Back to Journals » International Journal of Women's Health » Volume 12
The Comparative Study of Cervical Shear Wave Elastography Between Twin and Singleton Pregnancy
Authors Diawtipsukon S , Bumrungphuet S , Dulyaphat W, Panburana P
Received 27 February 2020
Accepted for publication 22 July 2020
Published 21 August 2020 Volume 2020:12 Pages 649—656
DOI https://doi.org/10.2147/IJWH.S251522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Sanpon Diawtipsukon, Sommart Bumrungphuet, Wirada Dulyaphat, Panyu Panburana
Department of Obstetrics and Gynecology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Sommart Bumrungphuet
Department of Obstetrics and Gynecology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok 10400, Thailand
Tel +66 2 201 1412
Email [email protected]
Objective: To compare the cervical shear wave elastography (SWE) by using transvaginal ultrasound (TVS) between twin and singleton pregnant women.
Materials and Methods: This was a prospective cohort study involving the twin and singleton pregnant women who attended the antenatal care at Ramathibodi Hospital, Bangkok, Thailand. The participants who met the inclusion criteria were serially measured the shear wave speed (SWS) by using TVS at early, mid-, and third trimester. The changes in SWS with advancing gestational age between twin and singleton pregnancies were evaluated. The gestational age at delivery and spontaneous preterm delivery rate were also analyzed.
Results: A total of 36 twin pregnancies and 38 singleton pregnancies were analyzed. No significant difference in baseline characteristics, except the age of participants (twin pregnancies 33.1± 4.6 years, singleton pregnancies 29.9± 5.4 years, p-value = 0.006) was observed. The cervical SWS decreased with advancing gestational age in both twin and singleton pregnancy, but there was a statistically significant difference of cervical SWS at the lower point in mid-trimester (twin pregnancies 2.27± 0.4, singleton pregnancies 2.71± 0.6 m/s, p-value = 0.001). However, no significant difference in cervical SWS at the upper point and the lower point in the early and third trimester was demonstrated. Even though the gestational age at delivery between both groups revealed a significant difference (twin pregnancies 35.9± 2.8, singleton pregnancies 37.6± 2.9 wk., p-value = 0.008) but the spontaneous preterm delivery rate did not differ significantly (twin pregnancies 22.2%, singleton pregnancies 15.8%, p-value = 0.483).
Conclusion: The mid-trimester cervical SWS measurement at the lower point detects the difference in cervical softness between twin pregnancies and singleton pregnancies. The cervical SWS might be an additional option for monitoring the change in cervical softness in twin pregnancies.
Keywords: shear wave speed, shear wave elastography, cervical shear wave, cervical softness
Introduction
Preterm birth is a common problem in twin pregnancy worldwide. Progressively increase from 40 to 55% of twin and multiple pregnancy were born before 37 weeks of gestation, compare with 6% to 10% in singleton.1 The identification of twin pregnancies who are at risk of preterm birth remains an important problem. The history of prior singleton preterm birth carries increased risk of preterm birth in the subsequent twin pregnancy, with hazard ratio of 1.6 to 1.8 in one study.2 Although a nonmodifiable risk of previous preterm birth history was gathering, the effective tools are still required to access and monitor the other factors causing preterm birth in twin pregnancy.
A short cervix identified by using TVS at 16–24 weeks of gestation is associated with a high risk of preterm delivery (PTD). Eighteen percent of twin pregnant women with a short cervical length has increased the risk of spontaneous preterm birth by 8-fold.3 The mechanical alteration of the cervix in the final common pathway regarding multiple etiologies leading to PTD; therefore, maintaining a healthy gestation is mandatory for the mechanical integrity of the cervical tissue.4 However, there was conflicting evidence of the benefit of routine cervical length screening in multiple pregnancies because there is no effective intervention for preventing PTD in multiple pregnancies.5
In addition to cervical length, cervical softness might be a useful parameter to stratify the risk of PTD in twin pregnancy. Cervical softening is related to the viscoelastic properties of the cervix determined by the collagen content, tissue hydration, and concentration of proteoglycans in the extracellular matrix.6–10 Sundtoft et al found that the collagen content obtained by cervical biopsies was significantly lower in women with a history of short cervix or cervical insufficiency in pregnancy at 1-year-prior cervical sampling.11
Ultrasound elastography has been widely studied and used to estimate the elastic properties of tissues. Two main techniques of ultrasound applied for evaluating cervical softness include strain elastography (SE), of which a mechanical force is applied to create the displacement of tissues, and shear wave elastography (SWE), of which an acoustic force produces a mechanical impulse that generates tissue displacement.12–16
A significant drawback of SE is the unstandardized mechanical force required to displace the cervix for the developing stress to the cervical area.16,17 SWE can overcome the limitations of SE by generating an acoustic force automatically through cervical tissue that can be calculated as speed in meters per second (m/s) or as an indirect estimate of Young’s modulus of elasticity in kilopascals (kPa). The lower SWS can indicate the softer cervix.13,15,16 SWE maintained the same thermal effect as pulsed Doppler ultrasound already authorized in obstetrics18 and previously directed SWE with neonatal tissue studied by Kim et al did not report any adverse outcome.19
Several previous studies have widely employed SWE in singleton pregnancy. Carlson et al reported that treatment of nonpregnant cervix with prostaglandins, and the induction of labor with prostaglandins which showed to diminish the shear wave speed in the cervix.20 Muller et al found that SWS was significantly reduced in women who had symptoms of preterm labor and who delivered preterm.21 Peralta et al documented that SWS in the cervix of pregnant women was progressively decreased during cervical ripening.22 Hernandez-Andrade et al reported that pregnant women who had decreased cervical SWS while normal length increased the risk of spontaneous preterm delivery.23
Numerous data on cervical SWS of singleton pregnancy have been obtained; however, there is a limited number of studies regarding SWS in twin pregnancy. Accordingly, we conducted a prospective cohort study to measure the cervical SWE of unselected twin pregnancies compared with singleton pregnancies.
Materials and Methods
Study Design and Participants
This prospective cohort study was conducted at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, between December 2018 and January 2020, in accordance with the Declaration of Helsinki. All patients voluntarily participated in the protocol approved by the Committee on Human Right Related to Research Involving Human Subjects and written informed consent were obtained from all participants. The study was approved by the Thai Clinical Trials Registry, RCT number TCTR20200226002.
After pregnancy dating had been performed, participants who met the inclusion criteria defined by age at least 18-year-old, attending antenatal care at about 12–15+6 weeks of gestation and willing to participate underwent three times serial TVS for SWE for measuring the cervical SWS from 12 to 32 weeks of gestation. All pregnant women with the following conditions, including history of cervical pathology and/or surgery, current cervical pathology, cervical length less than 25 millimeters at 18–24 weeks of gestation, lethal fetal anomaly, death fetus in utero, abortion, loss to follow up, failure to obtain SWE, and refusing or withdrawing to participate were excluded from the study.
A total of 79 unselected twin and singleton pregnant women were recruited. One participant refused to participate before first transvaginal ultrasound was performed. Therefore, the data from 78 pregnant women were available for analysis. Demographic data, baseline characteristics, SWS, maternal outcomes, and neonatal outcomes were recorded. Gestational age and expected date of confinement were confirmed by using the ultrasound result at first antenatal care visit.
The sample size was calculated by the equation for comparing two independent means where α determine type I (alpha) error = 0.05, β determine type II (beta) error or power of the test at 90%. The mean SWS of singleton and twin pregnant women have been provided by Muller et al,21 and Ono et al,24 respectively. Therefore, 36 participants of twin and singleton pregnant women (72 participants in total) were required.
Ultrasound Examination
The TVS was performed by using a 7 MHz endovaginal probe (Aplio i700, Toshiba Medical System, Tochigi, Japan) by two sonographers, who have more than three years’ experience in cervical length measurement and certified by an online certificate of competence from the Fetal Medicine Foundation. All elastographic images were stored and evaluated by another obstetrician. To measure cervical SWS, we used the same sonographic view for the cervical length.
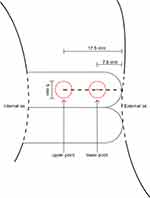
According to the Society for Maternal-Fetal Medicine (SMFM) Consult Series, the sonographers followed the eleven steps for proper cervical length measurement.5 After cervical length measurement had performed, we evaluated the cervical SWS by moving the transvaginal probe to the position that we can see both internal and external os clearly and adjusted the elastogram box to cover the entire cervix (Figures 1 and 2). The pressure was applied to the cervix as minimal as possible to ensure that the thickness of the anterior and posterior cervical lips remained equally, while the ultrasound probe remained in a fixed position, and the participant was asked to hold her breath for a very short period of time.5,13-15
Elastography was automatically generated from the system by using an acoustic impulse and estimated the SWS. SWS expressed as m/s, was calculated using a 5-mm-diameter circular region of interest and adjusted the center at measured points 7.5 mm, defined as lower point, and 17.5 mm, upper point, from the external cervical os of anterior lip of cervix,24 where Ono et al reported as the more relevant assessment point for cervical softening. All participants serially underwent TVS for cervical length and SWE measurement at 12–15+6 weeks, 18–23+6 weeks, and 28–31+6 weeks of gestation.
Statistical Analysis
Data were analyzed by IBM SPSS statistics 17.0. All continuous variables were tested for normality by Kolmogorov–Smirnov test and two independent engines were performed Student’s t-test and generated the mean and standard deviation (SD). The categorical data were analyzed using Chi-square and Fisher’s exact test as appropriate. Repeated measured ANOVA was performed for change in shear wave speed in the progression of gestational age. Variables that P-values less than 0.05 were defined as statistically significant.
Intra- and inter-rater reliability tests were acquired from 10% of participants to calculate the intraclass correlation coefficient (ICC). The first sonographer obtained two data sets from the average of three SWS measured at the upper point and lower point (Data A and Data B) and the second sonographer performed the three measurements of SWS at both points (Data C) from the same participant. To test the intra-rater reliability, Data set A and set B were analyzed. The Data set A and set C were calculated for the inter-rater reliability.
Results
A total of 79 pregnant women were enrolled. One participant refused to participate before 12 weeks of gestation, while each of 39 twin and singleton pregnant women were eligible for the study. Four pregnancies were excluded due to abortion, single fetal demise (in twins), refusal to undergo TVS, and loss to follow-up. Remaining 36 twin pregnant women and 38 singleton pregnant women were available for analysis as shown in Figure 3.
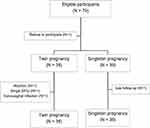 |
Figure 3 Participant eligibility diagram. |
Table 1 shows the demographic and baseline characteristics of the 74 participants. There was a significant difference in the age of participants (p-value = 0.006). Pre-pregnant body mass index (BMI), parity, route of the previous delivery, prior history of abortion and curettage, gestational age and cervical length at the cervical elastography measurement between both groups were not shown a statistically significant difference. Regarding the indicated preterm delivery in twin pregnancies, the gestational ages at delivery were significantly lower (p-value = 0.008). However, the spontaneous preterm delivery rate was not a significant difference (p-value 0.483).
 |
Table 1 Baseline Characteristics |
The main outcomes are shown in Table 2. At the lower point of cervix, the SWS in mid-trimester was significantly lower in twin pregnancies than that of singleton pregnancies (p-value = 0.001) but It did not show a difference in SWS between first and third measurements. There was no significant difference in SWS at the upper point for all measurements.
 |
Table 2 Cervical Shear Wave Speed (SWS, m/s) by Transvaginal Ultrasound Elastography |
Figure 4 shows, the SWS of the cervix at both upper and lower points were gradually decreased with advancing of gestational age. The reduction in SWS at the lower point of cervix represented the difference between first and second trimester (P-value =0.004), second and third trimester (P-value = 0.003), and first and third trimester (P-value < 0.001).
 |
Figure 4 Regression line of the mean SWS and 95% confidence interval of the lower point (A) and upper point (B). |
Sub-group analysis in participants with spontaneous preterm delivery was also performed. Interestingly, we found that significant difference in the mid-trimester SWS at the lower point between preterm and term twins (preterm 2.20±0.4, term 2.54±0.3 m/s, p-value = 0.042). On the contrary, the SWS of preterm and term singleton did not show a statistical difference (preterm 2.65±0.5, term 2.72±0.6 m/s, p-value = 0.818).
The intra- and inter-rather reliability assessed by the intraclass correlation coefficient (ICC) of two sonographers was evaluated in 10% of participants. The ICC at the upper point were 0.95, 0.99 and the lower point were 0.90, 0.93 for intra- and inter-rathe reliability, respectively.
Discussion
In this study, we found that the cervical softness in twin pregnancies progressed with advancing gestational age as that of singleton pregnancies. Moreover, the mid-trimester cervical SWS of twin pregnancies were significantly lower at the lower point compared with that of singleton pregnancies while there was no difference in cervical length. Likewise, several studies have revealed that there was no significant change in cervical length between 14 and 28 weeks of gestation25,28 whereas cervical softness has changed.
There was a significant difference in age between twin and singleton pregnant women which might affect the amount of collagen content in cervix. Only the study of Oxlund et al documented the increasing of collagen concentration by age29 However, there was a conflicting evidence regarding age related cervical change as described by Meyberg-Solomayer et al of which reported the softening of cervix associated with the increasing maternal age.30
We also agree with the established data from Hernandez-Andrade et al given that no significant differences in the SWS between the anterior, posterior, and lateral regions at any gestational period.31 The region of interest for measuring the SWS should be defined and reproduced. We therefore used the anterior region and external cervical os as an index point for examining the SWS. Similar to Ono et al,24 we modified the measured points at 7.5 mm (lower point) and 17.5 mm (upper point) from the external cervical os in this study. The interobserver correlation shows acceptable range of reliability for both measurements at upper and lower points. Several previous studies also documented the high reliability of quantitative elastography of the cervix.32,33
Surprisingly, the cervical SWS at 7.5 mm (lower point) obtained from external cervical os was significant difference between twin and singleton pregnancies. From the previous study, Ono et al described that the softening of cervix at 15–20 mm (upper point) from external cervical os was different from 26 twin pregnant participants24 without cervical length documentation. As previously known, the internal os of cervix must be firmly closed in early pregnancy and then gradually effaces and opens before the other part of cervix.6 Barnum et al demonstrated that the majority of the mechanical properties of murine cervix decrease at mid-gestation and not just at term.34 We hypothesize that the softness of cervix at the upper point had been likely changed in both twin and singleton cervix in the similar manner but at the lower point there are other factor such as smooth muscle cell organization which may also determine the softness. Joy et al found cervical smooth muscle cells were randomly scattered organized around the external cervical os when compare with circumferentially organized at the area of internal cervical os.35 Therefore, we could not detect the differences of SWS at the upper point in both twin and singleton pregnancies.
The advantage of SWE is a promising method for tissue evaluation due to quantitative determination of stiffness of the tissue without operator-dependence.13,15,16,26 Our data of SWS were prospectively developed and serially captured from the same participants. So, we could analyze the trends of SWS over time.
According to the study design, there are some limitations that we could not control, including baseline characteristics of all participants. Moreover, the sample size was inadequate for investigating the benefit of SWE in terms of preterm surveillance. We are still in need of further studies for evaluating the potential of SWE for preterm surveillance in twin pregnancy.
Conclusion
The mid-trimester cervical SWS measurement at the lower point detect the difference in cervical softness between twin pregnancies and singleton pregnancies. The cervical SWS might be an additional option for monitoring the change in cervical softness in twin pregnancies.
Data Sharing Statement
All available anonymized data can be obtained by contacting the corresponding author (Dr.Sommart Bumrungphuet, e-mail: [email protected]) until 5 years after publication.
Ethics Statement
The study protocol was approved by the Committee on Human Right Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. No. MURA2018/892). The study was approved by the Thai Clinical Trials Registry, RCT number TCTR20200226002.
Acknowledgments
The authors would like to thank Umaporn Udomsubpayakul, Statistician, Section for Clinical Epidemiology and Biostatistics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, who provided the statistical analysis.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Kogan MD, Alexander GR, Kotelchunk M, et al. Trends in twin birth outcomes and prenatal care utilization in the United States, 19811997. JAMA. 2000;284:335–341. doi:10.1001/jama.284.3.335
2. Ananth CV, Kirby RS, Vintzileos AM. Recurrence of preterm birth in twin pregnancies in the presence of a prior singleton preterm birth. J Matern Fetal Neonatal Med. 2008;21:289–295. doi:10.1080/14767050802010206
3. Golddenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol. 1996;175:1047–1053. doi:10.1016/S0002-9378(96)80051-2
4. Gravett MG, Rubens CE, Nunes TM. GAPPS Review Group. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S2. doi:10.1186/1471-2393-10-S1-S2
5. McIntosh J, Feltovich H, Berghella V, Manuck T. Society of maternal-fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215:B27. doi:10.1016/j.ajog.2016.04.027
6. Bohiltea RE, Munteanu O, Turcan N, et al. A debate about ultrasound and anatomic aspects of the cervix in spontaneous preterm birth. J Med Life. 2016;9:342–347.
7. Gedikbasi A, Yucel B, Arsaln O, Giris M, Gedikbasi A, Abbasoglu SD. Dynamic collagen changes in cervix during the first trimester and decreased collagen content in cervical insufficiency. J Matern Fetal Neonatal Med. 2016;29:2968–2972.
8. Myers KM, Feltovich H, Mazza E, et al. The mechanical role of the cervix in pregnancy. J Biomech. 2015;48:1511–1523. doi:10.1016/j.jbiomech.2015.02.065
9. Yao W, Gan Y, Myers KM, Vink JY, Wapner RJ, Hendon CP. Collagen fiber orientation and dispersion in the upper cervix of non-pregnant and pregnant women. PLoS One. 2016;11:e0166709. doi:10.1371/journal.pone.0166709
10. Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S829. doi:10.1016/j.ejogrb.2009.02.008
11. Sundtoft I, Langhoff-Roos J, Sandager P, Sommer S, Uldbjerg N. Cervical collagen is reduced in non-pregnant women with a history of cervical insufficiency and a short cervix. Acta Obstet Gynecol Scand. 2017;96:984–990. doi:10.1111/aogs.13143
12. Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol. 2018;43:773–785. doi:10.1007/s00261-018-1475-6
13. Tsuyoshi S, Kathryn RN, Mark LP, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principle and terminology. Ultrasound Med Biol. 2015;41:1126–1147. doi:10.1016/j.ultrasmedbio.2015.03.009
14. Barr RG. Elastography in clinical practice. Radiol Clin N Am. 2014;52:1145–1162. doi:10.1016/j.rcl.2014.07.002
15. Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB Guidelines and recommendations on the clinical use of ultrasound elastography part 1: basic principle and technology. Ultraschall in Med. 2013;34:169–184. doi:10.1055/s-0033-1335205
16. Swiatkowska-Freund M, Preis K. Cervical elastography during pregnancies: clinical perspectives. Int J Womens Health. 2017;9:245–254. doi:10.2147/IJWH.S106321
17. Maurer MM, Badir S, Pensalfini M, et al. Challenging the in-vivo assessment of biomechanical properties of the uterine cervix: a critical analysis of ultrasound based quasi-static procedures. J Biomech. 2015;48:1541–1548. doi:10.1016/j.jbiomech.2015.02.038
18. Issaoui M, Debost-Legrand A, Skerl K, et al. Shear wave elastography safety in fetus: a quantitative health risk assessment. Diagn Interv Imaging. 2018;99:519–524. doi:10.1016/j.diii.2018.04.013
19. Kim HG, Park MS, Lee JD, Park SY. Ultrasound elastography of the neonatal brain: preliminary study. J Ultrasound Med. 2017;36:1313–1319. doi:10.7863/ultra.16.06079
20. Carlson LC, Romero ST, Palmeri ML, et al. Changes in shear wave speed pre- and post-induction of labor: a feasibility study. Ultrasound Obstet Gynecol. 2015;46:93–98. doi:10.1002/uog.14663
21. Muller M, Ait-Belkacem D, Hessabi M, et al. Assessment of the cervix in pregnant women using shear wave elastography: a feasibility study. Ultrasound Med Biol. 2015;41:2789–2797. doi:10.1016/j.ultrasmedbio.2015.06.020
22. Peralta L, Mourier E, Richard C, et al. In vivo evaluation of cervical stiffness evolution during induced ripening using shear wave elastography, histology and 2 photon excitation microscopy: insight from an animal model. PLoS One. 2015;10:e0133377. doi:10.1371/journal.pone.0133377
23. Hernandez-Andrade E, Maymon E, Luewan S, et al. A soft cervix, categorized by shear-wave elastography, in women with short or with normal cervical length at 1824 weeks is associated with a higher prevalence of spontaneous preterm delivery. J Perinat Med. 2018;46:489–501. doi:10.1515/jpm-2018-0062
24. Ono T, Katsura D, Yamada K, et al. Use of ultrasound shear-wave elastography to evaluate change in cervical stiffness during pregnancy. J Obstet Gynaecol Res. 2017;43:1405–1410. doi:10.1111/jog.13379
25. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous preterm delivery. National institute of child health and development maternal fetal medicine unit network. N Engl J Med. 1996;334:567–572. doi:10.1056/NEJM199602293340904
26. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi:10.1056/NEJMoa067815
27. Bergelin I, Valentin L. Cervical changes in twin pregnancies observed by transvaginal ultrasound during the latter half of pregnancy: a longitudinal, observational study. Ultrasound Obstet Gynecol. 2003;21:556–563. doi:10.1002/uog.150
28. Oh KJ, Park KH, Jeong EH, Lee SY, Ryu A, Kim S. The change in cervical length over the time as a predictor of preterm delivery in asymptomatic women with twin pregnancies who have a normal mid-trimester cervical length. Twin Res Hum Genet. 2012;15:516–521. doi:10.1017/thg.2012.27
29. Oxlund BS, Ørtoft G, Bruel A, et al. Collagen concentration and biomechanical properties of samples from the lower uterine cervix in relation to age and parity in non-pregnant women. Reprod Biol Endocrinol. 2010;8:Epub82. doi:10.1186/1477-7827-8-82
30. Meyberg-Solomayer G, Gerlinger C, Hamza A, Schlaegel F, Takacs Z, Solomayer F. Cervical strain elastography in pregnancy and association with maternal factors. Ultraschall in Med. 2017;38:71–77.
31. Hernandez-Andrade E, Aurioles-Garibay A, Garcia M, et al. Effect of depth on shear-wave elastography estimated in the internal and external cervical os during pregnancy. J Perinat Med. 2014;42:549–557. doi:10.1515/jpm-2014-0073
32. Fruscalzo A, Schmitz R, Klockenbusch W, Steinhard J. Reliability of cervix elastography in the late first and second trimester of pregnancy. Ultraschall Med. 2012;33(Epub):101–107. doi:10.1055/s-0031-1299330
33. Fruscalzo A, Steinhard J, Londero AP, et al. Reliability of quantitative elastography of the uterine cervix in at-term pregnancies. J Perinat Med. 2013;41:421–427. doi:10.1515/jpm-2012-0180
34. Barnum CE, Fey JL, Weiss SN, et al. Tensile mechanical properties and dynamic collagen fiber re-alignment of the murine cervix are dramatically altered throughout pregnancy. J Biomech Eng. 2017;139:Epub061008. doi:10.1115/1.4036473
35. Joy YV, Sisi Q, Clifton OB, et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am J Obstet Gynecol. 2016;215:
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.