Back to Journals » Infection and Drug Resistance » Volume 15
The Correlation Between Biofilm-Forming Ability of Community-Acquired Methicillin-Resistant Staphylococcus aureus Isolated from the Respiratory Tract and Clinical Characteristics in Children
Authors Huang S , He J, Zhang Y, Su L, Tong L, Sun Y , Zhou M, Chen Z
Received 21 April 2022
Accepted for publication 17 June 2022
Published 12 July 2022 Volume 2022:15 Pages 3657—3668
DOI https://doi.org/10.2147/IDR.S370755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shumin Huang,1,2 Jing He,1,2 Yiting Zhang,1,2 Lin Su,1,2 Lin Tong,1,2 Ying Sun,1,2 Mingming Zhou,2,3 Zhimin Chen1,2
1Department of Pulmonology, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310052, People’s Republic of China; 2National Clinical Research Center for Child Health, National Children’s Regional Medical Center, Hangzhou, 310052, People’s Republic of China; 3Department of Clinical Laboratory, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310052, People’s Republic of China
Correspondence: Zhimin Chen, Email [email protected]
Objective: This study aimed to investigate the biofilm-forming ability, molecular typing, and antimicrobial resistance of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) strains isolated from the respiratory tract of children and their correlation with clinical characteristics.
Methods: All CA-MRSA strains were isolated from hospitalized children, and their presentation, molecular typing, antimicrobial susceptibility, and biofilm formation were investigated. The clinical characteristics were compared between the strong and weak biofilm producer groups.
Results: Fifty-three CA-MRSA strains were isolated from the respiratory samples of 53 children, with nearly half of them being young infants (0– 12 months). Approximately, 88.7% (47/53) of the isolates were resistant to four or more antibiotics, mainly β-lactam antibiotics, lincosamides, and macrolides. Twelve sequence types (STs) and 20 subtypes of staphylococcal protein A (spa) typing were identified, with ST59-t437 (39.6%, 21/53) as the predominant subtype. All strains showed the ability to form biofilms. When compared to children with weak biofilm-forming CA-MRSA strains, those with strong biofilm-forming strains had higher proportions of lower respiratory tract infections (LRTI) (88.5% vs 59.3%), obvious cough symptoms (84.6% vs 51.9%), and severe chest imaging manifestations (76.9% vs 37.0%). Furthermore, a strong biofilm-forming ability significantly increased the risk of prolonged cough in children with LRTI (44.4% vs 14.3%), and a positive correlation between the duration of cough and the extent of biofilm formation was observed. Medical history investigation revealed that the strong biofilm-forming group had a much higher percentage of macrolides intake than the weak biofilm-forming group in the last month before admission (61.5% vs 14.8%).
Conclusion: ST59-t437 was the most prevalent clone in CA-MRSA respiratory isolates among the hospitalized children. All CA-MRSA strains formed biofilms. The stronger the biofilm-forming ability, the more serious and prolonged were the respiratory symptoms.
Keywords: CA-MRSA, biofilm, respiratory infection, child, genotype
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a major pathogen and the most common multidrug-resistant bacterium that causes nosocomial infections, with high morbidity and mortality. MRSA is closely associated with health care settings.1 However, since the 1990s, MRSA infections have increased among people without hospital-related risk factors worldwide.2,3 Furthermore, there have been notable epidemics, such as the USA300, which spread internationally and caused many deaths.4,5 All of these are associated with the emergence of new MRSA clones known as community-acquired MRSA (CA-MRSA). Compared to hospital-acquired MRSA (HA-MRSA), CA-MRSA is more clonally diverse and highly virulent because it produces Panton-Valentine leukocidin (PVL) exotoxin and is more likely to cause severe infections, particularly among young, healthy individuals.6–9 Additionally, more CA-MRSA strains contain the staphylococcal cassette chromosome mec (SCCmec) element type IV or V, conferring resistance to β-lactam antibiotics.8,9 It can cause a wide spectrum of clinical diseases, from the skin and soft tissue infections (SSTIs) to severe invasive infections, accounting for a large proportion of the increased disease burden observed in the last decade, particularly among children.6,7,9–11
Biofilms are bacterial aggregates embedded in a matrix composed of extracellular polysaccharides, fibrin, and other substances produced by bacteria. They are highly resistant to antibiotics and host immunity, making biofilm-associated infections a major concern in clinical practice. A biofilm can be formed not only on the surface of medical implants but also on mucous membranes. The formation of bacterial biofilms on the respiratory tract has been linked to recurrent respiratory diseases and asymptomatic colonization.12,13 According to data reported in published studies, approximately 2–10% of healthy children have MRSA nasal colonization, and the figures are still on the rise.14–17 Children may be an important reservoir for the community transmission of CA-MRSA.6,18 However, the role of biofilms in respiratory diseases has not yet been fully explored.
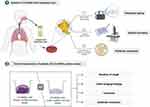
In this study, we examined the molecular typing, antimicrobial resistance, and biofilm-forming ability of CA-MRSA strains isolated from the respiratory tract of children. We mainly investigated the relationship between the biofilm-forming ability and the clinical characteristics of respiratory diseases, which might provide new insights for treating patients with CA-MRSA infections. A simplified description of the study protocol is shown in Figure 1.
Materials and Methods
Collection of Isolated Strains and Clinical Data
This retrospective study was conducted at a tertiary-care academic pediatric center. In this study, 53 non-duplicate CA-MRSA isolates were collected from inpatients at Children’s Hospital, Zhejiang University School of Medicine, from April 2015 to July 2020. All strains were isolated from respiratory specimens, including sputum, bronchoalveolar lavage fluid (BALF), pharyngeal swabs, and pleural fluids. MRSA identification is mediated by the mecA gene detection and the cefoxitin disk diffusion methods, which will be discussed later. In addition, we defined CA-MRSA according to the United States Centers for Disease Control and Prevention criteria: (1) positive culture for MRSA within 48 h after admission; (2) no medical devices or indwelling catheters permanently placed through the skin; (3) no history of MRSA infections; and (4) no history of hospitalization or residence in nursing homes or long-term care facilities in the previous year.19 Each strain was obtained independently and maintained as a stock culture at −80 °C in Luria broth (LB) supplemented with 20% glycerol until further examination.
Clinical data, including age, sex, length of hospital stay, clinical characteristics, laboratory examinations, chest imaging findings, treatment, and prognosis, were collected from patients’ medical records. To investigate the relationship between the biofilm-forming ability and medication history, the information on each child’s antibiotic medication in the month before admission was collected. This study was approved by the Ethics Committee of the Children’s Hospital, Zhejiang University School of Medicine (approval number: EC2022-IRB-005).
Identification and Antimicrobial Susceptibility Testing
Species confirmation was using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics GmbH, Germany). MRSA was identified using the mecA gene detection and disk diffusion method with a 30 μg cefoxitin disk according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. Standard S. aureus ATCC 25923 and ATCC 43300 strains (supplied by the Microbiology Laboratory of Children’s Hospital, Zhejiang University School of Medicine) were used as the quality controls.
The antimicrobial susceptibility of 14 different antimicrobial agents was determined among all the collected CA-MRSA isolates using the commercialized microdilution method (VITEK COMPACT, BioMérieux, Marcy-l’Étoile, France), and the results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines M100-Ed30. The antibiotics tested were penicillin, ceftaroline fosamil, oxacillin, sulfamethoxazole/trimethoprim, gentamicin, rifampicin, levofloxacin, moxifloxacin, clindamycin, daptomycin, erythromycin, linezolid, vancomycin, teicoplanin, and tigecycline. Staphylococcus aureus ATCC 29213 was used as the quality control.
DNA Extraction
The DNA was extracted by using a DNA extraction kit (Vazyme Biotech Co. Ltd., Nanjing, China) with lysozyme (20 mg/mL) according to the manufacturer’s instructions, and used as the template for all the polymerase chain reaction (PCR) products.
Molecular Typing Methods and Detection of PVL and mecA Genes
Spa Typing
Spa typing is mainly based on polymorphism of the X-region repeats of staphylococcal protein A (spa). After amplifying the X-region by PCR with primers (5’-AGACGATCCTTCGGTGAGC-3’; 5’-GCTTTTGCAATGTCATTTACTG-3’),20 and subsequent sequencing, the sequences were submitted to spaTyper (http://spatyper.fortinbras.us/) for spa typing.
Multilocus Sequence Typing (MLST)
MLST was performed by sequencing the PCR products of seven housekeeping genes (arcC, aroE, glpF, gmK, pta, tpi, and yqi). The sequence types (STs) and clonal complexes (CCs) of each strain were determined by comparison with known alleles from the MLST database (https://pubmlst.org/organisms/staphylococcus-aureus), which also provides the primers and conditions for PCR. The software PHYLOViZ 2.0 (http://www.phyloviz.net) was used to construct the dendrogram of STs and to assess the evolutionary relationship between the isolates.
Detection of PVL and mecA Genes
The PVL genes (lukS/F-PV) and mecA gene were detected by PCR amplification using primers (5’-GGCCTTTCCAATACAATATTGG-3’; 5’-CCCAATCAACTTCATAAATTG-3’) and (5’-GTGAAGATATACCAAGTGATT-3’; 5’- ATGCGCTATAGATTGAAAGGAT-3’) respectively, as previously described.21 The reference strains ATCC-43300-mecA+/PVL-(MRSA), ATCC-29213-mecA-/PVL-(MSSA) and ATCC-25923-mecA-/PVL+(MSSA) were used as positive or negative controls.
Biofilm Formation
CA-MRSA strains were inoculated onto Columbia blood agar (Bioivd, Zhengzhou, China) and incubated for 18–24 h at 35 °C in a 5% CO2-enriched atmosphere. A single clone was picked from each blood agar sample and cultured in tryptic soy broth (TSB; Oxoid, Basingstoke, UK) with shaking (200 rpm) for 16 h at 35 °C to reach the logarithmic growth phase. The cultures were inoculated into a sterile 0.9% NaCl solution to match a 0.5 McFarland turbidity of 100-fold dilution using TSB. Subsequently, 200 μL of this suspension was inoculated into triplicate in a 96-well polystyrene plate (Corning Costar, Acton, MA, USA) at 35 °C for 24 h. The blank group was treated with 200 µL of TSB. Biofilm formation was quantified by crystal violet staining as previously described.22 After incubation, the supernatant was removed, and each well of the microtiter plate was washed twice with phosphate buffer (0.1 M, pH 7.0), fixed with 100% methanol for 20 min, stained with crystal violet staining solution (Beyotime, Shanghai, China) for 15 min, and washed again. To solubilize the crystal violet, 150 μL of 33% acetic acid in water was added to each well.
The optical density (OD) of each well was measured at 590 nm using an automated ELx800 universal microplate reader (Bio-Tek, USA). According to previous studies,23,24 the results were categorized based on the calculated cut-off optical density (ODc) value. The ODC value was defined as the mean OD of the blank group plus three times the standard deviation (SD),25 and the results of the biofilm formation assay were analyzed as follows: non-biofilm producers (OD ≤ ODC), weak producers (ODC < OD ≤ 4ODC), and strong producers (OD > 4ODC).
Scanning Electron Microscopy (SEM)
After biofilm formation on a sterile slide after 24 h of culturing in TSB, the sample was fixed with 2.5% glutaraldehyde overnight, washed three times in phosphate buffer (0.1 M, pH 7.0) for 15 min prior to postfixing with 1% OsO4 for approximately 1–2 h, and then washed again. Subsequently, the sample was dehydrated using gradient ethanol, dried in a Hitachi Model HCP-2 critical point dryer, coated with gold-palladium, and observed using a Hitachi Model SU-8010 SEM at 30 kV.
Statistical Analysis
The results for continuous variables were presented as the means (SD) or median (IQR), and proportions were expressed as percentages. The chi-squared test, Fisher’s exact test and Mann–Whitney U-test were used for between-group comparisons. Statistical analyses were performed using the SPSS, version 19.0 (Chicago, IL, USA). A P value of ≤ 0.05 was considered statistically significant.
Results
General Information and Clinical Characteristics
Fifty-three CA-MRSA strains were isolated from the respiratory samples of 53 children aging from 0.3–144 months (median, 11.1). However, approximately half of these children (27/53, 50.9%) were young infants (0–12 months). The male to female ratio was 1.30. Of the 53 children, 92.4% (49/53) were previously healthy, three (5.7%) had a history of pneumonia, one (1.9%) had congenital heart disease, three (5.7%) had a history of premature delivery. The clinical features and demographic data are presented in Table 1.
 |
Table 1 General Information and Clinical Characteristics of Enrolled Children |
Regarding sample sources, 49.1% (26/53) of the CA-MRSA strains were collected from sputum samples. Of the enrolled pediatric patients, 41.5% (22/53) were from the respiratory department, followed by the neonatal department (20.8%), and the remaining were scattered in the various clinical departments in the hospital. The most common diagnosis was lower respiratory tract infection (LRTI; 39/53, 73.6%), including 36 cases of pneumonia and 3 cases of bronchitis. Moreover, eight children (15.0%) were classified as asymptomatic colonization because they had no obvious respiratory symptoms and the isolates were recovered from pharyngeal swabs on the day before they received planned elective surgical procedures.
Antibiotic Resistance
Antimicrobial susceptibility results are presented in Table 2. All CA-MRSA strains were resistant to penicillin and oxacillin. The rates of resistance were considerably high to erythromycin (47/53, 88.7%) and clindamycin (46/53, 86.8%) but were less than 10% to the other antibiotics. In addition, all strains were sensitive to linezolid, vancomycin, tigecycline, teicoplanin, rifampicin, and daptomycin. Overall, 88.7% (47/53) of the isolates were resistant to four or more antibiotics, mainly β-lactam antibiotics, lincosamides, and macrolides.
 |
Table 2 The Antimicrobial Resistance Profiling of 53 CA-MRSA Isolates |
Molecular Characteristics
Among the CA-MRSA isolates, 12 STs and 20 subtypes of spa were identified. In MLST, ST59 (60.4%, 32/53) was the most prevalent subtype, followed by ST22 (9.4%, 5/53), and ST398 (7.5%, 4/53). The spa typing analysis showed that t437 was the most common subtype (39.6%, 21/53), followed by t3592 (7.5%, 4/53), and the remaining subtypes occupied minor proportions. By integrating the results of MLST and spa typing, the ST59-t437 clone (39.6%, 21/53) was found to be predominant among the CA-MRSA isolates. According to gene detection, the mecA gene was present in all CA-MRSA strains in this study, while the PVL genes was found in only 35.8% (19/53) of the strains. Furthermore, no significant difference in the presence of the PVL genes was found between the dominant clone group and other clonal typing groups. The genotypic characteristics of the CA-MRSA isolates are shown in Figure 2.
Biofilm Assay and Its Effect on the Clinical Picture
All CA-MRSA strains were capable of forming biofilms. The ability of the CA-MRSA strains to form biofilms and representative SEM images are shown in Figure 3. According to the standard described in the Methods section, 50.9% (27/53) of the isolated CA-MRSA strains belonged to the weak producer group and 49.1% (26/53) to the strong producer group. Based on this classification, the correlation between the biofilm-forming ability and the clinical characteristics of these patients was analyzed (Table 3). Compared with the weak biofilm-forming group, the patients in the strong biofilm-producing group experienced a higher proportion of lower respiratory tract infections (88.5% vs 59.3%, P=0.026), more obvious cough symptoms (84.6% vs 51.9%, P=0.006), and more severe chest imaging (76.9% vs 37.0%, P=0.002). However, no significant differences in demographics, hospital stay duration, other symptoms, laboratory results, drug resistance rates, or positive rate of the PVL genes were found between the two groups.
 |
Table 3 Clinical Characteristics Between CA-MRSA Groups with Different Biofilm-Forming Capacity |
To further explore the adverse impact of biofilm formation in children with LRTI (n=39), a subgroup analysis was conducted. Intriguingly, as shown in Figure 4A, biofilm-forming significantly increased the risk of prolonged cough (44.4% vs 14.3%, P=0.037). Meanwhile, the line chart revealed a positive correlation between the duration of cough and the extent of biofilm formation (P=0.0496, Figure 4B). Furthermore, the strong biofilm-forming group had a significantly higher proportion of antibiotic changes owing to inefficient antimicrobial treatment than the weak producer group (50% vs 19%, P=0.041) (Figure 4C). However, no significant differences were found between the two groups in terms of oxygen demand or systemic glucocorticoid administration.
Additionally, in order to explore the factors underlying different biofilm-forming abilities of the two groups of CA-MRSA isolates, we investigated the relationship between their biofilm-forming ability and previous medication history. The strong biofilm-forming group had a much higher percentage of macrolides intake history in the last month before admission than the weak biofilm-forming group (61.5% vs 14.8%, P= 0.001, Supplementary Table 1).
Discussion
CA-MRSA infection remains a challenging issue worldwide. There has been an increase in the number of CA-MRSA infections among children.7,26 CA-MRSA is often associated with severe invasive infections characterized by acute exacerbation and high mortality.6,27 In addition, CA-MRSA can cause asymptomatic colonization of the nasopharynx in children, contributing to community transmission and recurrent infections. As a susceptible group, children play an important role in the transmission of CA-MRSA. Therefore, it is meaningful to characterize CA-MRSA respiratory strains from pediatric patients in terms of presentation, molecular characteristics, antibiotic resistance, and biofilm profiles.
In the present study, 53 CA-MRSA strains were isolated from the respiratory samples of 53 children, of which half were young infants (0–12 months) (27/53, 50.9%), including 11 neonates. Although our data did not directly reflect the incidence rate of CA-MRSA respiratory infection in this age group, it did indicate that the neonatal group was at a greater risk for CA-MRSA infection. Neonates are classified as a putative high-risk group in the USA, as reported in a previous study.6 Furthermore, several countries have reported outbreaks of CA-MRSA in the neonatal group, and some evidence has revealed that multiple sources of transmission may be involved, such as family members28–30 and health care workers who have asymptomatic MRSA colonization or current infection,31,32 as well as vertical transmission,33 breast milk,34 and household contacts.30,35 Environmental cleaning, hand hygiene, contact precautions, and active screening for colonization are fundamental for the prevention of CA-MRSA infection.26,36
ST59 was the most predominant clone among the CA-MRSA isolates in our study as well as in China.37,38 Owing to the clonal diversity of CA-MRSA strains and varied antimicrobial resistance patterns, understanding the antibiotic resistance trends of epidemic clones in different regions could be helpful for clinical treatment. In the current study, 88.7% (47/53) of the isolates were resistant to four or more antibiotics, mainly β-lactam antibiotics, lincosamides, and macrolides, similar to the antimicrobial profiles of a nationwide study conducted in China.37 Clindamycin was once effective for treating CA-MRSA infections in the USA and Europe, where the most prevalent clones are USA300 and ST80; however, drug resistance has been increasing, not only to clindamycin, but also to other non-β-lactams, such as levofloxacin and mupirocin, because of the carriage of the mupA gene and widespread antibiotics use.39–42 Linezolid, vancomycin, teicoplanin, and daptomycin remain effective against MRSA infections. Unfortunately, some strains with reduced susceptibility to glycopeptides have been identified in a few cases.43,44
Biofilm formation has been implicated in recurrent pediatric upper respiratory tract infections, such as tonsillitis, adenoiditis, and chronic rhinosinusitis (CRS).45 Since biofilm formation in the lower respiratory tract is difficult to observe directly and there are few sources of bioptic samples, biofilm studies in LRTI has not been fully explored. To our knowledge, this study firstly explored the correlation between the biofilm-forming ability of CA-MRSA strains isolated from the respiratory tract and the clinical characteristics of respiratory diseases in children. The results showed that all the isolated CA-MRSA strains could form biofilms, and patients with strong biofilm-forming isolates tended to have a higher incidence of LRTI, more severe chest imaging, higher antibiotic change rate, and prolonged cough duration. Previous studies have shown that biofilms not only serve as barriers for bacteria but also exhibit high virulence potential. Biofilm proteome studies have revealed that the extracellular proteins primarily consist of functional virulence factors, including hemolysins, leukotoxins, and lipases.46,47 In addition, excreted intracellular proteins, such as FbaA, can promote binding to cell surfaces and exhibit high cytotoxicity toward host cells.48 Polysaccharides and eDNA enhance biofilm formation and mediate numerous virulence traits.49,50 Furthermore, biofilm-related intracellular inflammation has an indispensable role in infections. Tan et al observed intracellular S. aureus infection in CRS patients and identified its association with surface biofilm.51 Jardeleza et al analyzed the biofilm features of sinonasal tissue samples from CRS patients and pointed out that an increasing intracellular inflammasome response with the upregulation of AIM2 was involved in the formation of nasal polyps and S. aureus biofilms.52
Several studies have proposed promising strategies for inhibiting or eliminating the formation of biofilms,53–55 such as antibiofilm coatings for implants, ultrasonic eradication, and functional compounds blocking biofilm-forming signalling pathways. However, these methods are more applicable to device-related biofilms, but not to infection tissues, for which antibiotics remain one of the few choices to combat biofilm-related infections. Nevertheless, previous studies have revealed that exposure to sub-inhibitory concentrations of antibiotics can enhance biofilm formation and promote the transmission of antibiotic resistance genes, thereby increasing bacterial tolerance to antibiotics.56,57 Similarly, we found that the CA-MRSA strains in the strong biofilm-forming group had a much higher percentage of macrolides medication history. Early improper use of antibiotics may be one factor contributing to the development of biofilms, although further research with a larger sample size is needed to verify this assumption.
Conclusion
ST59-t437 is the most prevalent clone of CA-MRSA respiratory isolates among children in Children’s Hospital at Zhejiang University School of Medicine and also belongs to the predominant lineage in China. Most of these strains showed multi-drug resistance, and all were able to form biofilms. Their biofilm-forming ability correlated well with clinical characteristics. The stronger the biofilm-forming ability, the more serious and prolonged were the respiratory symptoms. More studies are needed to confirm this relationship and develop applicable biofilm control measures.
Ethics Approval and Consent to Participate
This study complies with the Declaration of Helsinki and was approved by the ethics committee of the Children’s Hospital, Zhejiang University School of Medicine, (EC) approval number: 2022-IRB-005. The informed consent was waived as the retrospective and anonymous nature of this study.
Acknowledgments
We thank Dandan Song in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for her technical assistance on transmission electron microscopy. BioRender.com was used to create the schematic (Figure 1).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No.81870023), Grant from the Key Program of the Independent Design Project of National Clinical Research Center for Child Health(G20B0003), Zhejiang Provincial Key R & D Projects (No. 2020C03062), Zhejiang Provincial Major Medical and Health Science and Technology Plan (2018268955).
Disclosure
The authors declare no potential conflicts of interest in this work.
References
1. Lakhundi S, Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4):e00020–18. doi:10.1128/CMR.00020-18
2. Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56(1):
3. Saravolatz LD, Markowitz N, Arking L, et al. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96(1):11–16. doi:10.7326/0003-4819-96-1-11
4. Mediavilla JR, Chen L, Mathema B, et al. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012;15(5):588–595. doi:10.1016/j.mib.2012.08.003
5. Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2012;18(8):725–734. doi:10.1111/j.1469-0691.2012.03822.x
6. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi:10.1128/CMR.00081-09
7. Loewen K, Schreiber Y, Kirlew M, et al. Community-associated methicillin-resistant Staphylococcus aureus infection: literature review and clinical update. Can Fam Physician. 2017;63(7):512–520.
8. Liao F, Gu W, Fu X, et al. Comparison of virulence-related determinants between the ST59-t437 and ST239-t030 genotypes of methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2021;21(1):264. doi:10.1186/s12866-021-02329-5
9. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):2976–2984. doi:10.1001/jama.290.22.2976
10. Song JH, Hsueh PR, Chung DR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66(5):1061–1069. doi:10.1093/jac/dkr024
11. Centers for Disease C, Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus - Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48(32):707–710.
12. Nazzari E, Torretta S, Pignataro L, et al. Role of biofilm in children with recurrent upper respiratory tract infections. Eur J Clin Microbiol Infect Dis. 2015;34(3):421–429. doi:10.1007/s10096-014-2261-1
13. Boisvert AA, Cheng MP, Sheppard DC, et al. Microbial biofilms in pulmonary and critical care diseases. Ann Am Thorac Soc. 2016;13(9):1615–1623. doi:10.1513/AnnalsATS.201603-194FR
14. Tsai MS, Chen CJ, Lin TY, et al. Nasal methicillin-resistant Staphylococcus aureus colonization among otherwise healthy children aged between 2 months and 5 years in northern Taiwan, 2005–2010. J Microbiol Immunol Infect. 2018;51(6):756–762. doi:10.1016/j.jmii.2017.07.014
15. Liang B, Liang X, Gao F, et al. Active surveillance, drug resistance, and genotypic profiling of staphylococcus aureus among school-age children in China. Front Med. 2021;8:701494. doi:10.3389/fmed.2021.701494
16. Gesualdo F, Bongiorno D, Rizzo C, et al. MRSA nasal colonization in children: prevalence meta-analysis, review of risk factors and molecular genetics. Pediatr Infect Dis J. 2013;32(5):479–485. doi:10.1097/INF.0b013e3182864e4c
17. Lin J, Peng Y, Xu P, et al. Methicillin-resistant staphylococcus aureus nasal colonization in Chinese children: a prevalence meta-analysis and review of influencing factors. PLoS One. 2016;11(7):e0159728. doi:10.1371/journal.pone.0159728
18. Hisata K, Kuwahara-Arai K, Yamanoto M, et al. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J Clin Microbiol. 2005;43(7):3364–3372. doi:10.1128/JCM.43.7.3364-3372.2005
19. Aj K, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304(6):641–648. doi:10.1001/jama.2010.1115
20. Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41(12):5442–5448. doi:10.1128/JCM.41.12.5442-5448.2003
21. McClure JA, Conly JM, Lau V, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44(3):1141–1144. doi:10.1128/JCM.44.3.1141-1144.2006
22. O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;(47). doi:10.3791/2437
23. Ghasemian A, Najar Peerayeh S, Bakhshi B, et al. Comparison of biofilm formation between methicillin-resistant and methicillin-susceptible isolates of Staphylococcus aureus. Iran Biomed J. 2016;20(3):175–181. doi:10.7508/ibj.2016.03.007
24. Cha JO, Park YK, Lee YS, et al. In vitro biofilm formation and bactericidal activities of methicillin-resistant Staphylococcus aureus clones prevalent in Korea. Diagn Microbiol Infect Dis. 2011;70(1):112–118. doi:10.1016/j.diagmicrobio.2010.11.018
25. Stepanovic S, Vukovic D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115(8):891–899. doi:10.1111/j.1600-0463.2007.apm_630.x
26. Kaplan SL. Implications of methicillin-resistant Staphylococcus aureus as a community-acquired pathogen in pediatric patients. Infect Dis Clin North Am. 2005;19(3):747–757. doi:10.1016/j.idc.2005.05.011
27. McAdams RM, Mazuchowski E, Ellis MW, et al. Necrotizing staphylococcal pneumonia in a neonate. J Perinatol. 2005;25(10):677–679. doi:10.1038/sj.jp.7211364
28. Al-Tawfiq JA. Father-to-infant transmission of community-acquired methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2006;27(6):636–637. doi:10.1086/505097
29. Sax H, Posfay-Barbe K, Harbarth S, et al. Control of a cluster of community-associated, methicillin-resistant Staphylococcus aureus in neonatology. J Hosp Infect. 2006;63(1):93–100. doi:10.1016/j.jhin.2005.11.016
30. Hollis RJ, Barr JL, Doebbeling BN, et al. Familial carriage of methicillin-resistant Staphylococcus aureus and subsequent infection in a premature neonate. Clin Infect Dis. 1995;21(2):328–332. doi:10.1093/clinids/21.2.328
31. David MD, Kearns AM, Gossain S, et al. Community-associated meticillin-resistant Staphylococcus aureus: nosocomial transmission in a neonatal unit. J Hosp Infect. 2006;64(3):244–250. doi:10.1016/j.jhin.2006.06.022
32. Saunders A, Panaro L, McGeer A, et al. A nosocomial outbreak of community-associated methicillin-resistant Staphylococcus aureus among healthy newborns and postpartum mothers. Can J Infect Dis Med Microbiol. 2007;18(2):128–132. doi:10.1155/2007/617526
33. Rutar T. Vertically acquired community methicillin-resistant Staphylococcus aureus dacryocystitis in a neonate. J AAPOS. 2009;13(1):79–81. doi:10.1016/j.jaapos.2008.08.005
34. Gastelum DT, Dassey D, Mascola L, et al. Transmission of community-associated methicillin-resistant Staphylococcus aureus from breast milk in the neonatal intensive care unit. Pediatr Infect Dis J. 2005;24(12):1122–1124. doi:10.1097/01.inf.0000189983.71585.30
35. Mean M, Mallaret MR, Andrini P, et al. A neonatal specialist with recurrent methicillin-resistant Staphylococcus aureus (MRSA) carriage implicated in the transmission of MRSA to newborns. Infect Control Hosp Epidemiol. 2007;28(5):625–628. doi:10.1086/513616
36. Cho SY, Chung DR. Infection prevention strategy in hospitals in the era of community-associated methicillin-resistant Staphylococcus aureus in the Asia-Pacific Region: a review. Clin Infect Dis. 2017;64(suppl_2):S82–S90. doi:10.1093/cid/cix133
37. Chen Y, Sun L, Ba X, et al. Epidemiology, evolution and cryptic susceptibility of methicillin-resistant Staphylococcus aureus in China: a whole-genome-based survey. Clin Microbiol Infect. 2022;28(1):85–92. doi:10.1016/j.cmi.2021.05.024
38. Chen H, Yin Y, van Dorp L, et al. Drivers of methicillin-resistant Staphylococcus aureus (MRSA) lineage replacement in China. Genome Med. 2021;13(1):171. doi:10.1186/s13073-021-00992-x
39. Buescher ES. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr Opin Pediatr. 2005;17(1):67–70. doi:10.1097/01.mop.0000147906.30720.4d
40. Chua K, Laurent F, Coombs G, et al. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA - its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52(1):99–114. doi:10.1093/cid/ciq067
41. Copin R, Sause WE, Fulmer Y, et al. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2019;116(5):1745–1754. doi:10.1073/pnas.1814265116
42. Desroches M, Potier J, Laurent F, et al. Prevalence of mupirocin resistance among invasive coagulase-negative staphylococci and methicillin-resistant Staphylococcus aureus (MRSA) in France: emergence of a mupirocin-resistant MRSA clone harbouring mupA. J Antimicrob Chemother. 2013;68(8):1714–1717. doi:10.1093/jac/dkt085
43. Sievert DM, Rudrik JT, Patel JB, et al. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46(5):668–674. doi:10.1086/527392
44. Linares J. The VISA/GISA problem: therapeutic implications. Clin Microbiol Infect. 2001;7(Suppl 4):8–15. doi:10.1046/j.1469-0691.2001.00054.x
45. Hamilos DL. Biofilm formations in pediatric respiratory tract infection: part 1: biofilm structure, role of innate immunity in protection against and response to biofilm, methods of biofilm detection, pediatric respiratory tract diseases associated with mucosal biofilm formation. Curr Infect Dis Rep. 2019;21(2):6. doi:10.1007/s11908-019-0658-9
46. Graf AC, Leonard A, Schauble M, et al. Virulence factors produced by Staphylococcus aureus biofilms have a moonlighting function contributing to biofilm integrity. Mol Cell Proteomics. 2019;18(6):1036–1053. doi:10.1074/mcp.RA118.001120
47. Lei MG, Gupta RK, Lee CY. Proteomics of Staphylococcus aureus biofilm matrix in a rat model of orthopedic implant-associated infection. PLoS One. 2017;12(11):e0187981. doi:10.1371/journal.pone.0187981
48. Ebner P, Rinker J, Nguyen MT, et al. Excreted cytoplasmic proteins contribute to pathogenicity in Staphylococcus aureus. Infect Immun. 2016;84(6):1672–1681. doi:10.1128/IAI.00138-16
49. Campoccia D, Montanaro L, Arciola CR. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int J Mol Sci. 2021;22(16):9100. doi:10.3390/ijms22169100
50. Karygianni L, Ren Z, Koo H, et al. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28(8):668–681. doi:10.1016/j.tim.2020.03.016
51. Tan NC, Foreman A, Jardeleza C, et al. The multiplicity of Staphylococcus aureus in chronic rhinosinusitis: correlating surface biofilm and intracellular residence. Laryngoscope. 2012;122(8):1655–1660. doi:10.1002/lary.23317
52. Jardeleza C, Miljkovic D, Baker L, et al. Inflammasome gene expression alterations in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Rhinology. 2013;51(4):315–322. doi:10.4193/Rhino13.045
53. Yin W, Xu S, Wang Y, et al. Ways to control harmful biofilms: prevention, inhibition, and eradication. Crit Rev Microbiol. 2021;47(1):57–78. doi:10.1080/1040841X.2020.1842325
54. Ribeiro SM, Felicio MR, Boas EV, et al. New frontiers for anti-biofilm drug development. Pharmacol Ther. 2016;160:133–144. doi:10.1016/j.pharmthera.2016.02.006
55. Xu Y, Jones JE, Yu H, et al. Nanoscale plasma coating inhibits formation of Staphylococcus aureus biofilm. Antimicrob Agents Chemother. 2015;59(12):7308–7315. doi:10.1128/AAC.01944-15
56. Penesyan A, Paulsen IT, Gillings MR, et al. Secondary effects of antibiotics on microbial biofilms. Front Microbiol. 2020;11:2109. doi:10.3389/fmicb.2020.02109
57. Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–543. doi:10.1128/MMBR.00013-14
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.