(9):e005930. doi:10.1161/JAHA.117.005930
57. Rosjo H, Varpula M, Hagve TA, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37(1):77–85. doi:10.1007/s00134-010-2051-x
58. Chen FC, Xu YC, Zhang ZC. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J Zhejiang Univ Sci B. 2020;21(7):537–548. doi:10.1631/jzus.B2000049
59. Webb AL, Kramer N, Stead TG, et al. Serum procalcitonin level is associated with positive blood cultures, in-hospital mortality, and septic shock in emergency department sepsis patients. Cureus. 2020;12(4):e7812. doi:10.7759/cureus.7812
60. Richardson JS, Thomas KA, Richardson R, Richardson DC. Crystal structure of bovine Cu, Zn superoxide dismutase at 3 angstrom resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975;72(4):1349–1353. doi:10.1073/pnas.72.4.1349
61. Rahman S, Waheed S. Blood-copper and zinc levels and consequences of cardiovascular complications: a study by INAA and FAAS. J Radioanal Nuclear Chem. 2011;287(2):657–664. doi:10.1007/s10967-010-0843-7
62. Laine JT, Tuomainen TP, Salonen JT, Virtanen JK. Serum copper-to-zinc-ratio and risk of incident infection in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Epidemiol. 2020;35(12):1149–1156. doi:10.1007/s10654-020-00644-1
63. Escobedo-Monge MF, Barrado E, Alonso Vicente C, et al. Copper and copper/zinc ratio in a series of cystic fibrosis patients. Nutrients. 2020;12(11):3344. doi:10.3390/nu12113344
64. Wisniewska M, Cremer M, Wiehe L, et al. Copper to zinc ratio as disease biomarker in neonates with early-onset congenital infections. Nutrients. 2017;9(4):343. doi:10.3390/nu9040343
65. Mortada WI, Awadalla A, Khater S, et al. Copper and zinc levels in plasma and cancerous tissues and their relation with expression of VEGF and HIF-1 in the pathogenesis of muscle invasive urothelial bladder cancer: a case-controlled clinical study. Environ Sci Pollut Res Int. 2020;27(13):15835–15841. doi:10.1007/s11356-020-08113-8
66. Ji HB, Pan SS, Zhang ZQ, Xie YD, Lei Y. Investigation on reference values of whole blood trace elements among pregnant women in Hangzhou area (in Chinese). Int J Labor Med. 2016;37(17):2366–2368. doi:10.3969/j.issn.1673-4130.2016.17.003
67. Hastuti AAMB, Costas-Rodríguez M, Anoshkina Y, Parnall T, Madura JA, Vanhaecke F. High-precision isotopic analysis of serum and whole blood Cu, Fe and Zn to assess possible homeostasis alterations due to bariatric surgery. Anal Bioanal Chem. 2019;8(3):727–738. doi:10.1007/s00216-019-02291-2
68. Stojsavljević A, Jagodić J, Vujotić L, et al. Reference values for trace essential elements in the whole blood and serum samples of the adult Serbian population: significance of selenium deficiency. Environ Sci Pollut Res. 2019;27(2):1397–1405. doi:10.1007/s11356-019-06936-8
69. Barany E, Bergdahl IA, Bratteby LE, et al. Relationships between trace element concentrations in human blood and serum. Toxicol Lett. 2002;134(1–3):177–184. doi:10.1016/s0378-4274(02)00187-x
70. Li HT, Wei LF. Study on the relationship between trace elements in whole blood and serum (in Chinese). Zhejiang J Prev Med. 1994;6(5):41.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.