Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
The Combination of Insulin Resistance and Serum Interleukin-1β Correlates with Post-Stroke Depression in Patients with Acute Ischemic Stroke
Authors Yi X, Zhu X, Zhou Y, Zhang D, Li M, Zhu Y, Guo X
Received 9 November 2020
Accepted for publication 7 February 2021
Published 9 March 2021 Volume 2021:17 Pages 735—746
DOI https://doi.org/10.2147/NDT.S291164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiaoyi Yi,1 Xiangyang Zhu,1 Yong Zhou,1 Dongmei Zhang,2 Mengmeng Li,2 Yuting Zhu,1 Xiaoming Guo1
1Deparment of Neurology, Affiliated Hospital 2 of Nantong University, Nantong, People’s Republic of China; 2Clinical Research Center, Affiliated Hospital 2 of Nantong University, Nantong, People’s Republic of China
Correspondence: Xiangyang Zhu
Department of Neurology, Affiliated Hospital 2 of Nantong University, 6 Haier-Xiang North Road, Nantong, 226000, Jiangsu, People’s Republic of China
Fax +86 513-85061148
Email [email protected]
Purpose: Previous studies have shown that insulin resistance and inflammation may be associated with the pathophysiological mechanisms of mood disorders. Here, we investigated whether homeostatic model assessment of insulin resistance (HOMA-IR) and serum interleukin-1β (IL-1β) in acute ischemic stroke patients might be associated with post-stroke depression (PSD).
Materials and Methods: The prospective study was conducted in China from February 2019 to September 2020. HOMA-IR and clinical data were collected at the time of admission. Serum levels of IL-1β were determined with enzyme-linked immunosorbent assays. Symptoms of depression and anxiety were screened by using the Hamilton Depression and Anxiety Scale at 6 months after stroke, and PSD was diagnosed on the basis of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria. The association of potential risk factors with PSD was analyzed with multivariate logistic regression analysis. Finally, the ability of HOMA-IR and IL-1β to predict PSD was assessed with receiver operating characteristic curve.
Results: A total of 305 people was included in the study; 65% were male, and the median age was 69.5± 11.8 years. At the 6-month follow-up, 113 patients (37.5%) showed depressive symptoms. In multivariate logistic regression analysis, HOMA-IR and IL-1β as graded variables were associated with an increased risk of PSD (P < 0.05). Receiver operating characteristic curve analysis indicated the highest sensitivity and specificity when the HOMA-IR and IL-1β were 1.96 and 38.71 pg/mL, respectively (P < 0.001). IL-1β improved the ability of HOMA-IR to diagnose PSD combined model area under the curve (AUC): 0.78; 95% CI: 0.72– 0.83; P < 0.001).
Conclusion: This study suggests that HOMA-IR and IL-1β are strongly associated with PSD at 6 months after stroke in patients with acute ischemic stroke. These two factors together improve the ability for early PSD assessment.
Keywords: insulin resistance, interleukin-1β, acute ischemic stroke, post-stroke depression, inflammation
Introduction
Stroke is a permanent tissue injury caused by sudden interruption of the cerebral blood supply because of occlusion or hemorrhage. At least 17 million cases of stroke occur every year worldwide.1 Stroke mood disorder, a common complication after stroke, mainly includes symptoms of depression, anxiety, mania, aphasia, emotional apathy, cognitive impairment, and fatigue.2 The most common manifestation is post-stroke depression (PSD). On the basis of the most recent available data, the overall prevalence of PSD is 31%.3 Initial and subsequent treatments in patients with stroke are usually administered in the department of neurology; however, most neurologists are unable to recognize and treat PSD in a timely and correct manner, thus affecting neural functional recovery in patients with stroke and potentially leading to cognitive impairment and mental behavior, severely diminished patient quality of life or even depressed caregivers.4 Therefore, early identification, accurate diagnosis, and timely treatment of PSD are of substantial clinical significance.
Although the mechanism of PSD remains unclear, in recent years, inflammatory mechanisms have become an increasingly important topic of investigation. Insulin resistance (IR), involving chronic low-grade inflammation, is characterized by diminished sensitivity of insulin target tissues to normal insulin concentrations, thus decreasing the body’s ability to control glucose levels.5 IR is associated not only with the onset of type 2 diabetes but also with cardiovascular and cerebrovascular diseases. As a marker of disease diagnosis and treatment, IR has attracted increasing attention. IR is higher in patients with all major subtypes of acute cerebral infarction, at both the acute stage and after 3 months, than in non-stroke patients.6 Woo has explained the mechanisms potentially underlying the association between IR correction and improvement in depressive symptoms.7
Inflammation is believed to be the main pathophysiological link between depression and metabolic syndrome, both of which are characterized by elevated pro-inflammatory cytokines and C-reactive protein and leptin, as well as the development of insulin and glucocorticoid resistance.8 Interleukin (IL) −1β was the first inflammatory cytokine identified in the brain. IL-1, particularly IL-1β, is produced by activated microglia after various forms of neurodegeneration and central nervous system inflammation, and plays a major role in cerebral ischemia injury.9 After stroke, peripheral inflammatory factors such as IL-1β are elevated within 24 hours and remain elevated for several months.10 Murata et al have reported that elevated IL-1β levels are associated with treatment-resistant bipolar depression.11
Therefore, we propose that serum levels of homeostatic model assessment of IR (HOMA-IR) and serum IL-1β in patients with ischemic stroke might be associated with the prevalence of PSD, and that the combination of these two factors may provide prognostic information for early assessment of PSD. In this study, we examined serum HOMA-IR and IL-1β levels in patients with AIS and assessed the relationship between these two factors and PSD at 6 months after stroke.
Materials and Methods
Study Population
A total of 465 patients were enrolled in this study at the Department of Neurology, Affiliated Hospital 2 of Nantong University, between February 2019 and February 2020. The inclusion criteria were as follows: (1) older than 18 years; (2) acute ischemic stroke occurring within 7 days; and (3) ischemic stroke confirmed after admission by computed tomography (CT) or magnetic resonance imaging (MRI). The exclusion criteria included: (1) CT scan confirming intracranial hemorrhage; (2) transient ischemic attack (TIA); (3) personal or family history of mental illness; (4) impaired consciousness or severe cognitive impairment; (5) presence of complete aphasia; (6) severe liver or kidney disease; and (7) autoimmune diseases. The study was approved by the ethics committee of the Affiliated Hospital 2 of Nantong University according to the principles of the Declaration of Helsinki. All participants provided written informed consent before their participation.
Participant Characteristics
Demographic data were collected from the patient medical records, including age, sex, body mass index (BMI), number of years of education, and whether they lived alone. In addition, vascular risk factors were collected, including history of smoking, drinking, hypertension, diabetes, atrial fibrillation and previous history of stroke. The location of the cerebral infarction was assessed with brain CT or MRI within 24 h after admission. Stroke severity was assessed within 24 h after admission by a trained neurologist using the National Institutes of Health Stroke Scale (NIHSS) score. NIHSS scores ranged from 0 to 42, with higher scores indicating more severe neurological impairment: 0–2, minor stroke; 3–5, mild stroke; 6–10, moderate stroke; and >10, severe stroke. Functional independence was assessed at discharge with the Barthel Index (BI) and cognitive function was assessed with the Mini-Mental State Examination (MMSE). Functional outcomes were assessed with the modified Rankin scale (mRS) at 6 months after stroke.
Blood Collection and Laboratory Tests
The quantification of insulin resistance (IR) was performed by estimating the peripheral insulin sensitivity in vivo with the Homeostasis Model Assessment (HOMA), HOMA-IR was calculated as follows: fasting insulin (mU/L) × fasting blood glucose (FBG) (mmol/L)/22.5.12 IR was defined by a HOMA-IR index in the top quartile (Q4). Fasting blood was collected in the morning on the second day after admission, then centrifuged at 1000×g at 4 °C immediately for 20 min, and frozen at −80 °C until measurement. Serum levels of IL-1β were determined by enzyme-linked immunosorbent assays with a human Interleukin-1β ELISA kit (Elabscience Biotechnology Co., Ltd) according to the kit protocol. IL-1β was measured in the range of 7.81 pg/mL to 500 pg/mL. The coefficients of variation for in-batch and inter-batch repeatability were less than 10. In addition, standard laboratory methods were used to test the hypersensitive c-reactive protein (Hs-CRP), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), homocysteine (Hcy), and free T3 (FT3).
Diagnosis of PSD
Clinical depression was diagnosed with the 24-item Hamilton Depression Scale (HAMD-24) (score ≥ 8) during a 6-month follow-up. PSD was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V). Clinical anxiety (HAMA score ≥7) was assessed with the 14-item Hamilton Anxiety Scale (HAMA-14) (score ≥7) according to anxiety symptoms. Both tests were performed by trained neurologists (Yi X and Guo X).
Statistical Analysis
Statistical analyses were conducted in IBM SPSS 22 (SPSS Inc., Chicago, IL, USA). Continuous measurement data with a normal distribution are expressed as mean ± standard deviation, and an independent sample T-test was used for comparison between groups. Count data are expressed as percentages, and chi-square (χ2) test was used for comparison between the two groups. Continuous measurement data with non-normal distribution are expressed as median (interquartile range), and the Mann–Whitney test was used for comparison between groups. Binary logistic regression was used to preliminarily analyze the relationships between different factors and PSD. Multivariate logistic regression was used to analyze variables with P < 0.1, and the results are presented as odds ratio (OR) and 95% confidence interval (CI). The area under the receiver operating characteristic curve (ROC) was computed to measure the utility of both HOMA-IR and IL-1β as potential markers for PSD. Diagrams were constructed in GraphPad Prism v8.0. A P value less than 0.05 indicated statistical significance.
Results
Baseline Characteristics of Patients with and without PSD
A total of 465 patients with ischemic stroke were included in the analysis. At the 6-month follow-up, 10 had incomplete data, 43 were lost to follow-up, 25 died, and 305 were included in the final study (Figure 1). A total of 113 patients (37.5%) were eventually diagnosed with PSD. In the study population, 198 (65%) were male, with an average age of 69.5±11.8 years. Table 1 lists the baseline characteristics of patients with and without PSD, including demographic, clinical, and laboratory characteristics. The analysis indicated no significant differences in age and sex between groups. Compared with patients in the post-stroke no depression (PSND) group, patients with PSD had higher blood biochemical indexes such as FBG, insulin, HbA1c, Hs-CRP, FT3, TC, LDL-C, HOMA-IR, and serum IL-1β. They also had more years of education and less alcohol consumption. Furthermore, patients with PSD had lower BI scores at baseline. In terms of cognition, patients with PSD were more likely to have cognitive impairment. At the 6-month follow-up, patients with PSD had higher mRS and HAMA scores. Moreover, no differences were observed in the locations of cerebral infarction between groups. Besides, the median NIHSS score was higher in patients with PSD (Table 1), and patients with PSD were more likely to be in the groups with higher NIHSS score (Supplementary Table S1).
 |
Table 1 Baseline Characteristics of Patients with and without PSD |
 |
Figure 1 Flow chart of the study. Abbreviations: AIS, acute ischemic stroke; PSD, post-stroke depression; PSND, post-stroke no depression. |
Prevalence of PSD in Both Sexes Grouped by Age at Different Time Points
In Figure 2, the prevalence of PSD in both sexes, grouped by age, are shown. In the groups under 40 (n = 6), 41–50 (n = 16), 51–60 (n = 42), and 71–80 (n = 110) years of age, the prevalence of PSD was higher in male than in female, but the differences were not statistically significant. In patients 61–70 years old (n = 78), the prevalence of PSD in female was 63.6%, a value higher than that in male 26.8% (P = 0.002). In patients 41–50 years old (n = 16), the prevalence of PSD was higher in female than in male, and in patients over 80 years old (n = 41), the prevalence of PSD was higher in female, but the differences were not statistically significant.
Baseline Characteristics of Patients with AIS According to Serum HOMA-IR and IL-1β
Table 2 summarizes the demographic characteristics and distributions of baseline clinical variables for low levels of HOMA-IR (Q1–Q3) and high levels of HOMA-IR (Q4) and low levels of IL-1β (< 38. 71 pg/mL) and high serum levels of IL-1β (> 38.71 pg/mL). The analysis showed that FBG, insulin, and HbAc1 levels were significantly higher than those in HOMA-IR (Q1–Q3) patients. In addition, LDL-C was higher and less alcohol was consumed by the patients with higher serum levels of IL-1β. Moreover, compared with HOMA-IR (Q1–Q3) patients, HOMA-IR (Q4) patients had lower MMSE scores at admission, lower BI scores at discharge, higher mRS scores, and higher HAMD and HAMA scores at the 6-month follow-up. Similar results were found for serum IL-1β.
 |
Table 2 Baseline Characteristics of Patients with HOMA-IR Quartiles and IL-1β Levels |
Serum HOMA-IR and IL-1β are Associated with PSD
HOMA-IR and IL-1β were significantly higher in the PSD group (P < 0.001; Figure 3A and B), and a correlation was observed between HOMA-IR and serum IL-1β (rs = 0.378; P < 0.001; Figure 3C). In addition, the proportion of patients in the lowest HOMA-IR quartile (< 1.32) was markedly lower in the PSD group (P < 0.05), whereas the proportion of patients in the highest HOMA-IR quartile (> 3.13) was markedly higher in the PSD group (P < 0.05). The same result was also found for serum IL-1β (Table 3).
 |
Table 3 HOMA-IR Quartiles and IL-1β Levels of Patients |
In the multivariate logistic regression analysis, after adjustment for various other confounding factors, and taking HOMA-IR Q1 or lower serum levels of IL-1β (< 38.71 pg/mL) as a reference, we found that HOMA-IR Q3 and Q4 were associated with development of PSD, and the risk of PSD increased 269% (OR = 3.69) and 403% (OR = 5.03), respectively. A high serum level of IL-1β (> 38.71 pg/mL) increased the risk of PSD by 349% (OR = 4.49). In addition, in contrast to all other parameters, a higher BI score, MMSE score, Hs-CRP, LDL-C, and lower FT3 were found to be important prognostic indicators of PSD (Table 4).
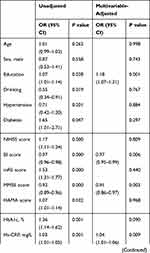 |
Table 4 Multivariate Logistic Regression of Risk Factors in Patients with PSD |
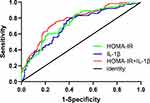
ROC curve analysis indicated the highest sensitivity and specificity for predicting depression 6 months after stroke when the HOMA-IR was 1.96 [85% and 55%, respectively; AUC = 0.74, 95% CI: 0.69–0.80; P < 0.001]. We additionally found that 38.71 pg/mL of IL-1β predicted the highest sensitivity and specificity for depression at 6 months after stroke [80% and 59%, AUC = 0.72, 95% CI: 0.66–0.78, P < 0.001]. In the HOMA-IR and IL-1β combination model, the AUC increased to 0.78 [75%, 71%, 95% CI: 0.72–0.83, P < 0.001] (Figure 4).
Discussion
Although the roles of inflammation and diabetes in depression are known, the roles of IR and cytokines in depression, particularly in patients with PSD, has not been fully explored. In our study, we observed that patients with PSD had higher levels of HOMA-IR and IL-1β at admission than PSND patients, and that those with higher, rather than lower, levels of HOMA-IR and serum IL-1β had a 5.03-fold and 4.49-fold greater risk of developing PSD, respectively. In addition, we found that IL-1β improved the ability of HOMA-IR to diagnose PSD and that both factors together added prognostic information enabling early assessment of PSD. Thus, for patients with acute stroke, particularly those with high levels of HOMA-IR and IL-1β, targeted lifestyle interventions and early antidepressant treatment should be emphasized.
The cumulative incidence of depression within 5 years after a stroke is 39% to 52%.13 A recent meta-analysis3 has reported that 31% of patients develop depression at some point in the 5 years following a stroke. In this study, we found a 6-month incidence of PSD of 37.5%. Regional differences, variations in the diagnostic and evaluation methods, and evaluation times may have led to differences in the reported prevalence of PSD. In addition, we found no difference in the prevalence of PSD between male and female. When the patients of each sex were grouped by age, the prevalence of PSD among patients 61–70 years old was higher for female than for male. Furthermore, previous studies have found that the severity of stroke is correlated with the prevalence of PSD,14 in agreement with our study. We also found that patients with PSD had more years of education. No clear conclusion can be drawn regarding the relationship between education and the occurrence of PSD.15,16 We speculate that this may be because patients with higher education have higher stress and expectations, and therefore are less able to accept the sequelae of stroke.
IR, as chronic low-grade inflammation, is the basis of a variety of metabolic syndromes and is closely associated with type 2 diabetes and depression. Matulewicz has shown that IR activates pro-inflammatory pathways, induces oxidative stress, and promotes the development of intra-arterial inflammation and atherosclerotic plaques.17 In addition, pro-inflammatory factors (IL-1β, IL-6 and TNF) indirectly block insulin signaling, thus leading to IR.18 Our study found that HOMA-IR and IL-1β remained independent risk factors for PSD after adjustment for other confounding factors (Table 4), and an association was found between HOMA-IR and IL-1β (rs = 0.378; p < 0.001; Figure 3C), thus suggesting a possible association between IR and pro-inflammatory cytokines in patients with PSD. In other studies, the association between IL-1β and PSD has been inconsistent. Jimenez et al have not found any association between IL-1β levels and PSD in the first month after stroke.19 However, Kim et al have found an association between higher IL-1β levels and the −511T allele and PSD at 2 weeks.20 We found that, compared with PSND patients, patients with PSD had higher IL-1β levels (Figure 3B), and after the exclusion of other confounding factors, high IL-1β remained an independent risk factor for the development of PSD (Table 4). Further studies have found that using HOMA-IR and IL-1β together, rather than individually, results in an AUC of 0.78, and a sensitivity and specificity of 75% and 71%, respectively. This result strongly suggests the diagnostic value of a combination of HOMA-IR or IL-1β. For stroke patients, this combination may be useful for the early identification and treatment of PSD.
Mitzi has demonstrated that IR adversely affects memory and executive functioning in Hispanic/Latino individuals without diabetes.21 Studies have shown that IR is independently associated with poor functional outcomes after acute ischemic stroke.22 Our results confirm these conclusions and suggest that HOMA-IR levels are significantly associated with cognitive impairment (as defined by MMSE), stroke severity (as defined by the NIHSS score) and functional outcome at 6 months (as defined by the mRS score) (Table 2).
The mechanism of poststroke depression is unclear. First, studies have shown that inflammatory mediators stimulate NF-κB and JNK signaling pathways in insulin-sensitive tissues and activate many pro-inflammatory molecules, thus promoting chronic low-activity inflammatory responses and IR,14 which may be associated with depressive states. Anti-inflammatory therapy has been shown to reduce the risk of PSD.23 Second, in mice with brain-specific insulin receptor gene knockout, IR directly leads to depression-like behavior and anxiety disorders.24 Marks et al have found that insulin delivery into the nasal cavity in awake mice transmits regulatory hormones and metabolic hormones through the blood-brain barrier, thus significantly improving memory and reducing anxiety levels.25 Interestingly, animal experiments have shown that changes in the intestinal flora can modulate insulin signals and metabolites in the brain, thereby leading to changes in neural behavior.26 Finally, IR in the brain can worsen mitochondrial function, and increase monoamine oxidase levels and dopamine clearance, which may lead to depression.24 Therefore, inhibiting inflammation to improve central insulin signaling may provide a new target for the treatment of mood disorders.
This study has several limitations. First, this study was a single-center study with a small sample size (n = 305), and it excluded patients with aphasia and severe stroke, which may have contributed to selection bias. Second, the gold standard for IR is the high-insulin-normal blood sugar clamp technique, which is complex, time-consuming, and expensive. However, Bonora et al have used this technique to validate an indirect indicator in this study, HOMA-IR, and have found a high correlation (r = −0.820, P < 0.001).27 HOMA is therefore considered an effective method for assessing peripheral insulin sensitivity in epidemiological studies. In our study, the HOMA-IR (P75) >3.13 was similar to previous results.28 Third, the HAMA and HAMD scales were used to identify anxiety and depression, respectively, in another limitation of this study. However, HAMA and HAMD have been widely verified and have advantages in application reliability, validity, and practicality. Fourth, whether IR is involved in the development of post-stroke mood disorders or is just a marker is not yet known, and because of the limitations of this type of study, we were unable to establish a causal relationship. Finally, HOMA-IR and IL-1β were measured on the second day after admission but not at other time points; thus, the relationship between dynamic changes and PSD could not be observed.
Conclusion
We found that HOMA-IR and IL-1β are closely associated with PSD at 6 months after stroke in patients with acute ischemic stroke and may lead to PSD development through a mechanism of severe inflammation. Use of these two factors together increases the prognostic information for early PSD evaluation. Treatment of inflammation and IR may provide a potential therapeutic target.
Acknowledgments
We would like to express heartfelt thanks to Professor Xiangyang Zhu and Yong Zhou for their guidance in writing, and to Mengmeng Li and Professor Dongmei Zhang for their guidance in the experiment.
Funding
Funding was provided by the Scientific Research Project of the Health Commission of Jiangsu Province (H2019057) and the Nantong Science and Technology Project (MS12018042).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Uzdensky AB. Regulation of apoptosis in the ischemic penumbra in the first day post-stroke. Neural Regen Res. 2020;15(2):253–254. doi:10.4103/1673-5374.265546
2. Robinson R, Jorge R. Post-stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi:10.1176/appi.ajp.2015.15030363
3. Hackett M, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–1025. doi:10.1111/ijs.12357
4. Kotila M, Numminen H, Waltimo O, et al. Depression after stroke: results of the FINNSTROKE study. Stroke. 1998;29(2):368–372. doi:10.1161/01.STR.29.2.368
5. Hauner H. Insulin resistance and the metabolic syndrome-a challenge of the new millennium. Eur J Clin Nutr. 2002;56(Suppl 1):S25–S29. doi:10.1038/sj.ejcn.1601350
6. Åberg D, Åberg N, Jood K, et al. Homeostasis model assessment of insulin resistance and outcome of ischemic stroke in non-diabetic patients - a prospective observational study. BMC Neurol. 2019;19(1):177. doi:10.1186/s12883-019-1406-3
7. Woo Y, Lim H, Wang S, Bahk W. Clinical evidence of antidepressant effects of insulin and anti-hyperglycemic agents and implications for the pathophysiology of depression-A literature review. Int J Mol Sci. 2020;21(18):6969. doi:10.3390/ijms21186969
8. Leonard B, Wegener G. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 2020;32(1):1–9. doi:10.1017/neu.2019.17
9. Spalletta G, Bossù P, Ciaramella A, et al. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11(11):984–991. doi:10.1038/sj.mp.4001879
10. Mena H, Cadavid D, Rushing E. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004;108(6):524–530. doi:10.1007/s00401-004-0918-z
11. Murata S, Murphy M, Hoppensteadt D, et al. Effects of adjunctive inflammatory modulation on IL-1β in treatment resistant bipolar depression. Brain Behav Immun. 2020;87:369–376. doi:10.1016/j.bbi.2020.01.004
12. Matthews D, Hosker J, Rudenski A, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
13. Ayerbe L, Ayis S, Wolfe C, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. doi:10.1192/bjp.bp.111.107664
14. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. 2014;9(8):1026–1036. doi:10.1111/ijs.12356
15. Lorant V, Deliège D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi:10.1093/aje/kwf182
16. Gan Z, Li Y, Xie D, et al. The impact of educational status on the clinical features of major depressive disorder among Chinese women. J Affect Disord. 2012;136(3):988–992. doi:10.1016/j.jad.2011.06.046
17. Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw. 2016;70:1245–1258.
18. Stošić-Grujičić S, Saksida T, Miljković Đ, et al. MIF and insulin: lifetime companions from common genesis to common pathogenesis. Cytokine. 2020;125:154792. doi:10.1016/j.cyto.2019.154792
19. Jiménez I, Sobrino T, Rodríguez-Yáñez M, et al. High serum levels of leptin are associated with post-stroke depression. Psychol Med. 2009;39(7):1201–1209. doi:10.1017/S0033291709005637
20. Kim J, Kang H, Kim J, et al. Associations of tumor necrosis factor–α and interleukin-1β levels and polymorphisms with post-stroke depression. Am J Geriatr Psychiatry. 2017;25(12):1300–1308. doi:10.1016/j.jagp.2017.07.012
21. Gonzales M, Durazo-Arvizu R, Sachdeva S, et al. Associations of insulin resistance with cognition in individuals without diagnosed diabetes: results from the Hispanic Community Health Study/Study of Latinos. Diabetes Res Clin Pract. 2019;150:38–47. doi:10.1016/j.diabres.2019.01.030
22. Ago T, Matsuo R, Hata J, et al. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. 2018;90(17):e1470–e1477. doi:10.1212/WNL.0000000000005358
23. Wium-Andersen I, Wium-Andersen M, Jørgensen M, et al. Anti-inflammatory treatment and risk for depression after first-time stroke in a cohort of 147 487 Danish patients. J Psychiatry Neurosci. 2017;42(5):320–330. doi:10.1503/jpn160244
24. Kleinridders A, Cai W, Cappellucci L, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112(11):3463–3468. doi:10.1073/pnas.1500877112
25. Marks D, Tucker K, Cavallin M, et al. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29(20):6734–6751. doi:10.1523/JNEUROSCI.1350-09.2009
26. Soto M, Herzog C, Pacheco J, et al. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018;23(12):2287–2301. doi:10.1038/s41380-018-0086-5
27. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi:10.2337/diacare.23.1.57
28. Ascaso J, Romero P, Real J, et al. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non-diabetic population. Med Clin. 2001;117(14):530–533. doi:10.1016/S0025-7753(01)72168-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.