Back to Journals » Clinical Epidemiology » Volume 11
The Copenhagen Oral Cavity Squamous Cell Carcinoma database: protocol and report on establishing a comprehensive oral cavity cancer database
Authors Schmidt Jensen J , Jakobsen KK , Mirian C , Christensen JT, Schneider K, Nahavandipour A, Wingstrand VL, Wessel I , Tvedskov JF , Frisch T, Christensen A, Specht L , Andersen E , Lelkaitis G, Grønhøj C, von Buchwald C
Received 11 May 2019
Accepted for publication 16 July 2019
Published 19 August 2019 Volume 2019:11 Pages 733—741
DOI https://doi.org/10.2147/CLEP.S215399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Irene Petersen
Jakob Schmidt Jensen,1 Kathrine Kronberg Jakobsen,1 Christian Mirian,1 Julie Thor Christensen,1 Katrine Schneider,1 Arvin Nahavandipour,1 Vibe Lindeblad Wingstrand,1 Irene Wessel,1 Jesper Filtenborg Tvedskov,1 Thomas Frisch,1 Anders Christensen,1 Lena Specht,2 Elo Andersen,3 Giedrius Lelkaitis,4 Christian Grønhøj,1 Christian von Buchwald1
1Department of Otorhinolaryngology, Head and Neck Surgery, and Audiology, Rigshospitalet, University of Copenhagen, Copenhagen 2100, Denmark; 2Department of Oncology, Rigshospitalet, University of Copenhagen, Copenhagen 2100, Denmark; 3Department of Oncology, Herlev Hospital, University of Copenhagen, Herlev 2730, Denmark; 4Department of Pathology, Rigshospitalet, University of Copenhagen, Copenhagen 2100, Denmark
Correspondence: Christian von Buchwald
Department of Otorhinolaryngology, Head and Neck Surgery, and Audiology, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark
Tel +45 3 545 2370
Email [email protected]
Objective: The aim was to establish a large comprehensive database of patients with oral cavity squamous cell carcinoma (OSCC) to enable surveillance and research of the disease.
Methods: All patients diagnosed and/or treated for OSCC at Rigshospitalet, University of Copenhagen, Denmark in the period 2000–2014 were included. Rigshospitalet is a tertiary treatment center and covers the Eastern Denmark region, comprising nearly half of the approximately 5.8 million inhabitants of Denmark. Data on numerous variables regarding general information of the patients at diagnosis, their primary cancer, recurrence, treatment, prior cancers, and secondary cancers were collected from the Danish Pathology Register and by evaluation of medical charts.
Results: One thousand three hundred and ninety-nine OSCC patients were included in the database (62% males). The median age at diagnosis was 63 years (range: 23–99 years). The most common anatomical location was the floor of mouth (38%). Among patients with known stage, 70.0% were diagnosed in T-stage 1 or 2 and 64.9% were diagnosed in N-stage 0. Most patients were treated with primary surgery (81.7% among patients with known treatment), of these 44% received adjuvant radiotherapy after surgery. The overall age-standardized incidence of OSCC per 100,000 increased from 2.15 in 2000 to 3.04 in 2014, with a significant annual percent change of 3.2%.
Conclusion: We have established a consecutive, population-based database of 1,399 OSCC patients. This creates a basis for multiple studies that will elaborate our understanding of OSCC, and hopefully improve diagnosis, treatment, and rehabilitation of OSCC patients.
Keywords: head and neck cancer, oral cavity cancer, squamous cell carcinoma, OSCC, database, epidemiology
About the Copenhagen Oral Cavity Squamous Cell Carcinoma (COrCa) database
The global incidence of oral cavity squamous cell carcinoma (OSCC) is approximately 300,000 new patients per year.1 In Denmark, oral cavity cancer is the second most common head and neck cancer and has previously been reported with an incidence of 3.5 pr. 100,000 in 2014 corresponding to more than 300 patients, with a substantial increase in incidence in the past decades.2,3 OSCC is consistently more common among men compared to women, but the most common anatomical sublocations vary in prior reports, with both buccal mucosa, oral tongue, and floor of mouth (FOM) reported.1,2,4–6 These differences might be explained by differences in tobacco use geographically, with cancer of buccal mucosa being associated with betel nut chewing.7–9
With the COrCa database, we established a database of 1,399 consecutive OSCC patients diagnosed, treated, or both, at the University Hospital of Copenhagen, Rigshospitalet, Denmark in the period 2000–2014. Rigshospitalet is a tertiary treatment center and covers the Eastern Denmark region comprising nearly half of the approximately 5.8 million inhabitants of Denmark. Since 2003, all OSCC patients have been treated according to national guidelines.10,11
The Danish health care system provides free access to all diagnostics and treatments from general practitioners to hospitals, financed by general taxes, to all citizens of Denmark. The standards of treatment are uniform, and treatment is initiated when indicated, irrespective of economy and insurance of the patients. By law there is no private hospital alternative. In this manner, referral- and selection-bias is diminished as the Danish health care system provides an ideal basis for retrospective and large cohort analyses.
The purpose of the database was to make data available for surveillance and research of OSCC and to encourage collaboration with external head and neck centers, thereby enabling validation studies. The COrCa database is supplemental to the Danish Cancer Registry (DCR), the Danish Pathology Register (DPR), and the Danish Head and Neck Cancer (DAHANCA) database, yet also provides data on additional parameters making new investigations of etiology of OSCC and survival of patients possible.12–14 With the database as a platform, the next step will be to implement a prospective and on-going database for future studies.
Materials and methods
We included all patients registered with a primary OSCC in the DPR diagnosed and/or treated at the University Hospital of Copenhagen between 2000 and 2014. The DPR is a nationwide database including information on pathology investigations performed in Denmark since 1970, with samples coded according to a Danish version of the Systematized Nomenclature of Medicine (SNOMED).14
During a 2-year period from 2016–2018, data on numerous parameters were collected from the DPR and by examination of the medical charts of each individual patient. In Denmark, medical charts have been digitalized since 2007. Most medical charts from before 2007 were scanned and available electronically, the remaining were obtained from a central archive.
The following data were collected: regarding the primary cancer, we included information on date of diagnosis, age at diagnosis, anatomical sublocation (ie, FOM, oral tongue, gingiva, lip and buccal mucosa, hard palate, and retromolar trigone), TNM-stage based on the clinical examination and any scans available at time of diagnosis (ie, T1, T2, T3, T4a/b/x, N0, N1, N2a/b/c, N3, M0, M1, and overall stage I, II, III, Iva/b/c/x), and corresponding certainty factor according to Union of International Cancer Control (UICC) 7th edition, differentiation degree (ie, low, intermediate, or high), histology with invasion depth, invasive front (ie, cohesive or non-cohesive), vascular invasion (ie, yes or no), perineural invasion (ie, yes or no), p16-status (ie, positive or negative), biopsy-verified lymph node metastases (ie, yes or no, and at which level(s) if yes), type of lymph node metastases (ie, isolated tumor cells, micrometastases, macrometastases, or unspecified), perinodal extension (ie, yes or no), and distant metastases (ie, yes or no, and location if yes).15 For recurrence, second primary malignancies, and previous malignancies, we obtained information on date of diagnosis, anatomical location, and whether the diagnosis was based on biopsy or resection. M-stage was defined as M0 unless a distant metastasis was specified in the medical records.
Regarding treatment of the primary cancer, the following information was obtained: for patients treated with primary surgery, date of surgery and information on resection margins (ie, clear, dysplasia, or with tumor involvement, clear defined as 5 mm or more) including eventual supplementary resection margins. For patients undergoing neck dissection and/or sentinel node biopsy, date of operation, number of lymph nodes removed, and number of lymph nodes with metastases were registered. For patients treated with radiotherapy, intent of therapy (ie, curative, supplementary, or palliative), date of start and end of treatment, overall numbers of fractions, fractions per week, and overall dose were registered. For patients receiving chemotherapy, type was applied. Further, information on sex, date of birth, vital status, smoking status, number of packyears, and alcohol intake was collected.
Only patients with squamous cell carcinoma specifically located in the oral cavity were included, with the oral cavity demarcated anteriorly by the vermillion junction of the lips and posteriorly by the junction of the hard and soft palate, the anterior palatoglossal arches, and the line of the circumvallate papillae on the tongue.
Cases in which there was uncertainty were discussed with the data collection group or an experienced ENT surgeon was consulted until consensus was reached.
After completion of the database, information was validated to ensure quality of the included data. All records were checked for incongruent or incomplete counts and all included records were issued with personal record numbers to ensure that there was no duplication of data. Unknown information/missing data in medical files were categorized to ensure no incomplete records. Half-way through the data collection process, as well as after the data collection, 5% of all cases were randomly selected and manually reviewed independently by two separate, experienced members of the data collection group to ensure uniform data entry methods.
The contents of the database are stored in REDCap (Vanderbilt University, Nashville, TN, USA). REDCap is an online-based application for secure storage of data and meets the criteria from 21 CFR Part 11, FISMA, and HIPAA, among others.16
We have further obtained access to data from the Danish National Patient Registry (NPR), Central Population Registry (CPR), and the Cause of Death Registry (CDR) for all patients included in the database. Data from these registries are not included in the database but are available for future studies. The NPR provides every contact a patient has with the health care system with a record number, to which all information on the contact is linked. The CPR contains data on vital status and emigration, and the CDR contains information on the specific cause of every death in Denmark.
All required approvals from the Danish Data Protection Agency (Approval number: 05228), the Danish Health Data Authority (Approval number: 04280), and the Ethical Committee (Approval number: H-1-2014-053) to establish the database have been obtained. Due to regulations from the Danish Data Protection Agency and The Danish Health Data Authority, the data are not freely available. Potential transfer of the data requires specific approval from the Danish Data Protection Agency and the Danish Health Data Authority.
Statistical analysis
For initial evaluation of the contents of the database, from which results are presented in this article, we divided anatomical sublocation into four groups: FOM, tongue, gingiva, and others (including: lip, buccal mucosa, retromolar trigone, hard palate, and unknown).9 We defined excessive alcohol intake as above 14 units of alcohol per week for men and seven for women, in accordance with the recommendation from the Danish Health Authority.17
Statistical analyses were performed in R statistics version 3.4.3 (Stanford University, Stanford, CA, USA).18 The incidence rates were calculated as age-standardized incidence rates (ASIR) per 100,000. ASIR circumvent that the distribution of age-groups can be different between populations, thereby allowing the rates to be compared with any given population directly. The ASIR were calculated with the direct method and the EpiTools package.19 The Danish population weighted with the WHO world standard population distribution was used as reference. The WHO world standard population gives the world average distribution of each 5-year age-group.20
The average annual percent change (AAPC) and possible changes in trends were assessed with Joinpoint trend analysis software v. 4.2.0.2.21 The joinpoint regression analysis estimates possible joinpoints also called trend breaks, which are significant changes in trends. We allowed up to three trend breaks and assumed growth to be logarithmic with the formula ln(y)=xb.
An age-period-cohort (APC) model was estimated using the Epi package.22 This allowed evaluation of the effect of age, calendar period, and birth cohort on incidence. The model gives estimates for age-period effect, age-cohorts effect, and age-period-cohort-effect individually. Age was arranged into 5-year intervals, and calendar period into 3-year intervals. For the period effect and the cohort effect we defined 2001 and 1920 as references.
We considered p-values less than 0.05 to indicate statistical significance.
Results
We included 1,399 patients diagnosed with and/or treated for an OSCC at Rigshospitalet, University Hospital of Copenhagen, during 2000–2014 in our database. The median age at diagnosis was 63 years (range: 23–99 years) and 61.9% (n=866) were men. The most common tumor location was the FOM comprising 38.1% (n=564), followed by the tongue at 34.5% (n=503). The majority of the patients were diagnosed in either T-stage 1 or 2, comprising 35.9% (n=389) and 34.1% (n=369) among patients with known T-stage. Further, among patients with known N-stage, 35.1% (n=382) were diagnosed in N1 or above, while 64.9% (n=704) were diagnosed in N0. Merely nine patients were diagnosed in M-stage 1 (0.6%) (Table 1).
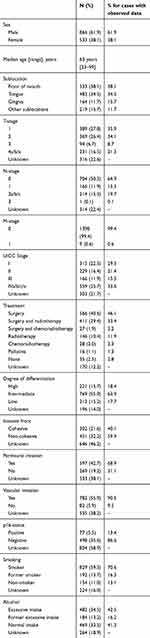 |
Table 1 Characteristics of 1,399 oral cavity squamous cell carcinoma patients diagnosed or treated at Rigshospitalet, University of Copenhagen, Denmark, in the period 2000–2014 |
 |
Table 2 Percentages of missing data among patients diagnosed before and after 2007 |
Among patients with known treatment, 81.7% (n=1,004) were treated with primary surgery and 438 (43.6%) of these patients received adjuvant radiotherapy after the surgery. One hundred and seventy-four patients were treated with primary radiotherapy (14.2% among patients with known treatment), 16.1% of these with concomitant chemotherapy (Table 1).
Among 607 patients with known invasion depth the average was 5.8 mm (range: 0.1–30 mm). The majority of patients with known status of invasive front of their tumor had a non-cohesive invasive front (59.9%). Above two thirds of cases (68.9%) evaluated for perineural invasion were positive. Among tumors evaluated for vascular invasion, 90.5% (n=782) was positive. Regarding p16-status, 77 patients were positive, and 498 were negative, corresponding to 13.4% and 86.6% among patients with known p16-status (Table 1).
At diagnosis 1,021 patients (86.9% among patients with known status) were smokers or former smokers and 482 (58.7% among patients with known status) had excessive alcohol intake or former excessive intake. The median number of packyears among smokers or former smokers was 40 (range: 1–183 packyears). For those with excessive alcohol intake the median number of units of alcohol per day was six (range: 3–50 units per day) for men, and three (range: 2–45 units per day) for women (Table 1).
The average percent of missing data per variable was 41.3% among patients diagnosed in 2007 or before, compared to 13.6% among patients diagnosed after 2007 (Table 2). Among complete cases the median age at diagnosis was 65 years (interquartile range [IQR]: 57–72) and the male to female ratio was 1.5. This was not substantially different from non-complete cases, with a median age at diagnosis of 62 years (IQR: 55–70) and a male to female ratio of 1.7.
The overall ASIR per 100,000 increased from 2.15 in 2000 to 3.04 in 2014, corresponding to an AAPC of 3.24% (95%CI: 1.6–5.0). There were no significant changes in trends. For men the increase was 3.14% per year (95%CI: 1.4–4-9) and for women it was 3.53% per year (95%CI: 0.7–6.5) (Figure 1). There was a significant increase in incidence for patients with cancer located to the FOM, tongue, and gingiva (AAPC: 2.73% [95%CI: 0.2–5.3], 5.74% [95%CI: 1.9–9.7], and 3.74% [95%CI: 0.9–6.6]), yet for the group of others there was no significant increase in incidence (Figure 1).
In the overall APC model we found a significant APC effect with a deviance of 8.9 (p=0.03). For women, the age cohort and age period deviance was 11.8 (p<0.01) and −14.4 (p<0.01) respectively, yet the deviance of the APC effect was 6.3 (p=0.1). This suggests a significant effect of age on the incidence of OSCC among women, yet no evident effect of cohort or period. For men, neither age cohort (p=0.09), age period (p=0.08), nor APC (p=0.12) deviance was significant, suggesting no significant effect of either age, period, or cohort on the incidence of OSCC among men (Figure 2).
Discussion
The COrCa is one of the largest known databases of OSCC originating from a well-defined geographic region, otherwise non-selective, consisting of 1,399 consecutive patients, and contains elaborate information on numerous parameters for each patient. In the evaluation of the content of the database presented in this article, we found median age at diagnosis and sex distribution among oral cavity cancer patients to be similar to previously reported results of a nationwide Danish study.2 The incidence of OSCC was however slightly lower in our evaluation. This difference is possibly because we specifically included solely squamous cell carcinoma, whereas Karnov et al included all oral cavity cancers. Further, there can be minor regional differences, with the Eastern Denmark region having a different incidence compared to nationwide incidence.
The overall incidence of OSCC increased with 3.24% on average per year in the study period. This is similar to prior reports from Denmark and other European countries, yet in contrast to findings from the USA and Canada, where the incidence of OSCC has been reported to be stable or decreasing.2,23,24
Alcohol consumption has been decreasing in Denmark the past decades, however the number of heavy drinkers is not known.25 Tobacco smoking has been decreasing as well, yet the number of heavy smokers remained stable among men and increased among women.26 This might explain the overall increase in incidence of OSCC and the marginally higher increase among women, compared to men.
The COrCA database provides more elaborate information on each OSCC patient compared to what has previously been available through the DCR, the DPR, and the DAHANCA database.12–14 The DAHANCA database is nationwide and includes patients with laryngeal, pharyngeal, sino-nasal, and salivary gland cancer in addition to oral cavity cancer, and contains the most elaborate information on each patient among the above-mentioned databases. However, regarding oral cavity cancer, the DAHANCA database does not provide information on, eg. alcohol intake, invasive front and depth, or vascular and neural involvement of the primary cancer. Further, for patients undergoing neck dissection or sentinel node biopsy, there is no information on how many lymph nodes have been removed or how many of these contained metastases. All this information is provided in the COrCa database.
The aim of the database was to make data available for surveillance and research of OSCC. We have planned a number of specific studies based on data from the database. An example is the investigation of the impact of p16 on survival. Evaluation of p16 status of all cases of OSCC treated at Rigshospitalet was implemented in 2010 with p16-positivity defined as immunohistochemistry staining above 70%. Opposite to the proportion of p16-positive cases in oropharyngeal cancer, only a minority of the evaluated oral cavity cancer cases in our database were p16-positive.
We plan to continuously update the contents of the database to include data on patients diagnosed with OSCC in the most recent years, thereby always making recent data available for future studies.
The APC model showed a significant effect of age on the incidence of OSCC among women, yet not among men. There is no obvious explanation to why the effect of age was not significant for men. It should however, be noted that the p-values for age cohort and age period deviance were both less than 0.1 and therefore not far from being significant. An effect of age on the incidence of OSCC among men might therefore not be dismissed completely.
Overall, there was a significant APC effect. This might be a result of the highly significant age effect for women. The cohort effect, with a decrease in the years leading up to, and following 1940, should also be noted, as this also has been observed in prior reports for head and neck cancer.27,28
All information in the database is based on registrations from the DPR and on reports by clinicians in medical charts. This will inevitably be associated with registration errors, and missing data are therefore a substantial limitation to the database. Further, all data were collected manually by the authors of this article, and hence is associated with registration bias as well. A substantial percentage of the data has however, as previously mentioned, been validated through two authors independently reviewing cases, and we will to continue to validate data from the database.
The decrease in missing data from 41.3% to 13.6% for patients diagnosed before and after 2007 is most likely explained by the digitalization of medical charts in 2007. Further, because the number of cases with missing data decreased every year after 2007, we expect future data collection to hold minimal levels of missing data. A minor level of missing data will however, be inevitable due to emigration.
In conclusion, with the COrCa database we have established one of the largest existing databases of OSCC patients at present, consisting of 1,399 consecutive patients. With its elaborate content, data for numerous retrospective studies are available, including advanced survival analyses, among others. Furthermore, it creates a basis for future prospective studies of OSCC. Hopefully it will expand our knowledge and understanding of OSCC and improve the survival of patients.
Acknowledgments
Kathrine Kronberg Jakobsen was funded by the Danish Cancer Society (grant number: R165-A10483-16-S7) and the University of Copenhagen (grant number: A5090). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
Professor Lena Specht reports non-financial support from Merck Serono (participation in international scientific conferences), personal fees from MSD and Kyowa Kirin, personal fees and non-financial support from Takeda, and grants from Varian, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015. doi:10.1002/ijc.29210
2. Karnov K, Grønhøj Larsen C, Jensen D, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol (Madr). 2017;56(9):1204–1209. doi:10.1080/0284186X.2017.1307516
3. Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol (Madr). 2018:1–9. doi:10.1080/0284186X.2018.1438657
4. Tandon P, Dadhich A, Saluja H, Bawane S. The prevalence of squamous cell carcinoma in different sites of oral cavity at our rural health care centre in Loni, Maharashtra – a retrospective 10-year study. Contemp Oncol (Pozn). 2017;21(2):178–183.
5. Aronberg R. The changing landscape of oral cavity cancer: analysis of epidemiological and genomic data. Yale Univ Sch Med - Yale Med Thesis Digit Libr. 2015;January:1–51.
6. Shield KD, Ferlay J, Jemal A, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. doi:doi:10.3322/caac.21384.
7. Anand R, Dhingra C, Prasad S, Menon I. Betel nut chewing and its deleterious effects on oral cavity. J Cancer Res Ther. 2014;10(3):499–505.
8. Hernandez BY, Zhu X, Goodman MT, et al. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. 2017;12(2):e0172196. doi:10.1371/journal.pone.0172196
9. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. doi:10.1016/j.soc.2015.03.006.CANCER
10. Dansk Selskab for Hoved- og Hals Onkologi; DSHHO. Behandling af planocellulært karcinom i mundhulen Nationale retningslinjer [National Guidelines for Treatment of Oral Cavity Sqaumous Cell Carcinoma]. Available from: https://www.dahanca.oncology.dk/assets/files/GUID_Mundhulekraeftretningslinjer.pdf. Published 2016.
11. Bilde A, Von Buchwald C, Johansen J, et al. The Danish national guidelines for treatment of oral squamous cell carcinoma. Acta Oncol (Madr). 2009;5(3):294–299. doi:10.1080/02841860600592998
12. Overgaard J, Jovanovic A, Godballe C, Grau Eriksen J. The Danish head and neck cancer database. Clin Epidemiol. 2016;8:491–496. doi:10.2147/CLEP.S103591
13. Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42–45. doi:10.1177/1403494810393562
14. Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39:72–74. doi:10.1177/1403494810393563
15. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours.
16. REDCap Consortium. REDCap. Available from: https://www.project-redcap.org/.
17. Sundhedsstyrelsen [Danish Health Authority]. Alcohol [Alkohol]. Available from: https://www.sst.dk/da/sundhed-og-livsstil/alkohol. Published 2018.
18. Team RC. R: A Language and Environment for Statistical Computing. Available from: https://www.r-project.org/. Published 2017.
19. Aragon TJ. Epitools: epidemiology Tools. R Package Version 0.5–9. Available from: https://cran.r-project.org/package=epitools. Published 2017.
20. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age Standardization of Rate: a New WHO Standard. Available from: http://www.who.int/healthinfo/paper31.pdf.
21. National Cancer Institute. Joinpoint Trend Analysis Software. Available from: https://surveillance.cancer.gov/joinpoint/.
22. Carstensen B, Plummer M, Laara E, Hills M. Epi: A Package for Statistical Analysis in Epidemiology. R package Version 2.37. Available from: https://cran.r-project.org/package=Epi. Published 2017.
23. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387–403. doi:10.1016/j.oraloncology.2014.01.016
24. Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States : 2000 – 2010. Cancer Epidemiol. 2019;39(4):497–504. doi:10.1016/j.canep.2015.04.007
25. Sundhedsstyrelsen [Danish Health Authority]. Alkoholstatistik 2015 - Nationale Data [Alcohol statistics 2015 - National Data]; Available from: https://www.sst.dk/da/udgivelser/2015/~/media/AD0E935AE31446C4AA09250B270A780B.ashx. Published 2015. Accessed January 12, 2019.
26. Osler M, Prescott E, Gottschau A, et al. Trends in smoking prevalence in Danish adults, 1964 – 1994. Scand J Soc Med. 1994;26(4):293–298.
27. Mirian C, Grønhøj C, Jensen DH, et al. Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980–2014. Cancer Epidemiol. 2018;55(May):81–87. doi:10.1016/j.canep.2018.05.009
28. Jensen JS, Jensen DH, Grønhøj C, et al. Incidence and survival of oropharyngeal cancer in Denmark: a nation-wide, population-based study from 1980 to 2014. Acta Oncol (Madr). 2018;57(2):269–275. doi:10.1080/0284186X.2017.1390251
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


