Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
The Combination of Serum BDNF, Cortisol and IFN-Gamma Can Assist the Diagnosis of Major Depressive Disorder
Authors Chen S , Zhang Y, Yuan Y
Received 27 May 2021
Accepted for publication 13 August 2021
Published 26 August 2021 Volume 2021:17 Pages 2819—2829
DOI https://doi.org/10.2147/NDT.S322078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Suzhen Chen,1,2 Yuqun Zhang,3 Yonggui Yuan1,2
1Department of Psychosomatics and Psychiatry, ZhongDa Hospital, School of Medicine, Southeast University, Nanjing, 210009, People’s Republic of China; 2Institute of Psychosomatics, School of Medicine, Southeast University, Nanjing, 210009, People’s Republic of China; 3School of Nursing, Nanjing University of Chinese Medicine, Nanjing, 210023, People’s Republic of China
Correspondence: Yonggui Yuan
Department of Psychosomatics and Psychiatry, ZhongDa Hospital, School of Medicine, Southeast University, No. 87 Dingjiaqiao, Gulou District, Nanjing, 210009, People’s Republic of China
Tel +86 25- 83285124
Email [email protected]
Purpose: Misdiagnosis and ineffective treatment are common in major depressive disorder (MDD) in current clinical practice, while the combination of various serum proteins may assist the correct diagnosis. The study aimed to explore whether the combination of serum inflammatory, stress, and neurotrophic factors could be helpful for the diagnosis of MDD and to investigate the predictors associated with early symptom improvements.
Patients and Methods: Baseline serum levels of C-reactive protein (CRP), interleukin (IL)-6, IL-10, IL-1beta, tumor necrosis factor (TNF)-alpha, interferon (INF)-gamma, cortisol, and brain-derived neurotrophic factor (BDNF) were detected in 30 MDD patients and 30 age- and gender-matched healthy controls. 17-item Hamilton Depression Rating Scale (HAMD-17) and Hamilton Anxiety Rating Scale (HAMA) were applied to assess symptoms both at baseline and two weeks after antidepressant treatment. Stepwise multiple linear regression was employed to identify the early efficacy predictors, and a logistic regression model was built with the above serum proteins. The area under the receiver operating characteristic (AUC) curve was calculated to evaluate the model’s diagnostic power.
Results: Multiple linear regression revealed that baseline scores of retardation (β = − 0.432, P = 0.012) and psychological anxiety (β = − 0.423, P = 0.014) factors were negatively associated with the reduction rate of HAMD-17. A simple and efficient diagnostic model using serum BDNF, cortisol, and IFN-gamma levels was established by the forward stepwise logistic regression, and the model achieved an AUC of 0.884, with 86.7% sensitivity and 83.3% specificity.
Conclusion: The results showed that combining serum BDNF, cortisol and IFN-gamma could aid the diagnosis of MDD, while baseline retardation and psychological anxiety may predict the poor early symptom improvement.
Keywords: major depressive disorder, inflammation factors, cortisol, neurotrophic factors, objective diagnostic platform, efficacy predictor
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders characterized by depressed mood, loss of interest, and reduced drive. It is also associated with sleep disturbance, diminished appetite, cognitive dysfunction, and motor symptoms. The lifetime prevalence of MDD is approximately 17%,1 affecting approximately 350 million people worldwide.2 It has been predicted that MDD would rank first in the total global burden of healthcare by 2030.3 According to the latest data in 2019, the lifetime prevalence rate of MDD in China has reached 3.4%, and it is estimated that about 44 million people suffer from MDD.4 More importantly, MDD is often recurrent and the leading risk factor for suicide.5 It has become a major mental disorder that has troubled people’s physical and mental health, bringing heavy burdens to patients, families, and society.
Accurate diagnosis and early efficacy prediction of MDD are prerequisites for effective treatment and a good prognosis. Unfortunately, as MDD is a complex disease with high heterogeneity, the diagnostic consistency is only 28%.6 Studies also report that less than half of MDD patients received effective treatment and only approximately 1/3 obtained remission.7,8 That is because the pathogenesis of MDD is still unclear and lacks objective biological diagnostic indicators. Current diagnoses still rely heavily on the subjective identification of depressive symptom clusters by psychiatrists. In addition, the antidepressant efficacy depends on the clinician’s assessments of the symptom changes over 6–8 weeks in clinical practice, which may lead to MDD patients taking ineffective drugs for long and delaying optimal treatment time. Happily, a report has been found, more than half the MDD patients who showed improvement at 8-week had begun to improve as early as 2-week,9 and the early improvement after two weeks of antidepressant treatment may be a predictor for eventual efficacy.10 Therefore, it is essential and urgent to develop objective tests for accurate diagnosis and early efficacy predictors for MDD.11
Although the brain tissue or cerebrospinal fluid (CSF) is an ideal resource for studying biomarkers of MDD, it is challenging to obtain CSF of MDD patients in clinical practice, and brain tissue is even more difficult to obtain. Therefore, peripheral blood proteins became more and more favored by researchers for they are easy to obtain and can reflect the function of the central system to some extent in recent years. Quite a few studies revealed that a platform composed of multiple peripheral blood proteins might be a promising objective diagnostic tool with a much higher diagnostic value than a single protein.12–17 Nevertheless, it is worth checking which peripheral blood proteins should be selected to construct the objective diagnostic platform.
Since the monoamine transmitter hypothesis has been questioned, the neurotrophic hypothesis, the inflammatory hypothesis, and the hypothalamic-pituitary-adrenal (HPA) axis dysfunction hypothesis have received more and more attention. Studies have found specific variations in inflammatory factors,18,19 cortisol20 and neurotrophic factors21–24 in peripheral blood of MDD patients. After conducting a comprehensive literature survey, we selected eight serum candidate markers based on the above three hypotheses. They, respectively, were C-reactive protein (CRP), interleukin (IL)-6, IL-10, IL-1beta, tumor necrosis factor (TNF)-alpha, interferon (INF)-gamma, cortisol, and brain-derived neurotrophic factor (BDNF).
In the present study, the main aims were (1) to explore the predictive factors that may be associated with the early depressive symptom improvement; (2) to establish a logistic regression model built with the above serum proteins and to explore whether the combination could help distinguish MDD patients from HC subjects with relatively good accuracy, specificity, and sensitivity.
Methods
Study Design and Participants
This study was a case-control study. From March 2018 to May 2018, thirty MDD inpatients that were drug-free for at least two weeks were recruited for the study from Zhongda Hospital Affiliated to Southeast University. Thirty gender- and age-matched healthy controls (HC) with no psychiatric or medical disorders were recruited through socially oriented advertising. We offered all the participants free biochemical tests and ¥100 as compensation. The study was carried out with the approval of the Ethics Committee of the Affiliated ZhongDa Hospital of Southeast University (Approval number: 2016ZDSYLL094-P01), and the study was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR-DOC-16010081). All participants provided written informed consent after being given a full explanation of the study following the Declaration of Helsinki.
All participants received semi-structured clinical interviews by two senior psychiatrists to determine whether they met the inclusion criteria for the patient or HC. The diagnosis was made with DSM-Axis 1 Disorders diagnostic criteria according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). Patients’ inclusion criteria were as follows: (1) age ≥18 years, (2) met the diagnostic criteria of DSM-IV with MDD, (3) 17-item Hamilton Depression Rating Scale (HAMD-17) score ≥17; while HC group that had a HAMD-17 score <7 were included. The exclusion criteria included: (1) in pregnancy or the puerperium, (2) with a comorbid axis I diagnoses (including bipolar depression), (3) with psychoactive substance including alcohol, tobacco or drug abuse and dependence, (4) with physical diseases (eg, tumor, organic brain diseases, cardiovascular diseases, endocrine disease, and autoimmune diseases), and (5) with a history of acute or chronic infections or nonsteroidal anti-inflammatory drugs used in the previous six weeks.
All enrolled patients were given antidepressant therapy according to their conditions, and 23 patients were treated with selective serotonin reuptake inhibitors (SSRIs) — nine with paroxetine, eight with escitalopram, three with sertraline, and three with fluoxetine; five patients with serotonin and norepinephrine reuptake inhibitors (SNRIs) — three with venlafaxine and two with duloxetine; one patient with noradrenergic and specific serotonergic antidepressants (NaSSAs) — mirtazapine; and one with serotonin modulator and stimulator (SMSs) — vortioxetine.
Clinical Assessment
A self-made general information questionnaire was used to collect the sociodemographic data of both patients and HC subjects. It included age, gender, education years, marital status, body mass index (BMI), alcohol, tobacco or substance use, familial history, physical or psychiatric illness, antidepressants and psychotropic medication history, number of episodes, and duration of recent disease. HAMD-17 and Hamilton Anxiety Rating Scale (HAMA) were applied to assess depressive and anxious severity of all the participants, respectively. HAMD-17 contains factors of anxiety/somatization, retardation, cognitive disorder, sleep disorder, and weight, while HAMA includes psychological anxiety factors and physical anxiety factors.
Sample Collection and Serum Proteins Measurement
Five milliliters of fasting blood were taken from the antecubital vein of each subject between 06:30 and 08:00 a.m., and the serum was obtained after centrifuging at 3500 rpm for 10 minutes. Then, serum samples were divided into equal parts of 0.5 mL and preserved at −80°C until further analysis. Measurements of serum CRP, IL-6, IL-10, IL-1beta, TNF-alpha, INF-gamma, cortisol, and BDNF were performed by enzyme-linked immunosorbent assay (ELISA) method using commercially available kits (CRP, IL-6, IL-10, IL-1beta, TNF-alpha, INF-gamma, and BDNF kits from RayBiotech, Norcross, GA, USA; cortisol kit from R&D Systems, Mutlukent Mah, Arda Sk, USA). The detection limits of CRP, IL-6, IL-10, IL-1beta, TNF-alpha, INF-gamma, cortisol and BDNF ELISA kits were 34 pg/mL, 3 pg/mL, 1 pg/mL, 0.3 pg/mL, 0.384 pg/mL, 15 pg/mL, 0.2 ng/mL and 80 pg/mL, respectively.
Statistical Analysis
SPSS statistical software (version 22, IBM, Armonk, NY, USA) was used to analyze the clinical and assay data. Continuous variables were presented as mean ± standard deviation (M ± SD) and discrete variables were expressed as number and percentage. The abnormal data determined by M ± 3 SD were excluded, and subsequently, replaced the missing values with the means of k nearest neighbors (KNN), which is simple but regarded to reduce imputation errors and give more accurate intake estimates.25–27 To examine whether the continuous variables were normally distributed, the Kolmogorov–Smirnov test was used. The Independent samples t-test or Chi-square test was applied to compare demographic and assay data between MDD patients at baseline and HC subjects as applicable. One-way analysis of variance (ANOVA) and Bonferroni post hoc test were performed to compare HAMD-17 and HAMA scores among pre-treatment and post-treatment MDD patients and HC subjects. At the same time, a paired-samples t-test was employed to examine the five factors (anxiety/somatization, retardation, cognitive disorder, sleep disorder, and weight) of HAMD-17 and the two factors (psychological and physical anxiety) of HAMA. Pearson’s correlation analysis was also used to test the correlations among different serum proteins, demographic, and clinical data. Stepwise multiple linear regression analysis was employed to identify and quantify the relationships of the reduction rate of HAMD-17 with the factor scores of HAMD-17 and HAMA, and the serum protein levels at baseline. Forward logistic stepwise regression analysis was performed to find the most appropriate multi-protein combination model for distinguishing MDD patients from HC subjects. The stepping criteria applied for entry and removal were based on the significance level of the F-value and set at 0.05 and 0.10, respectively. The receiver operating characteristic (ROC) curve analysis was employed to evaluate the model’s differential capacity by calculating the area under the curve (AUC: 0.9–1 = excellent; 0.8–0.9 = good; 0.7–0.8 = fair; 0.6–0.7 = poor; 0.5–0.6 = fail). Statistical significance was set at a 2-tailed P < 0.05 for all statistical tests.
Results
Demographic and Clinical Data
The demographic and clinical data of all participants are presented in Table 1. No statistically significant differences were observed between the MDD and HC groups concerning age, gender, education years, marital status, and BMI (Table 1, all P > 0.05). There were significant differences in HAMD-17 and HAMA scores among pre-treatment MDD, post-treatment MDD, and HC groups. Post hoc multiple comparisons showed that HAMD-17 and HAMA scores of both the pre-treatment MDD group (both P < 0.001) and post-treatment MDD group (both P < 0.001) were significantly higher than the HC group. After two weeks of antidepressant treatment, HAMD-17 and HAMA scores in MDD patients significantly declined but were still much higher than those in HC subjects.
 |
Table 1 Demographic and Clinical Data of Participants |
Serum Proteins Measurements
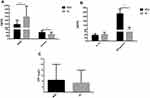
Among the eight selected serum proteins, serum levels of IL-6 in eight MDD patients and 15 HC, IL-1beta in 20 MDD patients and 22 HC, and TNF-alpha in 10 MDD patients and 14 HC were below the detectable limits of the ELISA kits. These three serum proteins were removed from subsequent analysis for the missing values exceeded 1/3 of the measurement values. Serum IFN-gamma levels were below the minimum detectable concentration of the kit in seven participants and higher than 3 SD in two participants. Serum levels of BDNF, cortisol, CRP, and IL-10 were, respectively, higher than 3 SD in one, two, one, and three participants. Since the missing values in these four serum proteins were less than 20% of the total measurement values, we replaced the deletions with the KNN. Serum levels of IFN-gamma and cortisol in the baseline MDD group were higher than those in the HC group, while serum BDNF levels were lower in MDD patients than those in HC (Figure 1, P < 0.05). However, there were no significant differences in serum levels of IL-10 and CRP between the two groups were only found in the trends to decline and rise, respectively (Figure 1, P > 0.05).
Correlation Analysis in MDD Patients
In MDD group, baseline HAMD-17 scores were significantly positively correlated with serum IFN-gamma levels (r = 0.378, P = 0.039); while the reduction rate of HAMD-17 was negatively correlated with baseline retardation factor (r = −0.399, P = 0.032) and psychological anxiety factor scores (r = −0.390, P = 0.037). In addition, significant positive correlations between serum levels of IFN-gamma and CRP (r = 0.497, P = 0.005), and negative correlations between serum levels of CRP and BDNF were also observed (r = −0.377, P = 0.040).
Multiple Linear Regression Analysis and Logistic Regression Analysis
Stepwise multiple linear regression analysis revealed that, in patients with MDD, scores of retardation factor and psychological anxiety factor at baseline were negatively associated with the reduction rate of HAMD-17 scores (β =−0.432, P =0.012 and β =−0.423, P =0.014, respectively). However, no association was found between the baseline levels of the remaining five serum proteins and the reduction rate of HAMD-17 scores.
Forward logistic stepwise regression analysis was employed to determine a regression model to maximize the identification ability with the minimum numbers among the remaining serum proteins. Three independent variables, BDNF, cortisol, and IFN-gamma, were entered into the logistic regression equation. The partial regression coefficients were −0.005, 0.049, and 0.007, respectively. Their corresponding p-values were 0.005, 0.002 and 0.013, respectively. The corresponding regression equation based on the three serum proteins was 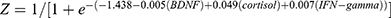 .
.
Performance of the Serum Proteins in the Differentiation of MDD and HC Subjects
As described in Table 2, ROC curve analysis revealed that the AUCs of single serum BDNF, cortisol, and IFN-gamma were 0.759 with a sensitivity of 70.0% and a specificity of 83.3%, 0.766 with a sensitivity of 96.7% and a specificity of 50.0%, and 0.716 with a sensitivity of 63.3% and a specificity of 76.7%, respectively (Figure 2A–C). While the AUCs of the combination of any two serum proteins increased to larger than 0.8 (Figure 2D–F). Unfortunately, however, the sensitivity or specificity of both single serum protein and combination of two serum proteins was poor. Interestingly, when combined with all of these three serum proteins, the AUC reached 0.884, with the sensitivity and specificity of 86.7% and 83.3%, respectively (Figure 3).
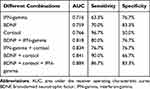 |
Table 2 The Comparison of AUCs of Each Single Serum Protein and Different Combinations of Serum Proteins |
Discussion
To date, the current diagnosis of MDD still depends on clinical symptomatologic criteria, like commonly used the International Statistical Classification of Diseases (ICD) and DSM diagnostic systems, and there are no objective diagnostic tools for clinical use. Consequently, the misdiagnosis of MDD is very common, which may lead to inappropriate treatment of patients. Furthermore, clinicians need at least 6–8 weeks to estimate the antidepressant treatment effect, resulting in patients receiving inappropriate treatment for long. Therefore, a convenient, effective objective diagnostic tool and reliable early efficacy predictors could help to reduce misdiagnosis, choose medication and treatment, and even improve prognosis. In this study, we innovatively combined the representative or key proteins involved in different hypotheses of the pathogenesis of MDD. We demonstrated that a logistic regression model based on serum BDNF, cortisol, and IFN-gamma could show a better differential efficacy between MDD and HC. In addition, early depressive symptom improvement was found to be associated with retardation and psychological anxiety degree of MDD patients at baseline.
The neurotrophic hypothesis, the HPA axis dysfunction hypothesis, and the inflammatory hypothesis are the currently highly accepted pathogenesis hypotheses of MDD, and many of the involved proteins have specific changes to be considered strong candidates for being biomarkers of MDD. The neurotrophic hypothesis suggests that MDD is closely related to the decreased expression of BDNF and other neurotrophic factors in the limbic system.28 A meta-analysis including 9484 subjects indicated that serum BDNF levels of MDD patients decreased significantly compared to HC,21 which was consistent with our current result. Previous studies showed a robust relationship between stress and MDD,29 stress was positively correlated with cortisol levels, and these two factors interacted with each other to predict depressive behaviors.30 Therefore, increased cortisol levels were observed in MDD patients,20 so as the results of our present study.
There is growing evidence that MDD is correlated with the upregulation of the inflammatory factors/cytokines.18,31–34 Simon et al35 assessed 20 cytokines simultaneously in both MDD and HC individuals and found that multiple pro-inflammatory cytokines, including IFN-gamma, IL-1beta, IL-6, IL-10 elevated significantly in MDD. They also found no significant difference in TNF-alpha levels between MDD and HC.35 In the present study, serum IFN-gamma levels were significantly higher in MDD than those in HC, which was consistent with some prior studies.18,33,36–38 Simultaneously, there was no significant difference in serum levels of IL-10 and CRP between MDD and HC groups, and serum levels of IL-6, IL-1beta, and TNF-alpha were even lower than the minimum detectable concentration of the ELISA kits in more than 1/3 participants. It was inconsistent with several meta-analysis studies.18,33 We speculated that there were several possible reasons for these conflicts in the results. First, MDD itself is a highly heterogeneous complex disease, it is more like a class of diseases than a single disease. Second, unlike inflammatory diseases, the inflammatory response of MDD is low-grade and the levels of some cytokines are below the minimum detectable limits of the typical commercial ELISA kits not only in HC subjects but also in MDD patients. Third, the ELISA kits we used in this study were different from those used in previous studies. Cassano et al39 believed that the inconsistency of the results may also be related to the sampling bias of non-inflammatory MDD. They found that the detected cytokines/chemokines showed no significant difference between the MDD and HC groups in their study, neither before nor after multiple corrections.39
In the present study, only serum IFN-gamma levels were found significantly positively correlated with HAMD-17 scores representing the severity of depressive symptoms in the MDD group. Schmidt and his colleagues40 found no significant correlations between CRP, IFN-gamma, and HAMD-17 total scores, but found IFN-gamma significantly correlated with “work and activities (item 7)” and “genital symptoms (item 14)”, which to some extent also reflected the severity of MDD. To all appearances, this result is inconsistent with ours. An animal study showed that serum IFN-gamma level was significantly correlated with anhedonia, the core symptom of MDD, and the higher the serum IFN-gamma level was, the less the sucrose consumption was in the depressed model mice.41 Kim et al42 found that IFN-gamma level only significantly positively correlated with HAMD in MDD patients without suicidal behavior, but not those with suicidal behavior. We also observed that serum CRP was significantly positively correlated with serum IFN-gamma and negatively correlated with serum BDNF. Considering that CRP is a nonspecific inflammatory marker of the systemic inflammatory response, it is often associated with the occurrence and severity of inflammation, including MDD. Furthermore, the inflammatory factors lead to abnormal synapses and reduce the synthesis of neurotrophic factors like BDNF through attacking neuronal cells.43
In addition, scores of retardation factor and psychological anxiety factor at baseline were observed to negatively predict the reduction rate of HAMD-17 scores in MDD patients. The more severe MDD patients’ baseline retardation and psychological anxiety were, the more minor improvements of the symptoms were after antidepressants treatment for two weeks. These findings are in line with the results of some studies and contrary to others.44,45 Retardation is one of the basic features of MDD,46 and results of several clinical studies suggested that MDD patients with severe baseline retardation should be treated with electroconvulsive therapy (ECT) as early as possible.47–49
For a useful diagnostic biomarker or test to be used in everyday clinical practice, it needs to be reproducible, reliable, inexpensive, and non-invasive. The serological examination is a convenient and straightforward method to evaluate the changes of different serum proteins involved in the pathogenesis of MDD in the body. As has been reported before, to be a clinically helpful biomarker or test for diagnosing and classifying a disorder correctly, at least 80% sensitivity and specificity must be provided.50 Schneider and Prvulovic50 pointed out that there were still no useful diagnostic biomarkers for MDD because none of the candidate markers fulfilled criteria as diagnostic biomarkers and a multivariable approach might be feasible/helpful to develop diagnostic biomarkers. In the present study, we detected serum levels of multiple proteins and assessed the diagnostic power of each serum protein and different combinations of these serum proteins to find a suitable diagnostic model of MDD for clinical use. Although all AUCs were larger than 0.7, either sensitivity or specificity was lower than 80% when serum BDNF, cortisol, or IFN-gamma was used to distinguish MDD from HC alone. In other words, single serum BDNF, cortisol, or IFN-gamma cannot be used to diagnose MDD for lacking sufficient sensitivity and specificity. Gratifyingly, the model based on these three serum proteins met the criteria of diagnostic tests for clinical practice, achieved an AUC of 0.884 with a sensitivity of 86.7% and specificity of 83.3%. Although the sensitivity, specificity, and diagnostic efficacy of the diagnostic platform for MDD in our present study are slightly less than those in some previous studies,12,15 we only need to combine three serum proteins to obtain the relatively superior diagnostic sensitivity, specificity, and diagnostic efficacy, which is less than five or nine different indicators required in those previous studies.12,15 Therefore, the diagnostic biomarker platform we proposed is more valuable for clinical application, both in economy and simplicity.
Besides, to explore the possibility of a more economical diagnostic platform, we also compared the combinations of any two in serum BDNF, cortisol, and IFN-gamma. The results revealed that the diagnostic effectiveness of the combination of serum cortisol and BDNF was highest (AUC = 0.834), and it also had the highest sensitivity of 90%. Unfortunately, however, the specificity of the combination was only 66.7%. Consequently, the combination of serum BDNF and cortisol is suitable for screening, while the combination of serum BDNF, cortisol, and IFN-gamma conduce to diagnose MDD. The clinicians may select the most appropriate one according to different needs.
Certain limitations should be considered in this study. Firstly, we included only a few serum proteins from the neurotrophic, the HPA axis dysfunction, and the inflammatory hypothesis in the study. Given that proteins involved in other MDD pathogenesis were also abnormal, a more comprehensive selection would be helpful to build such a diagnostic platform with much higher effectiveness, sensitivity, and specificity. Secondly, the sample size was relatively small which might result in false negatives. Thirdly, more female participants might lead to gender bias in this model. Fourthly, the ELISA kits we used were not hypersensitive, which led to the serum levels of three cytokines (TNF-alpha, IL-6, and IL-1beta) were below the minimum limits of the kits and failed to obtain exact detection values, so they did not enter the later stepwise logistic regression analysis. In future studies, hypersensitive ELISA kits can be used to detect cytokine levels in larger samples with different mental disorders to validate the current preliminary results or even to obtain an improved objective diagnostic biomarker platform for MDD.
Conclusion
In conclusion, the current study demonstrates that serum BDNF, cortisol, and IFN-gamma levels changed significantly in MDD patients, and combined these three serum proteins assist in distinguishing MDD patients from HC subjects. Baseline degrees of retardation and psychological anxiety could negatively predict patients’ symptom improvement after two weeks of antidepressant treatment. These findings could help improve the accuracy of the diagnosis and switch earlier to another effective therapeutic regimen.
Data Sharing Statement
The data and material supporting the results of this article are included within the article.
Acknowledgments
The authors would like to thank all participants for their time. This work was supported by the National Key Research and Development Program of China (2016YFC1306700), Jiangsu Provincial Key Research and Development Program (BE2019748), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_0116).
Author Contributions
Yuan Y was the principal investigator, designed the study protocol, and was involved in the recruitment of subjects and the revision of the manuscript. Chen S collected the samples of the subjects, performed the experiment, analyzed the statistical analysis, and wrote the manuscript. Zhang Y also participated in the collection of the subjects’ samples and revised the manuscript. All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ota KT, Liu RJ, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20(5):531–535. doi:10.1038/nm.3513
2. Smith K. Mental health: a world of depression. Nature. 2014;515(7526):181. doi:10.1038/515180a
3. Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2331–2371. doi:10.1016/j.neubiorev.2012.12.007
4. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
5. McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174(5):ITC65–65ITC80. doi:10.7326/AITC202105180
6. Freedman R, Lewis DA, Michels R, et al. The initial field trials of DSM-5: new blooms and old thorns. Am J Psychiatry. 2013;170(1):1–5. doi:10.1176/appi.ajp.2012.12091189
7. Rzezniczek S, Obuchowicz M, Datka W, et al. Decreased sensitivity to paroxetine-induced inhibition of peripheral blood mononuclear cell growth in depressed and antidepressant treatment-resistant patients. Transl Psychiatry. 2016;6(5):e827. doi:10.1038/tp.2016.90
8. Williams LM, Rush AJ, Koslow SH, et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12:4. doi:10.1186/1745-6215-12-4
9. Nierenberg AA, McLean NE, Alpert JE, Worthington JJ, Rosenbaum JF, Fava M. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry. 1995;152(10):1500–1503. doi:10.1176/ajp.152.10.1500
10. Vermeiden M, Kamperman AM, Vulink ME, van den Broek WW, Birkenhäger TK. Early improvement as a predictor of eventual antidepressant treatment response in severely depressed inpatients. Psychopharmacology (Berl). 2015;232(8):1347–1356. doi:10.1007/s00213-014-3765-1
11. Huang TL, Lin CC. Advances in biomarkers of major depressive disorder. Adv Clin Chem. 2015;68:177–204. doi:10.1016/bs.acc.2014.11.003
12. Bilello JA, Thurmond LM, Smith KM, et al. MDDScore: confirmation of a blood test to aid in the diagnosis of major depressive disorder. J Clin Psychiatry. 2015;76(2):e199–206. doi:10.4088/JCP.14m09029
13. Chen S, Jiang H, Liu Y, et al. Combined serum levels of multiple proteins in tPA-BDNF pathway may aid the diagnosis of five mental disorders. Sci Rep. 2017;7(1):6871. doi:10.1038/s41598-017-06832-6
14. Haenisch F, Cooper JD, Reif A, et al. Towards a blood-based diagnostic panel for bipolar disorder. Brain Behav Immun. 2016;52:49–57. doi:10.1016/j.bbi.2015.10.001
15. Jiang H, Chen S, Li C, et al. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl Psychiatry. 2017;7(4):e1079. doi:10.1038/tp.2017.43
16. Xiong P, Zeng Y, Wu Q, et al. Combining serum protein concentrations to diagnose schizophrenia: a preliminary exploration. J Clin Psychiatry. 2014;75(8):e794–801. doi:10.4088/JCP.13m08772
17. Yamamori H, Ishima T, Yasuda Y, et al. Assessment of a multi-assay biological diagnostic test for mood disorders in a Japanese population. Neurosci Lett. 2016;612:167–171. doi:10.1016/j.neulet.2015.12.019
18. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–387. doi:10.1111/acps.12698
19. Huang TL, Lee CT. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin Neurosci. 2007;61(4):415–420. doi:10.1111/j.1440-1819.2007.01686.x
20. Wichmann S, Kirschbaum C, Böhme C, Petrowski K. Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology. 2017;83:135–141. doi:10.1016/j.psyneuen.2017.06.005
21. Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19(7):791–800. doi:10.1038/mp.2013.105
22. Chiou YJ, Huang TL. Serum brain-derived neurotrophic factors in Taiwanese patients with drug-naïve first-episode major depressive disorder: effects of antidepressants. Int J Neuropsychopharmacol. 2017;20(3):213–218. doi:10.1093/ijnp/pyw096
23. Hsieh MT, Lin CC, Lee CT, Huang TL. Abnormal brain-derived neurotrophic factor Exon IX promoter methylation, protein, and mRNA levels in patients with major depressive disorder. J Clin Med. 2019;8(5):568. doi:10.3390/jcm8050568
24. Lin CC, Huang TL. Brain-derived neurotrophic factor and mental disorders. Biomed J. 2020;43(2):134–142. doi:10.1016/j.bj.2020.01.001
25. Steuer R, Morgenthal K, Weckwerth W, Selbig J. A gentle guide to the analysis of metabolomic data. Methods Mol Biol. 2007;358:105–126. doi:10.1007/978-1-59745-244-1_7
26. Parr CL, Hjartåker A, Scheel I, Lund E, Laake P, Veierød MB. Comparing methods for handling missing values in food-frequency questionnaires and proposing k nearest neighbours imputation: effects on dietary intake in the Norwegian Women and Cancer study (NOWAC). Public Health Nutr. 2008;11(4):361–370. doi:10.1017/S1368980007000365
27. Troyanskaya O, Cantor M, Sherlock G, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17(6):520–525. doi:10.1093/bioinformatics/17.6.520
28. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi:10.1016/j.biopsych.2006.02.013
29. Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi:10.1001/jama.2009.878
30. Qin DD, Rizak J, Feng XL, et al. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci Rep. 2016;6:30187. doi:10.1038/srep30187
31. Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl). 2016;233(9):1623–1636. doi:10.1007/s00213-016-4214-0
32. Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1044–1053. doi:10.1016/j.pnpbp.2007.03.004
33. Köhler CA, Freitas TH, Stubbs B, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55(5):4195–4206. doi:10.1007/s12035-017-0632-1
34. Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One. 2018;13(6):e0197267. doi:10.1371/journal.pone.0197267
35. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230–233. doi:10.1016/j.euroneuro.2007.06.004
36. Dahl J, Ormstad H, Aass HC, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi:10.1016/j.psyneuen.2014.03.019
37. Schmidt FM, Lichtblau N, Minkwitz J, et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29–34. doi:10.1016/j.jpsychires.2014.04.021
38. Eidan AJ, Al-Harmoosh RA, Al-Amarei HM. Estimation of IL-6, INFγ, and lipid profile in suicidal and nonsuicidal adults with major depressive disorder. J Interferon Cytokine Res. 2019;39(3):181–189. doi:10.1089/jir.2018.0134
39. Cassano P, Bui E, Rogers AH, et al. Inflammatory cytokines in major depressive disorder: a case-control study. Aust N Z J Psychiatry. 2017;51(1):23–31. doi:10.1177/0004867416652736
40. Schmidt FM, Schröder T, Kirkby KC, et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. 2016;239:85–91. doi:10.1016/j.psychres.2016.02.052
41. Géa LP, Colombo R, Rosa EDD, et al. Anhedonic-like behavior correlates with IFNγ serum levels in a two-hit model of depression. Behav Brain Res. 2019;373:112076. doi:10.1016/j.bbr.2019.112076
42. Kim YK, Lee SW, Kim SH, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):356–361. doi:10.1016/j.pnpbp.2007.08.041
43. Wu Z, Wang G, Wei Y, Xiao L, Wang H. PI3K/AKT/GSK3β/CRMP-2-mediated neuroplasticity in depression induced by stress. Neuroreport. 2018;29(15):1256–1263. doi:10.1097/WNR.0000000000001096
44. Schrijvers D, Hulstijn W, Sabbe BG. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord. 2008;109(1–2):1–20. doi:10.1016/j.jad.2007.10.019
45. Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):395–409. doi:10.1016/j.pnpbp.2010.10.019
46. Schrijvers D, de Bruijn ER, Maas Y, De Grave C, Sabbe BG, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44(5):569–579. doi:10.1016/j.cortex.2007.08.014
47. van Diermen L, Vanmarcke S, Walther S, et al. Can psychomotor disturbance predict ect outcome in depression. J Psychiatr Res. 2019;117:122–128. doi:10.1016/j.jpsychires.2019.07.009
48. Heijnen WT, Kamperman AM, Tjokrodipo LD, Hoogendijk WJ, van den Broek WW, Birkenhager TK. Influence of age on ECT efficacy in depression and the mediating role of psychomotor retardation and psychotic features. J Psychiatr Res. 2019;109:41–47. doi:10.1016/j.jpsychires.2018.11.014
49. Belge JB, Van Diermen L, Sabbe B, et al. Improvement of psychomotor retardation after electroconvulsive therapy is related to decreased IL-6 levels. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110146. doi:10.1016/j.pnpbp.2020.110146
50. Schneider B, Prvulovic D. Novel biomarkers in major depression. Curr Opin Psychiatry. 2013;26(1):47–53. doi:10.1097/YCO.0b013e32835a5947
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.