Back to Journals » Cancer Management and Research » Volume 14
The Combination of Anlotinib and Gemcitabine/Docetaxel in Patients with Metastatic Osteosarcoma Who Have Failed Standard Chemotherapy
Authors Wang T , Lin F, Huang Y, Qian G, Yu W, Hu H, Ji T, Tang L , Yao Y
Received 19 June 2022
Accepted for publication 13 September 2022
Published 4 October 2022 Volume 2022:14 Pages 2945—2952
DOI https://doi.org/10.2147/CMAR.S378264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Tian Wang,1,* Feng Lin,2,* Yujing Huang,2 Guowei Qian,2 Wenxi Yu,2 Haiyan Hu,2 Tong Ji,3 Lina Tang,2 Yang Yao2
1The Eighth People’s Hospital of Shanghai, Shanghai, People’s Republic of China; 2Shanghai Sixth People’s Hospital, Shanghai Jiaotong University, Shanghai, People’s Republic of China; 3Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Yao; Lina Tang, Shanghai Sixth People’s Hospital, Shanghai Jiaotong University, No. 600 Yishan Road, Xuhui Distinct, Shanghai, 200233, People’s Republic of China, Tel +86 2164369181 ; +86 2164701361, Email [email protected]; [email protected]
Purpose: The options for the second-line treatment of metastatic osteosarcoma are still limited. Anlotinib is a multi-kinase inhibitor which has shown promising efficacy and good tolerability in various cancer types. This retrospective study was conducted to evaluate the efficacy and safety of anlotinib combined with gemcitabine/docetaxel (GD) in patients with metastatic osteosarcoma who have failed first-line chemotherapy.
Patients and Methods: The data of patients who received anlotinib combined with GD or GD were collected. The primary endpoint was progression-free survival. Secondary endpoints included objective response rate and safety.
Results: From July 2013 to November 2020, a total of 32 patients were enrolled, 13 received anlotinib combined with GD and 19 received GD. Median PFS was 9.0 months (95% CI 6.7– 39.1) in the combination group and 5.0 months (95% CI 1.2– 6.7) in the chemotherapy group. ORR were 38.4% and 15.8%, DCR were 69.2% and 38.1% in the combination and chemotherapy group, respectively. The most common adverse events included fatigue (78.9% in the combination group vs 69.2% in the chemotherapy group), hypertension (46.2% vs 10.5%), diarrhea (38.5% vs 21.1%), hypothyroidism (38.5% vs 15.8%), neutropenia (23.1% vs 36.8%) and AST elevation (30.8% vs 21.1%). The most common grade 3 or worse adverse events included hand-foot reaction (7.7% vs 5.3%), hypothyroidism (15.4% vs 0), neutropenia (0 vs 10.5%).
Conclusion: The combination of anlotinib and GD showed favorable efficacy with manageable toxicities compared with GD in the second-line treatment for metastatic osteosarcoma. This combination therapy deserves further investigations in patients with osteosarcoma.
Keywords: anlotinib, refractory metastatic osteosarcoma, combination therapy
Introduction
Osteosarcoma is the most common malignant bone tumor for children and young adults.1 About 18% of the patients have developed metastatic disease at diagnosis,2 and about 30% of the patients with local disease will develop metastatic or recurrent disease after definite therapy.3 The prognosis of patients with metastatic or recurrent osteosarcoma is poor4 with limited progress in the past decades.5 The first-line treatment for metastatic osteosarcoma is still based on chemotherapy including doxorubicin, cisplatin, methotrexate, ifosfamide, while the efficacy is limited as the 5-year survival rates were lower than 30% in various attempts.6 In NCCN guidelines, the recommended second-line and subsequent regimens for patients with metastatic osteosarcoma include chemotherapy and multi-kinase inhibitors. The survival time was shorter than 1 year with chemotherapy regimens including ifosfamide and etoposide, docetaxel and gemcitabine, cyclophosphamide and topotecan.7 Sorafenib, regorafenib and cabozantinib are the recommended multi-kinase inhibitors, while in most of the studies, response rate (RR) was lower than 15%, median progression-free survival (PFS) and overall survival (OS) were only 4 months and 10 months, respectively.8–11 Osteosarcomas carry complex genetic alterations and have extensive intra-tumoral heterogeneity, while the essential driver mutations are still not revealed and no agent with precise target is available.12 The preliminary exploration of PD-1 inhibitors in metastatic osteosarcoma showed very limited activity.13 Effective strategies to improve the survival of patients with metastatic disease are still in urgent need.
As many other tumors, osteosarcoma depends on new blood vessels for development. A number of tyrosine kinase receptors involved in angiogenesis including vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), KIT, MET are overexpressed and activated in osteosarcomas14 and high levels of VEGF have been demonstrated to correlate with disease progression and poor survival in patients with osteosarcoma.15 Therefore, antiangiogenic therapy is attractive for osteosarcoma. Multi-kinase inhibitors have been investigated in advanced osteosarcoma. While in most of the available studies, the efficacy of single-agent therapy is still not satisfied, and the combination of chemotherapy, immunotherapy and VEGFR TKIs is under investigation.5
Anlotinib is an oral multi-targeted receptor tyrosine kinase inhibitor which suppresses tumor cell proliferation via the inhibition of VEGFR, fibroblast growth factor receptor (FGFR), PDGFRα/β and c-Kit.16 Anlotinib has shown promising efficacy and favorable safety in advanced soft tissue sarcoma with an RR of 13% and OS of 1 year.17 In a preclinical study, anlotinib inhibits the growth, metastasis, angiogenesis of osteosarcoma by blocking VEGFR2 and MET pathways.18 These support the exploration of anlotinib in osteosarcoma. Due to the limited efficacy of single-agent VEGFR TKI in osteosarcoma, we did a retrospective study to investigate the combination of anlotinib and GD, and here report the efficacy and safety of this combination regimen in patients with metastatic osteosarcoma who have failed standard treatment.
Materials and Methods
Patients
This is a retrospective study including patients with metastatic osteosarcoma who have failed standard chemotherapy. Eligible patients were diagnosed with measurable, metastatic and histologically confirmed osteosarcoma. All patients experienced disease progression on standard chemotherapy (completed >4 weeks before study entry), including cisplatin and doxorubicin, high-dose methotrexate and ifosfamide, and received GD as second-line or subsequent treatment, with or without anlotinib. Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1 and adequate organ function, based on laboratory tests including hematology, serum chemistry, lipids, coagulation, thyroid function, urinalysis and electrocardiograph, and had a life expectancy of ≥3 months.
The main exclusion criteria included uncontrolled blood pressure, active myocardial ischemia, previous history of arterial infarction, QT interval ≥440 ms or cardiac insufficiency, 24-hour urine protein >1.0 g; major surgery within 4 weeks; venous thrombosis within 6 months; clinically significant hepatic or gastrointestinal dysfunction, wound healing and infectious comorbidities. The study was approved by the Ethics Committees of Shanghai Sixth People’s Hospital, following the principles of Declaration of Helsinki. All subjects provided written informed consent to participate in the study.
Treatment
GD has been investigated in several studies and shown synergistic antitumor activity, with disease control rate (DCR) ranged from 20% to 50%, OS about 9–11 months.7 We enrolled patients received GD as second-line or subsequent treatment as the GD group. For the limited efficacy of VEGFR TKI as monotherapy, we chose patients who received both GD and anlotinib as the combination group. Patients received oral anlotinib once daily at an initial dose of 12 mg, day 1–14 in a 3-week cycle. Chemotherapy was delivered with gemcitabine at a dose of 675 mg/m2 on day 1 and 8, docetaxel at a dose of 100 mg/m2 on day 1 in a 3-week cycle. Treatment was continued until disease progression or intolerable toxicity were observed. The dose of anlotinib was reduced to 10 mg per day when grade 3 or 4 adverse events occurred and could be further reduced to 8 mg when the adverse events occurred again. The dose adjustment of GD was conducted as the clinical practice.
Endpoints
The primary endpoint was PFS. Secondary endpoints included objective response rate (ORR), DCR, and safety. PFS was defined as the time from the start of study treatment to the time of disease progression or death due to any cause, whichever occurred first. OS was measured from the start of study treatment to the date of death for any cause. ORR was the proportion of patients who achieved complete response (CR) and partial response (PR). DCR was the proportion of patients with CR, partial response PR, and stable disease (SD) lasting for 12 weeks or more. Tumor responses were assessed by the investigators according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Statistical Analysis
Statistical analyses were performed with SPSS 26.0. The baseline data and AE data were calculated by the direct counting method, in terms of median (range) or frequency (percentage). Survival analysis was estimated by the Kaplan–Meier method with a 95% confidence interval (CI) and compared by Log rank test. Statistical significance was defined as an alpha level of 0.05 (p < 0.05).
Results
Study Population
The patients diagnosed as metastatic osteosarcoma in our hospital were reviewed. Between July 2013 and Nov 2021, a total of 32 eligible patients from our hospital were enrolled, among which 13 received GD and anlotinib, 19 received GD alone as second-line or subsequent treatment. Twelve (92.3%) and 13 (68.4%) patients had grade 3 tumors in the combination group and in the GD group, respectively. Two (15.4%) and 4 (21%) patients had unilateral lung metastasis, 5 (38.5%) and 11 (58%) had bilateral lung metastasis, while 6 (46.1%) and 4 (21%) had synchronous metastasis outside lungs in the combination and GD groups, respectively. Baseline characteristics of the patients are listed in Table 1.
 |
Table 1 Baseline Characteristics |
Treatment
In the combination group, five patients received anlotinib simultaneously with GD, two received anlotinib since the start of the third cycle of GD, four received anlotinib immediately after the completion of GD, while two began to receive GD since the start of the second cycle of anlotinib. By the data cutoff date, 6 (46.1%) and 5 (26.3%) patients remained on treatment in the combination and GD groups, respectively. Disease progression was confirmed in 6 (31.6%) and 6 (46.1%) patients, death occurred in 1 (7.7%) and 6 (31.6%) patients in the combination and GD groups, respectively. In the combination group, four patients received the anlotinib for more than 1 year.
Efficacy
The preliminary analysis was conducted in May 2021. Median PFS was 9.0 months (95% CI 6.7–39.1) in the combination group and 5.0 months (95% CI 1.2–6.7) in the GD group (hazard ratio, 0.45; 95% CI, 0.19–1.07; p=0.048; Figure 1A and B).
In the combination group, one patient achieved CR, four patients achieved PR and four had SD. PD occurred in four patients in the initial evaluation. The confirmed ORR and DCR were 38.4% and 69.2%. In the GD group, 3 patients achieved PR, 5 had SD and 11 had PD. The confirmed ORR and DCR were 15.8% and 38.1%, resppectively (Table 2). The best response of patients in the two groups is shown in Figure 2.
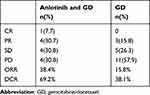 |
Table 2 Treatment Response of the Two Groups |
In the four patients with synchronous metastases outside lungs of the GD group, PFS was dismal (2.7m, 5m, 1.3m and 2.5m), while in the six patients with synchronous metastases outside lungs in the combination group, PFS ranged from 4 to 18 months.
Safety
In the combination and GD groups, adverse events of any grade occurred in 100% and 84.2% of patients, respectively. In the combination group, the most common adverse events were fatigue (69.29%), hypertension (46.2%), diarrhea (38.5%), hypothyroidism (38.5%), aspartate transaminase (AST) elevation (30.8%), hand and foot reaction (23.1%), alanine transaminase (ALT) elevation (23.1%), hyponatremia (23.1%) and neutropenia (23.1%). While in the GD group, the most common adverse events (>20%) included fatigue (78.9%), neutropenia (36.8%), ALT elevation (26.3%), hypertriglyceridemia (21.1%) and diarrhea (21.1%). In the combination group, grade 3 AEs included hypothyroidism (15.4%), hand and foot reaction (7.7%), diarrhea (7.7%), fatigue (7.7%) and ALT elevation (7.7%). The most frequent grade 3 AEs in the GD group included neutropenia (10.5%), thrombocytopenia (5.3%) and hand-foot reaction (5.3%). No grade 4 or worse adverse event was observed in either group (Table 3). Three (23.1%) and 5 (26.3%) patients in the combination and GD group experience dose interruptions due to adverse events.
 |
Table 3 The Most Common Adverse Events in the Two Groups |
Discussion
In this retrospective study, the combination of anlotinib and GD as second-line or subsequent therapy in patients with osteosarcoma achieved an ORR of 38.4%, DCR of 69.2%, and median PFS of 9.0 months. In the GD group, ORR and DCR were 15.8% and 38.1%, respectively, PFS was 5.0 months, which were comparable with the previous reports of second-line chemotherapy.7 In the combination group of our study, the proportions of patients who experienced more than one line of chemotherapy, had grade 3 tumors and metastases outside of the lungs were higher than in the GD group. Although the results of the two groups could not be compared directly, the numerically longer PFS in the combination group indicating the promising efficacy. The ORR and DCR in the combination group were about two times of that in the GD group. In a Phase 1/2 study, the combination of lenvatinib and etoposide/ifosfamide achieved a PFS of 8.7 months, ORR of 9% and DCR of 25% as the second-line and subsequent treatment,19 suggesting the potential synergistic effect of VEGFR TKI and chemotherapy in osteosarcoma. As GD and etoposide/ifosfamide are both recommended regimens of second-line chemotherapy, the best chemotherapy regimen for the combination with VEGFR TKI may be further explored.
The proportion of patients achieved long-lasting response in the combination group was encouraging, as 9 (69.2%) and 4 (30.8%) patients had PFS longer than 6 months and 18 months respectively. In the GD group, only 8 (42%) patients had PFS longer than 6 months, while none had PFS longer than 12 months. In the combination group, one case of CR was observed, and the patient was still on treatment with anlotinib at the last follow-up with a PFS exceeding 19 months. In the four patients who had a best response of PR, three were still on treatment and the PFS exceeded 7, 18 and 19 months, respectively. In the four patients who had SD, PFS reached 11.6 and 23 months in 2 patients, and response was ongoing in the others. These indicate the durable response with the combination of anlotinib and GD. For various genetic alterations in osteosarcoma, the combination of different regimens may be more efficient and helpful to overcome drug resistance. In a preclinical study, the combination of pazopanib and low dose metronomic topotecan showed significant enhancement of antitumor activity compared with single agent in osteosarcoma models.20 The combination of anti-angiogenic agents and chemotherapy in osteosarcoma worth further investigation.
In previous studies of VEGFR TKIs in osteosarcoma, only moderate efficacy was observed, as the ORR and DCR were approximately 10% and 30%, the median PFS was only around 4 months.5 In the Phase 2 study of sorafenib in unresectable osteosarcoma after standard treatment, the median PFS and OS were 4 months and 7 months, ORR and DCR were 8% and 48% of the patients.8 In the phase 2 studies of regorafenib, PFS was approximately 4 months while OS was slightly longer than 11 months, ORR was 8–13.6%.9,10 In phase 2 study of cabozantinib, the median PFS and OS were 6.7 and 10.6 months, ORR was 17%.11 The efficacy of VEGFR TKIs seemed to be various, which may partly attribute to the different spectrum of targets.21 The targets of anlotinib in the angiogenic pathway are more completed including both anti-angiogenic and stromal receptors may contribute to the favorable efficacy we observed.
The safety in the combination group is favorable, as no grade 4 adverse events were observed. In the phase 2 studies of sorafenib, regorafenib and cabozantinib, the incidence of dose interruption was 39–59%.8–11 In our study, the incidence of grade 3 adverse events was 46.2%, while dose discontinuation and reduction occurred in only 3 patients (23.1%), indicating most of the AEs were manageable in the combination group. No grade 3 hematological toxicity was observed in the combination group. The population of osteosarcoma is relatively younger than other tumor types, which may explain the better tolerability, and the deterioration of performance status are usually caused by the localized symptoms of tumor lesion.
Our study has limitations due to the retrospective nature. The sample size was relatively small, and the baseline characteristics could not be well balanced between the two groups. The comparison between the combination therapy and single-agent anlotinib was also lacking. The time and manner of GD and anlotinib combination were different among patients in the combination group, which may affect the results. Randomized trials with larger sample size are needed to further verify the activity of anlotinib and GD in this population.
Conclusion
In general, as second-line therapy, the combination of anlotinib and gemcitabine/docetaxel showed promising efficacy compared with GD. The combination regimen was well tolerated as grade 3 and worse events were rare and manageable. This supports further investigations of anlotinib and GD in osteosarcoma.
Acknowledgments
We are thankful to the investigators, the patients enrolled in this study and their families.
The study was funded by the National Natural Science Foundation of China (grant number 81900189). The funder had no role in the study design, data collection or analysis. The corresponding authors had full access to the data and had final responsibility for the decision to submit the manuscript for publication.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi:10.1002/ijc.24320
2. Marko TA, Diessner BJ, Spector LG. Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr Blood Cancer. 2016;63:1006–1011. doi:10.1002/pbc.25963
3. Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis. 2007;2:6. doi:10.1186/1750-1172-2-6
4. Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23:559–568. doi:10.1200/JCO.2005.04.063
5. Duffaud F. Role of TKI for metastatic osteogenic sarcoma. Curr Treat Options Oncol. 2020;21:65. doi:10.1007/s11864-020-00760-w
6. Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16:543–556.
7. Zhang Y, Yang J, Zhao N, et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16:6228–6237. doi:10.3892/ol.2018.9434
8. Grignani G, Palmerini E, Dileo P, et al. A Phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi:10.1093/annonc/mdr151
9. Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind Phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019;37:1424–1431. doi:10.1200/JCO.18.02374
10. Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–133. doi:10.1016/S1470-2045(18)30742-3
11. Italiano A, Mir O, Mathoulin-Pelissier S, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:446–455. doi:10.1016/S1470-2045(19)30825-3
12. Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–491. doi:10.1038/nrendo.2017.16
13. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–1501. doi:10.1016/S1470-2045(17)30624-1
14. Hassan SE, Bekarev M, Kim MY, et al. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–749. doi:10.1002/cncr.26339
15. Yu XW, Wu TY, Yi X, et al. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–160. doi:10.1007/s13277-013-1019-1
16. Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86. doi:10.1016/j.gene.2018.02.026
17. Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24:5233–5238. doi:10.1158/1078-0432.CCR-17-3766
18. Wang G, Sun M, Jiang Y, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. 2019;145:979–993. doi:10.1002/ijc.32180
19. Gaspar N, Venkatramani R, Hecker-Nolting S, et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): a multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021;22:1312–1321. doi:10.1016/S1470-2045(21)00387-9
20. Kumar S, Mokhtari RB, Sheikh R, et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17:5656–5667. doi:10.1586/14737140.2016.1168697
21. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. doi:10.1186/s13045-018-0664-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


