Back to Journals » International Journal of General Medicine » Volume 15
The Clinical Value of Lipid Abnormalities in Early Stage Cervical Cancer
Authors Jiang Q, Wang L, Jin M, Shou Y, Zhu H, Li A
Received 13 January 2022
Accepted for publication 30 March 2022
Published 11 April 2022 Volume 2022:15 Pages 3903—3914
DOI https://doi.org/10.2147/IJGM.S352934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Qi Jiang,1 Luhui Wang,1 Mengya Jin,1 Yueyao Shou,1 Haiyan Zhu,1,2,* Anyang Li1,*
1Department of Gynecology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325027, People’s Republic of China; 2Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, 200126, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Anyang Li, Department of Gynecology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325027, People’s Republic of China, Tel +86 19817583796, Email [email protected] Haiyan Zhu, Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, 2699 Gaoke West Road, Shanghai, 200126, People’s Republic of China, Tel +86 57755069162, Email [email protected]
Background: To describe the characteristics of plasma lipid proliferation in cervical cancer and further evaluate the prognostic significance of lipid levels in cervical cancer.
Methods: We retrospectively reviewed 1713 patients with cervical cancer in our hospital. The preoperative plasma lipid profile, including cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL), of 1713 cervical cancer patients was compared with that of 10,397 healthy women. Then, we evaluated the impact of lipids on overall survival (OS) and recurrence-free survival (RFS) in cervical cancer using univariate and multivariate Cox models.
Results: While plasma TC, TG, and LDL were significantly higher, HDL was lower in patients with cervical cancer than in healthy women. TG was identified as an independent predictor for RFS and OS among patients with cervical cancer. Further stratified by age, patients with higher TGs showed a significantly worse RFS and OS than those with lower TGs among patients ≥ 50 years old but not among those < 50 years old.
Conclusion: Cervical cancer was associated with a disordered lipid profile. Hypertriglyceridemia was an independent poor prognostic indicator for cervical cancer, especially for elderly patients. Strengthening lipid management may be beneficial for improving postoperative OS and RFS in patients with cervical cancer.
Keywords: cervical cancer, lipid profile, triglyceride, cholesterol, prognosis
Background
Cervical cancer is the most common gynecologic malignancy, with an estimated 604,127 new cases and 341,831 deaths in 2021 worldwide.1 High-risk subtypes of human papillomavirus (HPV) infection have been well established as the main cause of cervical carcinoma. In the past decade, the use of screening programs and HPV vaccination programs has dramatically reduced the incidence of cervical cancer in developed countries. However, these diseases remain a heavy health burden and a major public health problem in the developing world because of the high incidence and poor prognosis of recurrent disease. Therefore, it is imperative to develop some simple and valuable predictors that can identify high-risk cervical cancer patients with poor progression and prognosis, further facilitating novel treatment strategies and improving clinical outcomes.
A large body of evidence has shown a relationship between lipid abnormalities and cancer initiation.2 Lipids crucially contribute to cell proliferation and tumorigenesis due to their influence on chemical-energy storage, cellular signaling, cell membranes, and cell–cell interactions.2 Preclinical studies have revealed the ability of adipocytes to provide energy for rapid cancer growth and metastasis.3 A positive correlation was found between serum triglycerides (TGs) and the risk of endometrial cancer.4 High levels of total cholesterol (TC) and low-density lipoprotein (LDL) increase colorectal cancer risk.5 Hypertriglyceridemia increases the risk of prostate cancer and promotes the aggressiveness of these diseases.6,7 With regard to cervical cancer, triglycerides were reported to be elevated among cervical cancer patients in an Indian population.8 However, no significant difference in any lipid parameters was detected between the cervical cancer group and the uterine leiomyoma group in a study by Sun et al.9 Thus, the alterations of plasma lipid profiles in cervical cancer remain a matter of controversy.
Recently, lipid parameters were described as prognostic factors in various cancers, with contradictory findings. While increased levels of LDL were reported as a negative prognosis in nasopharyngeal carcinoma,10 higher preoperative LDL was related to an improved 5-year RFS of ovarian cancer.11 In addition, high-density lipoprotein (HDL) is a favorable prognostic indicator of lung cancer and breast cancer.12,13 To date, the impact of lipid abnormalities in cervical cancer has been poorly investigated. In the current study, we first evaluated the correlation between lipid parameters and cervical cancer and then further explored the prognostic value of the preoperative lipid profile in a large population of cervical cancer patients treated with radical hysterectomy and thus identified a reliable and convenient predictor.
Methods
Study Population
This study included 1713 patients with pathologically confirmed uterine cervical carcinoma who underwent radical hysterectomy between January 2008 and December 2018 at the First Affiliated Hospital of Wenzhou Medical University, China. The following exclusion criteria were used: (1) women who received any drugs that impacted lipid metabolism; (2) patients with chronic diseases that affected lipid levels (ie, diabetes); and (3) patients who received any treatments that affected lipid levels before serum collection (including cholesterol-lowering drugs and antihypertensive drugs, such as statins and thiazide diuretics). The information mentioned above was obtained from the electronic medical records. In addition, the control group included 10,397 healthy women. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, and patients signed informed consent forms before taking part in this study. A detailed review of patient history, general physical examination, pelvic examination (including bimanual pelvic and rectal examinations), and preoperative laboratory (plasma lipid profiles and serum squamous cell carcinoma antigen) and pathological data (tumor subtypes, metastasis, stage, and differentiation) of all patients were collected from electronic medical records and reviewed. The subtypes of tumors are classified as squamous carcinoma, adenocarcinoma, and others, including adenosquamous carcinoma and other special types, such as neuroendocrine carcinoma, and undifferentiated carcinoma. Detailed clinical data were collected within one week before the operation. Preoperative plasma levels of HDL, LDL, TC, TG, and SCC-Ag were measured in the early morning before surgery and immediately analyzed using a Hitachi 7600–020 automatic biochemical analyzer with the kinetic method.11 Body mass index (BMI) was calculated as body weight (kg)/height (m)2.
All 1713 cervical cancer patients were classified as high-risk, intermediate-risk, and low-risk after postoperative pathological evaluation. High-risk patients were defined as having tumor involvement of the parametria, positive margins, or lymph node metastases.14 Intermediate risk factors included depth of invasion, lymphovascular space invasion, and tumor size.15 Patients without high- and intermediate-risk factors were defined as low-risk.
In addition, we collected age, BMI, and plasma levels of HDL, LDL, TC, and TG from 10,397 healthy women as controls. Given that lipid levels were affected by confounders such as age and BMI, we matched the healthy women group and the cervical cancer group with age, with every 10 years as a subgroup. Then, by randomly matching the healthy control group and the cancer group at a ratio of 2:1, we obtained 3426 healthy women. We then controlled for potential confounders (age and BMI) by regression analysis and analyzed the difference in lipid levels between the healthy group and the cancer group.
Follow-up examinations were performed every 3 months in the first 2 years and then every 6 months for the next 3 years and every 1 year thereafter. Pelvic examination, cytology, the serum concentration of SCC-Ag, and imaging studies, including computed tomography, magnetic resonance imaging, or positron emission tomography-computed tomography, were performed during routine follow-up.
The last follow-up date was July 15, 2019. The endpoints of this study were overall survival (OS) and recurrence-free survival (RFS). Overall survival was determined from the date of surgery to death or last follow-up. RFS was calculated from the date of surgery to tumor recurrence or distant metastasis.
Statistical Analysis
Continuous data were presented as the median (Q1–Q3) or mean ± standard deviation based on their distribution. Then, differences between groups were tested using the Wilcoxon rank-sum test or a standard t-test. Categorical data were analyzed using Fisher’s exact test or χ2 test. The Kaplan–Meier method was used to calculate survival estimates for OS and RFS. Univariate and multivariate Cox regression analyses were used to evaluate the associations between clinical covariates and survival. A two-tailed P < 0.05 was considered as statistically significant. Analyses were performed using R (version 3.3).
Results
Characteristics of Cervical Cancer
The clinical and pathologic characteristics of cervical cancer are summarized in Table 1. This study included 1713 cervical cancer patients. The median age at diagnosis was 52 years (45, 60), and the median BMI was 23.24 (19.95, 26.53). The most common histological type was squamous cell carcinoma (85.9%), followed by adenocarcinoma (9.0%). All patients were diagnosed with FIGO stage I–II. Tumor sizes in 221 patients (18.7%) were ≥4 cm, 781 (66.0%) were 2–4 cm, and 181 (15.3%) were <2 cm. In addition, 274 patients showed lymph node metastasis, 342 patients had lymphovascular space invasion, and 40 patients had parametrial involvement. Based on pathology after radical hysterectomy, there were 319 (18.6%) patients with high-risk factors and 643 (37.5%) patients with intermediate-risk factors.
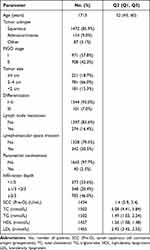 |
Table 1 The Baseline Patients’ Characteristics |
Lipid Profile in Healthy Women and Patients with Cervical Cancer
In 1713 patients with cervical cancer, the median preoperative levels of TC, TG, LDL, and HDL were 5.08 (4.41, 5.84), 1.49 (1.03, 2.24), 2.92 (2.42, 3.52), and 1.26 (1.08, 1.48) mmol/L, respectively. Figure 1 shows the comparisons of plasma lipid levels between healthy women and patients with cervical cancer. While plasma levels of TC, TG, and LDL were significantly higher, plasma HDL levels were significantly lower among patients with cervical cancer than among healthy women (Figure 1A).
Given that age may influence lipid levels, all the individuals were further divided into young (<50 years) and old groups (≥50 years). Similarly, patients with cervical cancer showed higher TC, TG, and LDL levels and lower HDL levels than healthy women in both the young and old groups (Figure 1B). Patients with cervical cancer showed higher TC, TG, and LDL levels and lower HDL levels than healthy women. The difference in lipid levels, including TC, TG, LDL, and HDL, between the healthy group and the cancer group remained statistically significant after further adjustment for age and BMI (P<0.001, P=0.001, P<0.001, P=0.008, respectively). Thus, compared with healthy women, patients with cervical cancer were coupled to a disordered lipid profile characterized by higher TG, TC, and LDL levels and lower HDL levels (Figure 2).
Lipid Profile Levels and Survival
Follow-up was available for 1499 cervical cancer patients. With a median follow-up of 4.76 (2.40, 8.21) years, 156 patients (10.41%) experienced relapse, and 102 patients (6.80%) died.
Initially, univariate analyses were employed to depict the prognostic value of clinicopathological features among patients with cervical cancer. As expected, the factors that were statistically significant in predicting poor RFS as well as OS were positive lymph node metastasis, parametrial involvement, late FIGO stage, positive lymphovascular space invasion, deeper infiltration depth, and increased preoperative level of SCC-Ag (Table 2). Our study found no significant correlation between tumor size and prognosis.
 |
Table 2 Univariate Cox Regression Analysis of Clinical Characteristics Regarding RFS and OS |
Next, we explored the prognostic value of the lipid profile among patients with cervical cancer. The cut-offs were determined based on the median of each lipid level (TC, TG, HDL, and LDL) in 1499 cervical cancer patients, with those above the cut-offs defined as “high” and those below the cut-offs defined as “low”. Table 3 shows the RFS and OS of the patients included in the study according to TC, TG, LDL, and HDL levels. As shown in Table 3, patients with high TG levels had a significantly worse RFS than those with low TG levels in both the univariate and multivariate Cox models. However, no association was detected between TC/LDL/HDL and RFS in cervical cancer.
 |
Table 3 Univariate Cox Regression Analysis and Multivariate Cox Regression Analysis of Lipid Profile Regarding Recurrence-Free Survival and Overall Survival |
With regard to OS, the univariate analysis revealed that increased TC and TG levels were significantly correlated with poor OS in cervical cancer. Further multivariate analysis showed that increased levels of TG were related to a significantly unfavorable OS of cervical cancer. The higher TC group had a trend toward higher HRs for OS than the lower group; however, the P value was 0.462. Nevertheless, HDL and LDL levels had no effect on OS (HR: 0.86; 95% CI: 0.44–1.69; P: 0.67; HR: 1.04; 95% CI: 0.88–1.23; P: 0.624, respectively) (Table 3). The Kaplan–Meier curves for RFS and OS of the two groups (high TG levels vs low TG levels) are further illustrated in Figure 3. Patients with higher TG levels had significantly worse RFS and OS than those with lower TG levels among patients ≥50 years old (Table 4).
 |
Table 4 Univariate Cox Regression Analysis of Lipid Profile Regarding Recurrence-Free Survival and Overall Survival Stratified by Age |
Cervical cancer patients with high-risk factors showed a worse prognosis than patients with intermediate-risk or low-risk factors, and we further analyzed patients with high-risk factors. As shown in Figures 4 and 5, a higher level of TG was an independent poor prognostic factor among cervical cancer patients with high risk (Figures 4 and 5).
Squamous cell carcinoma is the most common pathological type of cervical cancer, so we analyzed the correlation between lipid profiles and clinical outcomes among patients with cervical squamous cancer using both univariate and multivariate Cox regression analyses. We found that higher levels of TG were related to worse RFS and OS among squamous patients (hazard ratio =1.13, 95 CI: (1.03–1.43), P<0.05; hazard ratio =1.27, 95 CI: (1.09–1.45), P<0.05, respectively). Thus, TG was also an independent negative prognostic predictor among patients with squamous cervical cancer.
Discussion
In this study, we explored the clinical significance of lipid profiles among cervical cancer patients and made several important discoveries. We comprehensively investigated the difference in serum lipid levels between healthy women and patients with cervical cancer using a relatively large population and demonstrated that TC/TG/LDL levels were elevated and HDL was decreased in patients with cervical cancer. These results suggest that dyslipidemia could be correlated with cervical cancer. Our study showed that hyperlipidemia was an independent prognostic factor for both RFS and OS among patients with cervical cancer.
Lipids are essential components of cell membranes, and lipid metabolism essentially contributes to tumor cell bioenergetics and biomass formation.16 Cholesterol and triglycerides are the two main lipids in plasma.17 LDL and HDL are lipoproteins responsible for cholesterol transportation. While LDL leads cholesterol to the cells and facilitates the deposition of fat in the vessel, HDL promotes the removal of excess cholesterol.17 Epidemiological studies, despite being controversial, reported the correlation between plasma triglyceride levels and the risk for cancer. Higher levels of triglycerides were associated with an increased risk of lung cancer, thyroid cancer, renal cancer, prostate cancer, and gynecological cancer in a large cohort study in Austria.18 Similarly, triglycerides were reported to increase the risk for cervical cancer in females and colon and thyroid cancer in males in a cohort of 22,946 Icelanders.19 Conversely, lower triglyceride (< 1.70 mmol/L) was related to an evaluated cancer risk in Chinese type 2 diabetes mellitus patients.20 Our observation of higher lipid proliferation in cervical cancer was similar to a previous cohort study among Icelanders, which regarded triglycerides as a positive risk factor for cervical cancer.19 Further evidence for a relationship with triglycerides comes from India.8 Raju et al reported that TG was elevated in cervical cancer patients compared to healthy controls.8 Therefore, cervical cancer is coupled to a disordered lipid profile characterized by higher TG, TC, and LDL levels and lower HDL levels. Dyslipidemia could be associated with cervical cancer.
Our current study further explored the prognostic value of hyperlipidemia at diagnosis in cervical cancer. We provided the first evidence that higher plasma triglyceride levels correlated with worse RFS and OS among patients with cervical cancer. This observation was consistent with previous studies in prostate cancer and breast cancer. Elevated serum triglycerides increased the risk of prostate cancer recurrence.21 Hypertriglyceridemia was correlated with a decreased 5-year OS among patients with triple-negative breast cancer.22 In a recently published, monocentric, retrospective study, Vernieri et al reported that higher plasma triglyceride levels correlated with lower progression-free survival in everolimus-treated patients with advanced pancreatic neuroendocrine tumors.23 These findings, coupled with evidence that adipocytes provide energy for rapid cancer growth and metastasis in vivo and in vitro,3 indicate that increased utilization of extracellular lipids, or their de novo synthesis, could increase the recurrence risk and result in poor clinical outcomes among cervical cancer patients. Therefore, we recommend lipid management for patients with cervical cancer, especially those with hyperlipidemia. Accordingly, the lipid profile was recommended for routine evaluation during follow-up.
Stratification by age revealed a negative association between hypertriglyceridemia and prognosis in patients ≥50 years old rather than in patients <50 years old, suggesting that hypertriglyceridemia was associated with poor outcomes in cervical cancer patients, especially elderly patients. Subanalysis for cervical cancer patients with high-risk factors showed that a higher level of TG was an independent poor prognostic factor for this population. These subanalyses further supported the preoperative lipid profile as a promising prognostic predictor in cervical cancer by controlling for confounding data.
More recently, Aziz et al24 evaluated the prognostic significance of the absolute neutrophil count, absolute lymphocyte count, platelet count, C-reactive protein albumin, and total bilirubin levels in patients with pancreatic cancer by collecting preoperative laboratory data available closest to the time of surgery. They found that the systemic-immune-inflammation index independently predicted survival and recurrence in resectable pancreatic cancer and that its prognostic value depends on bilirubin levels.24 Similarly, Okugawa et al25 evaluated the prognostic significance of a combination of inflammatory factors using preoperative blood examination and reported that the preoperative lymphocyte-C-reactive protein ratio was a useful marker for predicting surgical and oncological outcomes in colorectal cancer. Therefore, we believe that collecting pretreatment lipid parameters is a rational way to explore the clinical value of lipid parameters in cervical cancer.
Our study has some limitations. As a retrospective study, collecting the change in lipid profile during follow-up was limited. This is because the serum lipid profile was not routinely evaluated in all patients during follow-up. A prospective randomized study evaluating the survival benefits of controlling lipids in primary cervical cancer is warranted.
Conclusions
In conclusion, a disordered lipid profile characterized by higher TG, TC, and LDL levels and lower HDL levels could be associated with cervical cancer. TG exerted an unfavorable influence either on RFS or on OS of cervical cancer. Hypertriglyceridemia was an independent negative prognostic predictor for cervical cancer. These simple and routinely tested parameters may be a convenient prognostic candidate for cervical cancer in the clinic. Strengthening lipid management may be beneficial for improving postoperative OS and RFS in patients with cervical cancer.
Abbreviations
HPV, human papillomavirus; TG, triglycerides; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SCC-Ag, serum squamous cell carcinoma antigen; OS, overall survival; RFS, recurrence-free survival; BMI, body mass index; SCC (Pre-o), serum squamous cell carcinoma antigen (preoperative).
Data Sharing Statement
Available on request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, and patients signed informed consent forms before taking part in this study. This study complies with the Declaration of Helsinki. All patients agreed to have their clinical data used for clinical research before being hospitalized.
Consent for Publication
Not applicable.
Acknowledgments
We thank Dr. Feiyun Zheng for her kind support to this manuscript in terms of data collection. We would like to thank all doctors, nurses, patients, and their family members for their kindness in supporting our study.
Author Contributions
All of the authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; have drafted, written, substantially revised, or critically reviewed the article; have agreed to submit to the current journal; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; gave final approval of the version to be published; and agree to take responsibility and be accountable for the contents of the article and all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Perrotti F, Rosa C, Cicalini I, et al. Advances in lipidomics for cancer biomarkers discovery. Int J Mol Sci. 2016;17(12):1992. doi:10.3390/ijms17121992
3. Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–1503. doi:10.1038/nm.2492
4. Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. Int J Cancer. 2009;124(12):2938–2941. doi:10.1002/ijc.24285
5. Agnoli C, Grioni S, Sieri S, et al. Colorectal cancer risk and dyslipidemia: a case-cohort study nested in an Italian multicentre cohort. Cancer Epidemiol. 2014;38(2):144–151. doi:10.1016/j.canep.2014.02.002
6. Wuermli L, Joerger M, Henz S, et al. Hypertriglyceridemia as a possible risk factor for prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(4):316–320. doi:10.1038/sj.pcan.4500834
7. Arthur R, Møller H, Garmo H, et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med. 2016;5(6):1307–1318. doi:10.1002/cam4.665
8. Raju K, Punnayanapalya SS, Mariyappa N, Eshwarappa SM, Anjaneya C, Kai LJ. Significance of the plasma lipid profile in cases of carcinoma of cervix: a tertiary hospital based s tudy. Asian Pac J Cancer Prev. 2014;15(8):3779–3784. doi:10.7314/APJCP.2014.15.8.3779
9. Sun Y, Meng H, Jin Y, et al. Serum lipid profile in gynecologic tumors: a retrospective clinical study of 1550 patients. Eur J Gynaecol Oncol. 2016;37(3):348–352.
10. Tang Q, Hu QY, Piao YF, Hua YH. Correlation between pretreatment serum LDL-cholesterol levels and prognosis in nasopharyngeal carcino ma patients. Onco Targets Ther. 2016;9:2585–2591. doi:10.2147/OTT.S98079
11. Zhu F, Xu X, Shi B, et al. The positive predictive value of low-density lipoprotein for recurrence-free survival in ovarian canc er. Int J Gynaecol Obstet. 2018;143(2):232–238. doi:10.1002/ijgo.12645
12. Chi PD, Liu W, Chen H, et al. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One. 2014;9(3):e91080. doi:10.1371/journal.pone.0091080
13. Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast ca ncer but not non-TNBCs. Int J Biol Markers. 2015;30(2):e200–e207. doi:10.5301/jbm.5000143
14. Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613. doi:10.1200/JCO.2000.18.8.1606
15. Rotman M, Sedlis A, Piedmonte MR, et al. A Phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169–176. doi:10.1016/j.ijrobp.2005.10.019
16. Vernieri C, Casola S, Foiani M, Pietrantonio F, de Braud F, Longo V. Targeting cancer metabolism: dietary and pharmacologic interventions. Cancer Discov. 2016;6(12):1315–1333. doi:10.1158/2159-8290.CD-16-0615
17. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of c ardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi:10.1093/eurheartj/ehr112
18. Ulmer H, Borena W, Rapp K, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101(7):1202–1206. doi:10.1038/sj.bjc.6605264
19. Tulinius H, Sigfússon N, Sigvaldason H, Bjarnadóttir K, Tryggvadóttir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6(11):863–873.
20. Yang X, Ma RC, So WY, et al. Low triglyceride and nonuse of statins is associated with cancer in type 2 diabetes mellitus: the Hon g Kong Diabetes Registry. Cancer. 2011;117(4):862–871. doi:10.1002/cncr.25455
21. Allott EH, Howard LE, Cooperberg MR, et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–2356. doi:10.1158/1055-9965.EPI-14-0458
22. Lofterød T, Mortensen ES, Nalwoga H, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival b y breast cancer subtypes. BMC Cancer. 2018;18(1):654. doi:10.1186/s12885-018-4568-2
23. Vernieri C, Pusceddu S, Fuc G, et al. Impact of systemic and tumor lipid metabolism on everolimus efficacy in advanced pancreatic neuroendo crine tumors (pNETs). Int J Cancer. 2019;144(7):1704–1712. doi:10.1002/ijc.32042
24. Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable p ancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter C ohort study. Ann Surg. 2019;270(1):139–146. doi:10.1097/SLA.0000000000002660
25. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological O utcomes in colorectal cancer. Ann Surg. 2020;272(2):342–351. doi:10.1097/SLA.0000000000003239
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





