Back to Journals » Medical Devices: Evidence and Research » Volume 7
The clinical utility of FibroScan® as a noninvasive diagnostic test for liver disease
Received 14 February 2014
Accepted for publication 10 March 2014
Published 3 May 2014 Volume 2014:7 Pages 107—114
DOI https://doi.org/10.2147/MDER.S46943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Julius Wilder,1,2 Keyur Patel1,2
1Division of Gastroenterology, Duke University School of Medicine, 2Duke Clinical Research Institute, Durham, NC, USA
Abstract: An important aspect of managing chronic liver disease is assessing for evidence of fibrosis. Historically, this has been accomplished using liver biopsy, which is an invasive procedure associated with risk for complications and significant sampling and observer error, limiting the accuracy for determination of fibrosis stage. Hence, several serum biomarkers and imaging methods for noninvasive assessment of liver fibrosis have been developed. In this article, we review the current literature on an important noninvasive imaging modality to measure tissue elastography (FibroScan®). This ultrasound-based technique is now increasingly available in many countries and has been shown to be a reliable and safe noninvasive means of assessing disease severity in chronic liver disease of varying etiology.
Keywords: elastography, ultrasound, fibrosis, cirrhosis
Introduction
The extent and progression of liver fibrosis is an important factor in the management of individuals with liver disease. Fibrosis is a wound healing response to injury and is a complex dynamic process involving fibrogenesis and fibrolysis. Chronic viral hepatitis or steatohepatitis leads to fibrogenesis through increased synthesis of extracellular matrix components such as collagens and glycoproteins. However, the wound response also initiates degradation and remodeling of the extracellular matrix, but persistent injury ultimately leads to fibrosis and cirrhosis. Assessment of fibrosis stage or the presence of cirrhosis will often dictate treatment options as well as provide an overall prognosis for patients with chronic liver disease.
Historically, liver biopsy has been the primary means of identifying fibrosis and monitoring for disease progression. However, liver biopsy is a painful, expensive, and invasive procedure with risk of potential complications.1 The accurate evaluation of fibrosis using liver biopsy is also complicated by sampling error and interobserver variation in staging.2,3 Given the risks of the procedure, the limited static and cross-sectional information provided in relation to overall disease progression, as well as the error rate, the development of noninvasive and reliable means of evaluating for the presence of fibrosis and fibrogenesis has been an important area of study. Reliable diagnostic noninvasive tests of fibrosis in viral hepatitis now include a combination of serologic markers (such as FibroTest; BioPredictive, Paris, France) as well as imaging modalities.4 As we progress into the era of safe and effective antiviral therapy for chronic hepatitis C (CHC), various serum biomarkers and imaging methods are now being validated for assessment of fibrosis in nonalcoholic liver disease.5 Several magnetic resonance imaging (MRI)-based techniques are currently being evaluated as noninvasive means of evaluating for cirrhosis. These MRI-based techniques include diffusion weighted imaging, perfusion MRI, magnetic resonance (MR) spectroscopy, and MR elastography.6 MR elastography shows significant promise. This imaging technique utilizes MRI to quantitatively measure liver stiffness (cirrhosis). MR elastography provides quantitative measures of liver stiffness over large areas, is less operator dependent than some other imaging modalities, and requires less than a minute of acquisition time.6 Further research on this promising technique in liver disease is required. The purpose of this review is to examine the current literature addressing the important imaging modality to measure tissue elastography (FibroScan®; Echosens, Paris, France), which has been shown to be a reliable and safe noninvasive means of assessing severity of fibrosis in patients with chronic liver disease.
Transient elastography
Transient elastography (TE) utilizing FibroScan now allows for a rapid measurement of liver stiffness. This technology had been used for some time in the food industry to assess maturity of cheese.7 Using an ultrasound transducer probe, vibrations of mild amplitude and low frequency (50 Hz) are transmitted through the liver tissue. This results in an elastic shear wave that propagates through the underlying liver tissue. The probe then utilizes pulse-echo ultrasound to follow the propagation of the shear wave and to measure its velocity. The velocity of the wave is directly related to tissue stiffness which correlates with fibrosis.8,9 This method allows for the evaluation of numerous parameters including velocity of vibration, velocity of wave propagation, and elastic modulus. TE allows for the identification of disease severity due to altered mechanical properties of the fibrotic liver.10,11 TE is a very simple and safe technique that takes 5–10 minutes and can be done in a specialty clinic or outpatient setting. The only preliminary preparation required is that patients fast for 2–3 hours prior to the procedure due to the potential increase in liver stiffness from postprandial blood flow.12 The patient is placed in a dorsal decubitus position with the right arm in maximal abduction. The exam then begins with placement of the probe along the intercostal space to obtain a view of the right lobe of the liver.11,13,14 Once an area of at least 6 cm thick and free of large vascular structures or gallbladder has been identified, ten measurements are obtained using the FibroScan probe. The actual area measured by the probe has a volume that is at least 100 times bigger than the average liver biopsy sample.14 A reliable exam should result in ten measurements with a 70% success rate, and the interquartile range should be less than 30% of the value of the median.11
An important aspect to any new technique is its cost effectiveness. A few studies have begun to evaluate for the cost effectiveness of TE and show promising results. TE has been found to be a cost-effective surveillance strategy to evaluate for the presence of fibrosis.15
Limitations of transient elastography
TE cannot be used in individuals with ascites, and is associated with higher failure rates or unreliable results in obese patients using the standard M probe, as the shear wave does not propagate through fluid, and fat also attenuates ultrasound and elastic waves.11,14,16 Newer XL probes have been developed that reduce failure rates in obese patients. Fibrosis thresholds are lower than the standard M probe, and further validation in larger cohorts of chronic liver disease patients is required.17 Children and lean patients with narrow intercostal spaces also have higher failure rates, and newer pediatric S2 probes are now available to improve reliability in this regard.18 Also of note, data suggest that liver stiffness values for TE may be 1.3–3 times higher in the setting of acute inflammation and/or moderate alanine aminotransferase (ALT) elevation.19 The stiffness values usually return to baseline along with the normalization of laboratory abnormalities.9,20 Hence, the use of TE in the setting of transaminitis is not recommended. Studies on measurement of liver stiffness in normal subjects have been done to establish patterns among the general population. Roulot et al showed that men and patients with a body mass index >30 kg/m2 had higher liver stiffness scores on average. After adjusting for sex and body mass index, liver stiffness values were also higher in subjects with metabolic syndrome.21 Other limitations for accurate stiffness reading include sinusoidal congestion,22 extra hepatic cholestasis,23 age,24 and steatosis (controversial).25,26 Finally, like many ultrasound-based techniques, TE is somewhat operator dependent. Therefore, there may be some variability in results depending on the operator. Nonetheless, there are new and exciting ultrasound technologies currently under development. Some of these will address some of the limitations of TE. One technique which uses advance spectral analyses for real-time automatic echographic tissue typing hopes to address the issue of differing compression of the ultrasound probe during the examination.27 Furthermore, a radiofrequency time series technique may eventually be used for tissue typing with ultrasound.28 Tissue typing allows for better differentiation of normal and abnormal tissue by augmenting ultrasound images with tissue-specific information.28
Hepatitis C
The first validation studies of TE and correlation with liver biopsy were performed in CHC. The first published study was by Sandrin et al in 2003.8 In this analysis, 91 CHC patients underwent liver biopsy as well as TE. TE was 99% effective in detecting cirrhosis and 88% effective in detecting fibrosis.8 Evaluation of the data on TE, particularly in patients with CHC, shows that the liver stiffness measurement (LSM) does correlate strongly with the METAVIR fibrosis stage. Multiple studies have validated TE in CHC, and a meta-analysis of 50 studies indicated characteristic area under the receiver operator curves (AUROCs) of 0.84, 0.89, and 0.94 for significant fibrosis (F≥2), F3–F4, and F4, respectively.29 Based on these findings, TE could be used as a means of both detecting severe fibrosis and cirrhosis (METAVIR score of F3 or F4) as well as a way to exclude significant fibrosis (METAVIR F≥2).30 Combined algorithms with both TE and serum markers add diagnostic confidence for mild or severe disease.4
Two areas of interest include the use of TE in patients with chronic hepatitis C virus (HCV) who have normal aminotransferases as well as the use of TE in patients who are post-transplantation and have positive HCV RNA.9 An interesting aspect to the natural history of CHC infection is the potential for progressive liver disease and development of extensive fibrosis or cirrhosis in the setting of persistently normal aminotransferase levels among a small proportion of patients. Due to this unique characteristic, patients with CHC may undergo a liver biopsy to evaluate for the presence of or progression of fibrosis. A noninvasive alternative would be ideal in this setting given the risk associated with liver biopsy.9 Hence, Colletta et al in 2005 prospectively evaluated how well TE predicted fibrosis in this cohort compared to liver biopsy, as well as how TE compared to the FibroTest.31 Among patients with chronic HCV and normal ALT, they found that FibroScan was superior to the FibroTest in the noninvasive identification of significant fibrosis.31
Liver transplantation is the only viable option for appropriate candidates with decompensated liver disease. With advances in surgical technique as well as immunosuppression regimens, liver transplantation improves clinical outcomes in eligible patients with end stage liver disease. However, in the setting of end stage liver disease related to CHC, reinfection of the graft is universal, and progression to significant fibrosis can be accelerated and hence is an important clinical concern.9 Historically, monitoring of progression of fibrosis posttransplant has relied on liver biopsy, associated with inherent procedural risks and sampling error. To evaluate the role of TE in transplant patients, Corradi et al compared TE to histology on liver biopsy and serological markers of fibrosis in HCV-infected transplant patients.32 Their results showed that using a stiffness cutoff of 10.1 kPa revealed 94% sensitivity, 89% specificity, 81% positive predictive value, and 94% negative predictive value in differentiating F1 from F2–F4. Furthermore, the AUROC in the assessment of fibrosis was significantly higher for TE (0.94) than for any of the other noninvasive indexes in the analysis.32
The morbidity and mortality associated with chronic HCV infection is mostly related to the rate of fibrosis progression and development of cirrhosis. The natural history of this progression is a complex and multifactorial process. Currently, there are numerous emerging new direct-acting antiviral treatment regimens, which show great potential for curing CHC, even in those who have failed previous treatment. However, there are socioeconomic issues that may limit global availability of these newer therapies. As such, an important decision point in targeted treatment is the assessment of degree of fibrosis and potential morbidity from advanced liver disease. Here, TE has been shown to be a valuable tool in establishing disease severity, prognosis, and subsequent management. A common consequence of CHC is the development of portal hypertension. This results in the formation of esophageal varices, gastric varices, and portosystemic encephalopathy. These increase morbidity and mortality in patients with chronic liver disease. Typically, the gold standards for evaluating these types of complications include upper endoscopy as well as hepatic venous pressure gradient (HVPG) measurement. However, these are both invasive procedures and have associated risk. The use of TE as a noninvasive means of evaluating for portal hypertension appears promising and has shown that LSM has a good correlation both with HVPG as well as the presence of esophageal varices.33 In a cross-sectional analysis of 117 patients with compensated cirrhosis by Berzigotti et al, LSM was the best single noninvasive variable for identifying patients with clinically significant portal hypertension (HVPG >10; AUROC, 0.88).34 The AUROC improved further when TE was combined with spleen size and platelet count. The literature has shown that the correlation between liver stiffness and measurement of HVPG declines with HVPG values <12 mm Hg. Hence, spleen stiffness measurement has been studied and shown to be a more accurate predictor of HVPG values.35 The combination of TE for LSM and spleen stiffness has been shown to identify patients with esophageal varices and different degrees of portal hypertension.36 The use of TE for evaluation of LSM and portal hypertension is known to predict 5-year survival in patients with CHC,37 and a meta-analysis by Singh et al showed that the degree of liver stiffness is associated with risk of decompensated cirrhosis, hepatocellular carcinoma (HCC), and death in patients with chronic liver disease.38
The role of TE as a diagnostic tool in other types of chronic liver disease including hepatitis B, primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), nonalcoholic fatty liver disease (NAFLD), and human immunodeficiency virus (HIV)-HCV coinfection has been evaluated. The data shows that TE is a useful noninvasive tool to evaluate for fibrosis in the setting of these causes of liver disease. However, because of differences in optimal TE cutoffs, sensitivity, specificity, and AUROC, the results of TE must be interpreted with caution.9,13,20,25,39–43
Hepatitis B
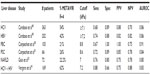
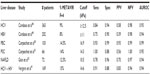
The data concerning the clinical utility of TE in hepatitis B appears promising. However, there are issues to consider when interpreting results. Using TE, Marcellin et al showed that in the setting of hepatitis B, TE performed as well as it does for hepatitis C, with 84% and 65% positive and negative predictive values, respectively, for a cutoff of 7.0 kPa.44 Furthermore, TE was included among the recommendations published by an Italian expert panel for monitoring fibrosis progression in patients with active hepatitis B virus (HBV) replication (HBV DNA >2,000 IU/mL) and normal ALT level.4,9,11,45 But, as suggested by Ganne-Carrié et al, the performance of TE in the diagnosis of cirrhosis may be less reliable in patients with HBV infection than HCV patients.46 To evaluate this Verveer et al prospectively studied the performance of TE compared to histology and examined whether there were differences between chronic hepatitis B and C in large biopsies (≥25 mm). In this analysis, they found that in the setting of the diagnosis of hepatitis B and fibrosis with stage F≤2, TE was suboptimal and that inflammation may induce higher values. However, they found that for stages F≥3, TE performed equally well in both chronic hepatitis B and C.47 These findings support the theoretical approach that different TE cutoff values should be employed depending on the etiology of liver disease. Furthermore, LSM must be cautiously interpreted in the setting of inflammation. Interestingly, in a cross-sectional analysis of TE in chronic hepatitis B and C, Cardoso et al found that TE measurement accurately predicted the absence or presence of significant fibrosis (Table 1) as well as advanced fibrosis or cirrhosis (Table 2), and did so similarly in patients with chronic hepatitis B and C.48 Furthermore, in their analysis, the use of TE cutoff values adjusted to ALT level did not improve performances for estimating liver fibrosis in patients with chronic hepatitis B.48 Based on these findings, clearly more research on the role and theoretical interpretation of TE in the setting of natural phase of chronic hepatitis B infection is needed.
One of the most concerning outcomes of chronic liver disease and cirrhosis from hepatitis B is HCC. This disease can carry a high morbidity and mortality. A meta-analysis of cohort studies by Singh et al evaluated the relationship between liver stiffness and HCC.38 They found that the degree of liver stiffness was associated with risk of HCC in patients with chronic liver disease and suggested that liver stiffness may be used for risk stratification. To more specifically evaluate the role of TE in HCC, Wong et al performed a prospective cohort study to evaluate the accuracy of an LSM-HCC score. This score was refined from the CU-HCC score with LSM based on TE, age, serum albumin, and HBV DNA level. They found that the AUROCs for the LSM-HCC score ranged from 0.83–0.89.49 This is a range that is higher than the traditionally used CU-HCC score.49 Hence, there is clearly a role for TE not only in the evaluation of fibrosis but possibly in the evaluation and risk stratification for HCC.
Primary biliary cirrhosis and primary sclerosing cholangitis
Using a cohort of 101 patients with either PBC or PSC, Corpechot et al showed that TE had a higher sensitivity and specificity for identifying cirrhosis rather than moderate fibrosis (F4: sensitivity 0.93 and specificity 0.95; F2: sensitivity 0.84 and specificity 0.87).42 To further characterize the use of TE in PBC, Corpechot et al in a later study performed a combined prospective analysis of TE for the diagnosis of METAVIR fibrosis stages in a diagnostic cohort of 103 patients and a retrospective longitudinal analysis of repeated examinations in a monitoring cohort of 150 patients followed-up for up to 5 years.50 In this analysis, they found that TE performed well for diagnosis of severe fibrosis or cirrhosis (Table 2) and was significantly superior to biochemical markers (aspartate aminotransferase/platelet ratio, FIB-4, hyaluronic acid, aspartate aminotransferase/ALT ratio, and Mayo score) in diagnosing significant fibrosis, severe fibrosis, or cirrhosis.50 The sensitivity for detecting significant fibrosis was lower in this analysis50 (Table 1). In another analysis, the role of TE in PSC was evaluated by Corpechot et al.51 In this prospective analysis, the diagnostic performance, reproducibility, longitudinal changes, and prognostic value of LSM using TE was evaluated in patients with PSC (Tables 1 and 2). Here, liver stiffness was independently linked to fibrosis stage and had a diagnostic accuracy for severe fibrosis and cirrhosis of 0.83 and 0.88, respectively.51 The AUROC for significant fibrosis in this analysis was 0.84 (Table 1) while the AUROC for cirrhosis was 0.95 (Table 2). Once again, they found that TE had a superior diagnostic performance compared to many of the serologic markers of fibrosis and cirrhosis.51 While the data here are promising for the use of TE in PBC and PSC, the above results are largely based on the work of one group. Additional research is needed to further clarify and define the role of TE in the management and treatment of PBC and PSC.
Nonalcoholic fatty liver disease
To assess the reliability of TE in the setting of NAFLD, Yoneda et al measured liver stiffness in 67 NAFLD patients for whom the diagnosis had been confirmed by liver biopsy and the severity of fibrosis had been scored according to Brunt classification.43 This analysis found that TE had the same accuracy as liver biopsy with an AUROC of 0.90 for F2 and 0.99 for F4.43 However, more recent analyses show that interpretation of LSM in the setting of NAFLD must be done with caution. In a prospective study of 219 patients who had undergone liver biopsy within 6 months, Gaia et al showed that while liver stiffness was related to fibrosis in the setting of NAFLD, the association was less impressive than seen in patients with hepatitis C.52 When evaluating for significant fibrosis in the NAFLD cohort, TE achieved a sensitivity of 0.76, specificity of 0.80, and AUROC of 0.80 (Table 1). The findings were more robust for cirrhosis, for which the sensitivity was 0.78, specificity 0.96, and AUROC 0.94. Furthermore, NAFLD patients with advanced fibrosis (F3) and severe steatosis (>33%) had LSM values that were lower than expected and were similar to those of patients with minimal fibrosis (F1) and less steatosis fat <33%. In this analysis, TE underestimated the stage of fibrosis in 75% of patients with F3 and steatosis >33%.52 Finally, an important issue with NAFLD is the potential impact of nonalcoholic steatohepatitis. The distinction between bland steatosis and steatohepatitis can only be reliably made on liver biopsy. There are still a relatively small number of studies looking at TE in the setting of NAFLD, and further research is needed.
Coinfection with HIV and Hepatitis C
A major issue in the management of patients with chronic viral hepatitis is coinfection with HIV and hepatitis C. Previous analyses have shown promising results in the use of TE in assessing fibrosis and disease progression in the setting of HIV-HCV coinfection. Vergara et al found a specificity of 88% and sensitivity of 91% (Table 2) when diagnosing cirrhosis (cutoff value 14.6 kPa) in coinfected HIV/hepatitis C patients, and 88% sensitivity and 66% specificity (Table 1) in diagnosing significant fibrosis of grade F2 (cutoff 7.2 kPa).41,53 Similarly, de Lédinghen et al performed a prospective analysis where they evaluated the accuracy of TE for identifying fibrosis in comparison to other noninvasive modalities in HIV-HCV patients.40 This study indicated that liver stiffness was significantly correlated to fibrosis stage, and for the diagnosis of cirrhosis, AUROC curves of LSM were significantly higher than those for other serology-based noninvasive markers.40
Conclusion
The availability of both serologic and imaging modalities for noninvasive measures of fibrosis and cirrhosis in the setting of chronic liver disease is an important step forward in the clinical management of patients with chronic liver disease. The global burden of chronic liver disease, with NAFLD and viral hepatitis, is significant. A significant number of patients with NAFLD will progress toward cirrhosis, and reliable assessment of fibrosis stage and disease progression remains clinically important. With the implementation of new and innovative curative treatments for hepatitis C, reliable noninvasive means of evaluating for fibrosis progression (or regression), assessment of prognosis, and efficacy of antifibrotic approaches, for example, in patients with virologic response and advanced stage disease, will become more important.
There are still issues that need to be addressed with the use of FibroScan. Besides technological limitations (ascites and obesity), questions related to variable cutoffs based on disease etiology, clinical utility in management of NAFLD, clinical approach to longitudinal assessment, and frequency of repeated measurements must be addressed. Furthermore, this is still a relatively new technology that carries with it the associated financial barriers including cost and widespread availability.
In summary, TE using FibroScan provides a viable alternative to the use of liver biopsy in the routine diagnostic assessment of significant fibrosis, and in particular cirrhosis, in chronic liver disease. This modality provides complementary information to other serologic noninvasive measures of significant fibrosis in chronic liver disease, and has certainly reduced the requirement for liver biopsies for routine staging of CHC patients. The increasing availability of this innovative noninvasive imaging technology for fibrosis assessment, progress with ongoing validation in NAFLD and clinical outcomes, along with new antiviral options in viral hepatitis, creates an exciting time in the diagnosis, treatment, and management of chronic liver disease patients.
Acknowledgments
While no funding was directed to obtaining the supply of data, JW was supported by the Duke NIDDK Grant No 5T32-DK7568-22, and KP is a consultant and/or has obtained grant/research support from Gilead Sciences, Santaris, Bristol-Myers Squibb, Benitec, and Merck & Co.
Disclosure
The authors report no conflicts of interest in this work.
References
Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32(3):477–481. | |
Afdhal NH. Diagnosing fibrosis in hepatitis C: is the pendulum swinging from biopsy to blood tests? Hepatology. 2003;37(5):972–974. | |
Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–2618. | |
Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–1302. e4. | |
Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10(11):666–675. | |
Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37(3):544–555. | |
Benedito J, Carcel J, Clemente G, Mulet A. Cheese maturity assessment using ultrasonics. J Dairy Sci. 2000;83(2):248–254. | |
Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. | |
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847. | |
Konofagou EE. Quo vadis elasticity imaging? Ultrasonics. 2004; 42(1–9):331–336. | |
Stasi C, Arena U, Vizzutti F, et al. Transient elastography for the assessment of liver fibrosis in patients with chronic viral hepatitis: the missing tool? Dig Liver Dis. 2009;41(12):863–866. | |
Arena U, Lupsor Platon M, Stasi C, et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013;58(1):65–72. | |
Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. | |
Beaugrand M. [Fibroscan: instructions for use]. Gastroenterol Clin Biol. 2006;30(4):513–514. French. | |
Canavan C, Eisenburg J, Meng L, Corey K, Hur C. Ultrasound elastography for fibrosis surveillance is cost effective in patients with chronic hepatitis C virus in the UK. Dig Dis Sci. 2013;58(9):2691–2704. | |
Foucher J, Castéra L, Bernard PH, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18(4):411–412. | |
Myers RP, Pomier-Layrargues G, Kirsch R, et al. Discordance in fibrosis staging between liver biopsy and transient elastography using the FibroScan XL probe. J Hepatol. 2012;56(3):564–570. | |
Pradhan F, Ladak F, Tracey J, Crotty P, Myers RP. Feasibility and reliability of the FibroScan S2 (pediatric) probe compared with the M probe for liver stiffness measurement in small adults with chronic liver disease. Ann Hepatol. 2013;12(1):100–107. | |
Tapper EB, Cohen EB, Patel K, et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2012;10(8):932–937. e1. | |
Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14(5):360–369. | |
Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48(4):606–613. | |
Lebray P, Varnous S, Charlotte F, Varaut A, Poynard T, Ratziu V. Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology. 2008;48(6):2089. | |
Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48(5):1718–1723. | |
Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46(4):628–634. | |
Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56(7):968–973. | |
Kim KM, Choi WB, Park SH, et al. Diagnosis of hepatic steatosis and fibrosis by transient elastography in asymptomatic healthy individuals: a prospective study of living related potential liver donors. J Gastroenterol. 2007;42(5):382–388. | |
Soloperto G, Conversano F, Greco A, Casciaro E, Franchini R, Casciaro S. Advanced spectral analyses for real-time automatic echographic tissue-typing of simulated tumor masses at different compression stages. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59(12):2692–2701. | |
Moradi M, Abolmaesumi P, Mousavi P. Tissue typing using ultrasound RF time series: experiments with animal tissue samples. Med Phys. 2010;37(8):4401–4413. | |
Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960–974. | |
Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57(9):1288–1293. | |
Colletta C, Smirne C, Fabris C, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42(4):838–845. | |
Corradi F, Piscaglia F, Flori S, et al; Bologna Liver Transplantation Group. Assessment of liver fibrosis in transplant recipients with recurrent HCV infection: usefulness of transient elastography. Dig Liver Dis. 2009;41(3):217–225. | |
Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56(3):696–703. | |
Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102–111. e1. | |
Sharma P, Kirnake V, Tyagi P, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108(7):1101–1107. | |
Colecchia A, Montrone L, Scaioli E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143(3):646–654. | |
Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140(7):1970–1979, 1979. e1–3. | |
Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(12):1573–1584. e1. | |
Gómez-Domínguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24(3):513–518. | |
de Lédinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41(2):175–179. | |
Vergara S, Macías J, Rivero A, et al; Grupo para el Estudio de las Hepatitis Viricas de la SAEI. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45(8):969–974. | |
Corpechot C, El Naggar A, Poujol-Robert A, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43(5):1118–1124. | |
Yoneda M, Fujita K, Inamori M, et al. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56(9):1330–1331. | |
Marcellin P dLV, Dhumeaux D, Poupon R, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis in chronic hepatitis B using FibroScan. Abstract presented at the 56th Annual Meeting of the American Association for the Study of Liver Disease, San Francisco, USA, November 11–15, 2005. Hepatology. 2005;42(Suppl 1):715A–716A. | |
Carosi G, Rizzetto M. Treatment of chronic hepatitis B: recommendations from an Italian workshop. Dig Liver Dis. 2008;40(8):603–617. | |
Ganne-Carrié N, Ziol M, de Ledinghen V, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44(6):1511–1517. | |
Verveer C, Zondervan PE, ten Kate FJ, Hansen BE, Janssen HL, de Knegt RJ. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012;32(4):622–628. | |
Cardoso AC, Carvalho-Filho RJ, Stern C, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32(4):612–621. | |
Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60(2):339–345. | |
Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56(1):198–208. | |
Corpechot C, Gaouar F, El Naggar A, et al. Baseline values and changes in liver stiffness, measured by transient elastography, are associated with fibrosis severity and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. Epub December 31, 2013. | |
Gaia S, Carenzi S, Barilli AL, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54(1):64–71. | |
Andersen ES, Christensen PB, Weis N. Transient elastography for liver fibrosis diagnosis. Eur J Intern Med. 2009;20(4):339–342. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.