Back to Journals » International Journal of General Medicine » Volume 15
The Clinical Significance of Deglycosylated PD-L1 Level Detection Using 28-8 Monoclonal Antibody in Lung Adenocarcinoma
Authors Wang H, Gu D, Chen D, Mei J, Yang X, Ding J, Xu J , Wang M, Liu C , Hua D
Received 7 July 2022
Accepted for publication 7 September 2022
Published 19 September 2022 Volume 2022:15 Pages 7383—7393
DOI https://doi.org/10.2147/IJGM.S381530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Huiyu Wang,1,* Dingyi Gu,1,* Datian Chen,2,* Jie Mei,1 Xuejing Yang,1 Junli Ding,1 Junying Xu,1 Meilin Wang,3 Chaoying Liu,1 Dong Hua1
1Comprehensive Cancer Center, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, 214023, People’s Republic of China; 2Department of Oncology, Haimen People’s Hospital Affiliated to Nantong University, Haimen, 226100, People’s Republic of China; 3The School of Public Health, Nanjing Medical University, Nanjing, 210000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meilin Wang; Chaoying Liu, Email [email protected]; [email protected]
Purpose: The aim of this study was to explore the clinical significance of deglycosylated PD-L1 level and its correlation with EGFR and ALK mutation in lung adenocarcinoma.
Materials and Methods: We estimated the intensity of both native and deglycosylated PD-L1 signals using a 28– 8 antibody on lung adenocarcinoma tissue microarray sections. We analyzed the difference in the H-score between tumor and paratumor tissues, as well as that before and after deglycosylation. Correlations between EGFR or ALK status and PD-L1 expression were analyzed. We also evaluated the differences among survival curves.
Results: The expression level of PD-L1 in lung adenocarcinoma tissues was significantly higher than that in paratumor tissues (P< 0.0001). Deglycosylation significantly enhanced the detection of PD-L1 in tumor tissues (P< 0.0001). There was no statistical significance between the signal intensity of deglycosylated PD-L1 and the survival of patients (P=0.9099). However, the response to deglycosylation of PD-L1 was significantly correlated with the survival of patients with stage N1-N3 (P=0.0435) and stage T3-T4 (P=0.0366) and male patients (P=0.0258). A statistical trend was found in the correlation between the response to deglycosylation of PD-L1 and the survival of patients with grade II–III plus grade III (P=0.0973). Correlation between EGFR or ALK status and the expression of PD-L1 was not found (P> 0.05).
Conclusion: PD-L1 deglycosylation enhances the detection of PD-L1 when utilizing a 28– 8 antibody. Moreover, the response to deglycosylation of PD-L1 may predict the survival of certain patients with lung adenocarcinoma.
Keywords: programmed death ligand 1, deglycosylation, lung adenocarcinoma, mutation, survival
Introduction
Programmed death ligand 1 (PD-L1) is one of the ligands of programmed death 1 (PD-1), which can cause tumor immune escape. Estimation of the PD-L1 signal intensity by utilizing immunohistochemistry (IHC) staining is used to guide immunotherapy in multiple cancer types.1 However, increasing evidence has shown that the response to immune checkpoint inhibitors (ICIs) is often inconsistent with the expression status of PD-L1.2 Therefore, a better PD-L1 detection method is needed to stratify patients more accurately in clinical practice.
A study by Lee et al showed that the removal of N-linked glycosylation can enhance the detection of PD-L1 and predict anti-PD-1/PD-L1 therapeutic efficacy more accurately in lung cancer, head and neck cancer, esophageal cancer, bladder cancer and et al.3 This inspired the detection of deglycosylated PD-L1 as a better diagnostic biomarker for cancer immunotherapy. N-linked glycosylation is a common post-translational modification of PD-L1, and it has been shown that PD-L1 with heavy N-linked glycans exists in a variety of cancer types. PD-L1 exhibits size differences on Western blots, mainly detected at ~ 45 kDa and ~ 33 kDa. Glycosylated PD-L1 is commonly detected at ~45 kDa, whereas PD-L1 without N-linked glycosylation is detected at ~33 kDa.4 A previous study by our team compared the signal intensity of native and deglycosylated PD-L1 utilizing different PD-L1 antibodies, including 28–8, 73–10, CAL10 and SPA142 in lung cancer.5 The results of that study indicated that PD-L1 expression detected by 28–8, CAL10 and SP142 monoclonal antibodies (mAbs) was significantly enhanced after deglycosylation, but the intensity of PD-L1 detected by 73–10 mAbs was slightly reduced after deglycosylation.
Lung adenocarcinoma is the most common form of lung cancer, and EGFR mutations are commonly detected in non-small cell lung cancer (NSCLC), especially in lung adenocarcinoma.6,7 ALK and KRAS mutations are also important oncogenic drivers in lung adenocarcinoma.8 It has also been shown that high PD-L1 expression is associated with a poor prognosis of lung adenocarcinoma patients.9
To learn more about the significance of detection of deglycosylated PD-L1 in lung adenocarcinoma, we improved the process of deglycosylation of PD-L1 by applying a thermal denaturation strategy, then compared the expression of native and deglycosylated PD-L1 with 28–8 mAbs, since it was previously shown that the intensity of PD-L1 detected by 28–8 mAbs was more enhanced than the other two mAbs in our previous research.5 We also analyzed the correlation between the survival time of patients and the response to deglycosylation of the samples. Moreover, a study by Ota et al found that EGFR and ALK mutations were correlated with higher PD-L1 expression levels,10 but another study reported that EGFR mutations were correlated with lower PD-L1 expression levels.11 The correlation between EGFR and ALK status and PD-L1 expression was also analyzed in this research. Overall, our study aimed to further understand the significance of deglycosylated PD-L1 detection in lung adenocarcinoma in clinical practice.
Materials and Methods
Reagents and Tissue Microarray
PD-L1 antibody (28–8), an IHC detection kit HRP/DAB (ab209101) and HIER antigen retrieval reagent (ab208572) were acquired from Abcam. Recombinant PNGase F (P0708) was acquired from New England Biolabs. Conventional reagents for IHC staining were obtained from Dako.
The lung adenocarcinoma tissue microarray (TMA) section (HLugA180Su07) was acquired from Outdo Biotech and it contained 98 lung adenocarcinomas and 82 paratumor samples. The TMA embodied several clinicopathological characteristics, including age, grading and staging of the tumor, EGFR and ALK status, and survival time. More precisely, the 98 patients included 55 men and 43 women with a median age of 60. They were diagnosed lung adenocarcinoma and underwent radical surgery for lung cancer between 2004 and 2009. After recurrence, all patients received standard first-line and second-line chemotherapy. The end of follow-up was August 2014, and 11 patients were lost. Disease staging and grading are according to the AJCC Cancer Staging Manual. Ethical approval for this study was granted by the Clinical Research Ethics Committee, Wuxi People’s Hospital affiliated with Nanjing Medical University.
Tissues Deglycosylation and Immunohistochemistry
IHC staining and tissue deglycosylation were performed on these TMA sections directly. The TMA sections were incubated at 60℃ for 1 hr after incubation at 40℃ overnight, deparaffinized with xylene and ethanol and hydrated with distilled water. HIER antigen retrieval buffer obtained from Abcam was used to perform antigen retrieval. To thoroughly remove the N-linked glycan of PD-L1, we applied a thermal denaturation strategy to improve Lee’s method3 for PD-L1 deglycosylation. After washing the TMA sections twice with PBS, the TMA sections were incubated with 1× glycoprotein denaturing buffer at 95℃ for 3 hr, and then the sections were washed with PBS three times. The TAM sections were then treated with or without 5% recombinant PNGase F (P0708, New England Biolabs) containing PBS at 37℃ for 12 hr and subjected to IHC staining. Antibody 28–8 (ab205921, Abcam) mixed with HIER antigen retrieval buffer (ab208572, Abcam) at a proportion of 1:500 was used in the current study. DAB and hematoxylin counterstaining were used to visualize the antibody staining. Aperio Digital Pathology Slide Scanners were used to scan the immunostained sections.
An established semiquantitative approach was used to evaluate the results of the IHC to assess the Histoscore (H-score). The H-score (0 to 300) was evaluated by the following method: H-score = [1×(the percentage of cells stained at intensity category 1) + 2×(the percentage of cells stained at intensity category 2) + 3×(the percentage of cells stained at intensity category 3)].
Statistical Analysis
Statistical analyses and visualization were performed by GraphPad Prism 8.0. All error bars denote the standard deviation (SD). The Mann–Whitney test was utilized to analyze the difference in H-score between the tumor and paratumor tissues, as well as that before and after deglycosylation. The Log rank test was used to evaluate the difference between survival curves. A two-tailed P value ≤ 0.05 was considered statistically significant.
Results
Comparability of PD-L1 Expression in Lung Adenocarcinoma Tissues and Paratumor Tissues Utilizing PD-L1 28-8 mAbs
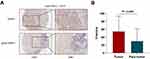
IHC assays are the most prevalent measure used to detect the signal intensity of PD-L1, which is used to stratify patients to guide anti-PD-1/PD-L1 therapy.12 In this research, we compared the signal intensity of PD-L1 between lung adenocarcinoma samples and paratumor samples utilizing PD-L1 28–8 mAbs. Representative images showing the characteristic staining pattern of PD-L1 located in the cytomembrane and cytoplasm are shown in Figure 1A. The H-score of PD-L1 in lung adenocarcinoma samples (mean±SD, 54.78±38.46) was significantly higher than that in paratumor samples (mean±SD, 30.31±31.38) when detected by PD-L1 28–8 mAbs (P<0.0001, Figure 1B).
Comparability of PD-L1 Expression in Lung Adenocarcinoma Before and After Deglycosylation Utilizing PD-L1 28-8 mAbs
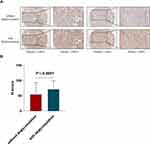
The study of Lee et al showed that removal of N-linked glycosylation could enhance the signal intensity of PD-L1.3 In this research, we applied a thermal denaturation strategy to thoroughly remove the N-linked glycosylation. Then, we compared the PD-L1 expression status between native and deglycosylated samples. Representative images showed that deglycosylation of PD-L1 remarkably enhanced PD-L1 detection (Figure 2A). We then analyzed the H-score of PD-L1 expression in native and deglycosylated samples. The H-score of deglycosylated PD-L1 (mean±SD, 54.78±38.46) samples was significantly higher (P<0.0001) than native PD-L1 (mean±SD, 71.86±27.77) samples (Figure 2B).
Correlation Between EGFR Status and the Expression of Native or Deglycosylated PD-L1
The EGFR status of 75 samples is provided. There were 13 EGFR mutation-positive samples as well as 62 EGFR mutation-negative samples among the 75 samples. To determine whether deglycosylation of PD-L1 could affect the relationship between EGFR status and the expression of PD-L1, we analyzed the signal intensity of native and deglycosylated PD-L1 in various samples with different EGFR statuses. We found no statistically significant correlation between either EGFR status and signal intensity of native PD-L1 (P=0.7767, Figure 3A) or EGFR status and signal intensity of deglycosylated PD-L1 (P=0.2457, Figure 3B).
Correlation Between ALK Status and the Expression of Native or Deglycosylated PD-L1
The ALK status of 80 samples is provided. Among these samples, 15 samples were ALK mutation-positive, and 65 samples were ALK mutation-negative. The signal intensities of the natural and deglycosylated PD-L1 in various samples with different ALK statuses were also analyzed. There was no statistically significant correlation between ALK status and the signal intensity of native PD-L1 (P=0.5334, Figure 4A) or between ALK status and the signal intensity of deglycosylated PD-L1 (P=0.4127, Figure 4B).
Correlation Between the Survival Time of Patients and the Response to Deglycosylation of the Lung Adenocarcinoma Tumor Tissue
We also studied the correlation between the survival time of patients and the signal intensity of deglycosylated PD-L1. Excluding 11 samples lost to follow-up, we analyzed the survival time of 87 samples. The results showed that there was no statistical significance between the deglycosylated PD-L1 signal intensity and the survival time of patients (P=0.9099, Figure 5).
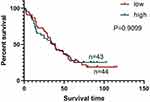 |
Figure 5 Correlation between survival time of patients and signal intensity of deglycosylated PD-L1 stained by PD-L1 28–8 mAb. |
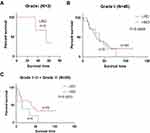
Then, we further analyzed the correlation between the response to deglycosylation of the samples and the survival time of patients with different pathological parameters. We divided the samples into two groups according to their response to deglycosylation. Among 87 samples, 17 samples exhibited a strong response to deglycosylation (HRD), with an H-score of deglycosylated PD-L1 that was 2.5 times higher than their H-score of native PD-L1, and 70 samples exhibited a low response to deglycosylation (LRD), whose H-score of deglycosylated PD-L1 was less than 2.5 times the H-score of the natural PD-L1 of those samples. However, we found that there was no significant correlation between survival time and the response to deglycosylation among the patients with tumors of grade I (Figure 6A) and grade II (P=0.6889, Figure 6B), but there was a statistical trend in the correlation between survival time and the response to deglycosylation among patients with tumors of grade II–III plus grade III (P=0.0973, Figure 6C). Then, we found that the survival time of LRD patients was significantly longer than that of HRD patients among patients with lymphatic metastasis (P=0.0435, Figure 7A), but there was no significant correlation among patients without lymphatic metastasis (P=0.3456, Figure 7B). Moreover, the survival time of LRD patients was significantly longer than that of HRD patients with tumors in stages T3-T4 (P=0.0366, Figure 8B), but there was no significant correlation among patients with tumors in stages T1-T2 (P=0.5420, Figure 8A). Finally, the results showed that the survival time of male patients varied significantly between HRD patients and LRD patients (P=0.0258, Figure 9A), but the survival time of female patients was not significantly correlated with the response to deglycosylation (P=0.8980, Figure 9B).
Discussion
PD-L1 is a vital biomarker for ICIs. Detection of the PD-L1 expression signal by IHC has been applied to guide immunotherapy for patients with different cancers in clinical work and can also be used to predict the response to immune checkpoint inhibitor (ICI) therapy. The American Food and Drug Administration (FDA) has confirmed that the results of PD-L1 IHC testing can guide whether ICIs should be used to treat patients suffering from NSCLC.13 The randomized Phase III KEYNOTE-042 study indicated that patients with PD-L1 expression ≥ 50% had longer overall survival (OS) than patients with PD-L1 expression < 50% after treatment with pembrolizumab.14 However, progression-free survival (PFS) was more improved by pembrolizumab than chemotherapy only in the PD-L1 tumor proportion score (TPS) ≥50% group. The PFS of patients treated with pembrolizumab in the PD-L1 TPS ≥20% group and PD-L1 TPS ≥1% group was similar to that of patients treated with chemotherapy in the KEYNOTE-042 China study.15 Another phase III Checkmate 078 trial showed that in advanced NSCLC patients treated with nivolumab, the median OS of patients with PD-L1 expression ≥ 1% was longer than the median OS of patients with PD-L1 expression < 1%.16
However, there are still some shortcomings in the application of PD-L1 detection, and researchers are looking for new detection methods to predict the curative effect of ICIs more accurately. Studies have shown that some PD-L1-negative patients can also benefit from ICIs.17–20 One study suggested that the positive rate of PD-L1 mRNA detected by in situ hybridization (ISH) was higher than that of PD-L1 protein expression detected by IHC,21 and more patients may benefit from ICIs if detected by ISH. For some patients, the tumor tissue sample is too small to enable a large number of molecular detections. The signal intensity of PD-L1 in circulating tumor cells (CTCs) has been suggested to be correlated with that in tumor tissue22 and is expected to be a new biomarker for ICIs, but treatment, including first-line treatment, may affect the uniformity between the signal intensity of PD-L1 in tumor tissue and that in CTCs.23 Moreover, the signal intensity of PD-L1 may be different between matched primary samples and metastatic samples,24 which can also affect the choice of treatment. Recently, exosomal PD-L1 and soluble forms of PD-L1 have also been detected. Cordonnier et al found that in melanoma, the positive rate of exosomal PD-L1 was higher than that of PD-L1 in biopsies, and the change in exosomal PD-L1 expression before and after treatment was negatively correlated with the tumor response to ICIs and the clinical outcome.25 Soluble PD-L1 may be able to trigger apoptotic signals in target T cells and promote tumor progression.26 Okuma et al found that in NSCLC, patients with low soluble PD-L1 expression had a longer OS than those with high soluble PD-L1 expression when treated with nivolumab.27 In metastatic urothelial cancer patients treated with atezolizumab, patients with lower peripheral T-cell receptor clonality at baseline were found to have longer PFS and OS than those with higher peripheral T-cell receptor clonality.28 Additional studies are needed to confirm the correlation between those biomarkers and the curative effect of immunotherapy.
Glycosylation modification could hamper the binding of 28–8 antibodies leading to underestimation of PD-L1 expression. This study showed that the response to deglycosylation of PD-L1 was associated with the survival of patients with tumors in stage N1-N3 and stage T3-T4 and male patients, and a statistical trend was found in the correlation between the response to deglycosylation of PD-L1 and the survival of patients with grade II–III plus grade III, which may help to guide the prognosis prediction in clinic. In contrast, no significant correlation was found between the response to deglycosylation of PD-L1 and the survival time of patients with other pathological parameters. A meta-analysis showed that poor tumor differentiation was associated with high PD-L1 signal intensity, but other clinicopathological characteristics, including tumor node metastasis (TNM) stage and sex, were not correlated with PD-L1 signal intensity. Moreover, high PD-L1 signal intensity was indicated to be associated with a poor prognosis.29 We speculated that it may be attributed to severe immunosuppressive environment in certain patients, but the mechanism needs to be further explored. Moreover, the subsequent use of deglycosylation detection may be more valuable for predicting the efficacy of ICIs treatment. Studies with more samples focusing on whether deglycosylated PD-L1 expression can more accurately predict ICIs efficacy are needed.
EGFR and ALK mutations are important oncogenic drivers in lung adenocarcinoma. Several studies have researched the correlation between EGFR and ALK status and the expression of PD-L1. Some researchers suggested that EGFR mutations were positively associated with high PD-L1 signal intensity in NSCLC.10,30,31 In contrast, a study by Takada et al showed that EGFR mutation-positive samples might have a lower signal intensity of PD-L1 in lung adenocarcinoma.11 Moreover, a study reported that there was no significant correlation between EGFR status and the expression of PD-L1.32 Hsu et al found that among EGFR mutation-positive lung adenocarcinoma patients, those with higher expression of PD-L1 were more likely to experience primary resistance to EGFR-TKIs.33 ALK mutation was also found to be positively related to high PD-L1 signal intensity in NSCLC.10,34 However, Zhang et al showed that there was no significant correlation between ALK status and the expression of PD-L1.9 Ota et al found that both mutant EGFR and EML4-ALK can activate the MEK-ERK and PI3K-AKT signaling pathways and upregulate PD-L1 signal intensity in NSCLC.10 In our research, no significant correlation was found between EGFR status and the expression of PD-L1, and ALK mutation also had no correlation with PD-L1 expression. The proportions of patients with EGFR mutations or ALK mutations in this research were 17.33% and 18.75%, respectively, which were different from the proportions of patients with EGFR mutations or ALK mutations in Asian patients, which were approximately 51.4% in lung adenocarcinoma and 4.9% in NSCLC.35,36 Moreover, insufficient specimens may also affect the results of the research. In general, studies containing more samples are needed for further study.
There are several limitations in this study. First, adenocarcinoma accounts for about 55% of NSCLC, researches focused on overall NSCLC patients are still needed. Secondly, there are several different antibodies for the detection of PD-L1 such as 28–8, 22C3, SP142 etc. These antibodies bind to different sites and show heterogeneous interference to the deglycosylation treatment. Our study discussed a common used 28–8 antibody and it is undeniable that there is a long way to go to form a unified gold standard PD-L1 detection method at present. Thirdly, our study did not analyze the relevance of deglycosylated PD-L1 detection and the efficacy of immunotherapy, which has vital value in clinical application as mentioned before. Finally, the sample size of this study is limited, and a larger sample size is needed for further verification and mechanism research.
Conclusions
In conclusion, our study indicated that the intensity of PD-L1 expression in lung adenocarcinoma tissues was higher than that in paratumor tissues. It also showed that PD-L1 deglycosylation could remarkably enhance the detection of PD-L1. Most importantly, there was a correlation between the response to deglycosylation of PD-L1 and the survival of patients with stage N1-N3 and stage T3-T4 and male patients. A statistical trend in the correlation between survival time and the response to deglycosylation among patients with grade II–III plus grade III was also found. In contrast, correlation between the response to deglycosylation of PD-L1 and the survival of patients with other pathological parameters was not found. No correlation was found between EGFR or ALK status and the expression of PD-L1. Overall, our research may improve the current method for the stratification of patients with lung adenocarcinoma for anti-PD-1/PD-L1 therapy, but more clinical samples are needed to prove the relationship between the response to deglycosylation of PD-L1 and the survival time of patients. More patients are expected to benefit from the new evaluation method.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Funding
This work was supported by the Postdoctoral research funding program of Jiangsu Province; the Six talent peaks project in Jiangsu Province (grant number WSN186); and the Taihu Talent Program (grant number HB2020005).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. 2020;126:260–270. doi:10.1002/cncr.32468
2. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. doi:10.1186/s40425-019-0768-9
3. Lee HH, Wang YN, Xia W, et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36:168–178.e164. doi:10.1016/j.ccell.2019.06.008
4. Li CW, Lim SO, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi:10.1038/ncomms12632
5. Mei J, Xu J, Yang X, et al. A comparability study of natural and deglycosylated PD-L1 levels in lung cancer: evidence from immunohistochemical analysis. Mol Cancer. 2021;20:11. doi:10.1186/s12943-020-01304-4
6. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–692. doi:10.1016/j.ccm.2011.08.005
7. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi:10.1093/jnci/dji055
8. Califano R, Abidin A, Tariq NU, et al. Beyond EGFR and ALK inhibition: unravelling and exploiting novel genetic alterations in advanced non small-cell lung cancer. Cancer Treat Rev. 2015;41:401–411. doi:10.1016/j.ctrv.2015.03.009
9. Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7:10255. doi:10.1038/s41598-017-10925-7
10. Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:4014–4021. doi:10.1158/1078-0432.CCR-15-0016
11. Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11:1879–1890. doi:10.1016/j.jtho.2016.06.006
12. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–1311. doi:10.1016/j.jtho.2018.05.013
13. Yang Q, Xu Z, Zheng L, et al. Multimodal detection of PD-L1: reasonable biomarkers for immune checkpoint inhibitor. Am J Cancer Res. 2018;8:1689–1696.
14. Cummings AL, Garon EB. KEYNOTE-042 rolls back programmed cell death ligand 1 threshold for non-small cell lung cancer pembrolizumab monotherapy without new insight into those deriving benefit. Transl Lung Cancer Res. 2019;8:S403–S406. doi:10.21037/tlcr.2019.07.06
15. Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer. 2021;148:2313–2320. doi:10.1002/ijc.33399
16. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: checkMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14:867–875. doi:10.1016/j.jtho.2019.01.006
17. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi:10.1200/JCO.2013.51.4802
18. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi:10.1056/NEJMc1808251
19. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005
20. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi:10.1056/NEJMoa1716948
21. Martinez Marti A, Martinez P, Navarro A, et al. Concordance of PD-L1 expression by different immunohistochemistry (IHC) definitions and in situ hybridization (ISH) in squamous cell carcinoma (SCC) of the lung. J Clin Oncol. 2014;32:7569.
22. Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29:193–199. doi:10.1093/annonc/mdx636
23. Cheng Y, Wang T, Lv X, et al. Detection of PD-L1 expression and its clinical significance in circulating tumor cells from patients with non-small-cell lung cancer. Cancer Manag Res. 2020;12:2069–2078. doi:10.2147/CMAR.S245425
24. Jilaveanu LB, Shuch B, Zito CR, et al. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer. 2014;5:166–172. doi:10.7150/jca.8167
25. Cordonnier M, Nardin C, Chanteloup G, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9:1710899. doi:10.1080/20013078.2019.1710899
26. Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi:10.1158/1078-0432.CCR-10-0250
27. Okuma Y, Wakui H, Utsumi H, et al. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer. 2018;19:410–417.e411. doi:10.1016/j.cllc.2018.04.014
28. Funt S, Snyder Charen A, Yusko E, et al. Correlation of peripheral and intratumoral T-cell receptor (TCR) clonality with clinical outcomes in patients with metastatic urothelial cancer (mUC) treated with atezolizumab. J Clin Oncol. 2016;34:3005.
29. Pan ZK, Ye F, Wu X, et al. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7:462–470. doi:10.3978/j.issn.2072-1439.2015.02.13
30. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi:10.1093/annonc/mdu242
31. D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi:10.1038/bjc.2014.555
32. Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi:10.1016/j.lungcan.2015.05.007
33. Hsu KH, Huang YH, Tseng JS, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naïve advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer. 2019;127:37–43. doi:10.1016/j.lungcan.2018.11.021
34. Ma L, Lv J, Dong Y, et al. PD-L1 expression and its regulation in lung adenocarcinoma with ALK translocation. Interdiscip Sci. 2019;11:266–272. doi:10.1007/s12539-019-00331-0
35. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–162. doi:10.1097/JTO.0000000000000033
36. Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi:10.1002/cncr.24181
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.