Back to Journals » Cancer Management and Research » Volume 12
The Clinical Impacts of Pretreatment Peripheral Blood Ratio on Lymphocytes, Monocytes, and Neutrophils Among Patients with Laryngeal/Hypopharyngeal Cancer Treated by Chemoradiation/Radiation
Authors Chuang HC , Tsai MH , Lin YT , Chou MH, Huang TL, Chiu TJ , Lu H , Fang FM, Chien CY
Received 10 August 2020
Accepted for publication 4 September 2020
Published 25 September 2020 Volume 2020:12 Pages 9013—9021
DOI https://doi.org/10.2147/CMAR.S275635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Hui-Ching Chuang,1,2 Ming-Hsien Tsai,1,2 Yu-Tsai Lin,1,2 Ming-Huei Chou,3,4 Tai-Lin Huang,2,5 Tai-Jan Chiu,2,5 Hui Lu,1 Fu-Min Fang,2,6 Chih-Yen Chien1,2,7
1Department of Otolaryngology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 2Kaohsiung Chang Gung Head and Neck Oncology Group, Cancer Center, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 3The Graduate Institute of Clinical Medical Sciences, Chang Gung University College of Medicine, Kaohsiung, Taiwan; 4Center for General Education, Cheng-Shiu University, Kaohsiung, Taiwan; 5Department of Hematology-Oncology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 6Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan; 7Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Correspondence: Chih-Yen Chien Email [email protected]
Purpose: This study aimed to investigate the clinical impacts of the pretreatment peripheral blood ratios of lymphocytes, monocytes and neutrophils among patients with hypopharyngeal cancer/laryngeal cancer.
Patients and Methods: A total of 141 people with cases of hypopharyngeal cancer/laryngeal cancer were enrolled to evaluate the clinical impacts of the systemic inflammation response index (SIRI), neutrophil–lymphocyte ratio (NLR) and lymphocyte–monocyte ratio (LMR) in pretreatment blood among patients with laryngeal/hypopharyngeal cancer between January 2012 and December 2014.
Results: Those patients with higher pretreatment LMR (> 2.99) showed a significantly higher 5-year complete response rate (CR) (69% vs 31%) than those with lower LMR (≤ 2.99, p = 0.006). Additionally, those patients with lower pretreatment SIRI (< 3.26) showed a significantly higher 5-year CR (90% vs 10%) than those with higher SIRI (≥ 3.26, p < 0.001). Patients with higher LMR had better 5-year overall survival (OS) (p = 0.01) and 5-year progression-free (PFS) (p = 0.005) rates than those with lower LMR in univariate analysis. Patients with lower SIRI had better 5-year OS (p < 0.001) and 5-year PFS (p < 0.001) than those with higher SIRI in univariate analysis. In the Cox regression analysis, SIRI (HR = 1.941, [95% CI: 1.223– 3.081], p = 0.005) and N classification (HR = 2.203, [95% CI: 1.327– 3.657], p = 0.002) were independent variables of 5-year OS. In addition, SIRI (HR= 2.127, [95% CI: 1.214– 3.725], p = 0.008), T classification (HR = 2.18, [95% CI: 1.072– 4.433], p = 0.031), and N classification (HR = 2.329, [95% CI: 1.395– 3.889], p = 0.001) were independent variables of 5-year PFS.
Conclusion: Pretreatment SIRI is superior to LMR in predicting treatment response and clinical outcomes among patients with laryngeal/hypopharyngeal cancer treated by CRT/RTO. SIRI may be adopted in the treatment of laryngeal/hypopharyngeal cancer by CRT/RTO.
Keywords: head and neck squamous cell carcinoma, hypopharyngeal cancer, laryngeal cancer, systemic inflammation response index, neutrophil–lymphocyte ratio, lymphocyte–monocyte ratio
Introduction
The host immune reaction is important in cancer’s development and is related to treatment outcomes. Infiltrating CD4+ T cells can show their anti-tumor response either by helping CD8+ T cells to recognize and process the antigens in the cancer cells for further immune response1 or by direct cytotoxicity to kill cancer cells.2 Monocytes make up only about 5% of circulating white blood cells in humans. Macrophages are the differentiated form of monocytes and have many functions in human tissue. Macrophages in internal organs present a lower activation threshold and are considered to be responsible for the induction of systemic inflammation in response to blood-borne pathogens, which can have deadly consequences in the case of sepsis.3 In addition, macrophages also have the role of either promoting tumor development or suppressing function in tumor progression.4,5 Nonetheless, in recent studies, tumor-associated macrophages (TAM) were reported to increase the tumor’s progression, including invasion, migration and angiogenesis.6,7 The neutrophil–lymphocyte ratio (NLR; N/L) reflects the status of systemic inflammation, which may be related to tumor progression and even prognosis. Several immune-related variables, such as NLR8,9 and the platelet–lymphocyte ratio10 in peripheral blood before treatment, were found to be relevant to clinical outcomes in head and neck squamous cell carcinoma (HNSCC) in past studies. The combination of neutrophils (N), monocytes (M) and lymphocytes (L) to form the systemic inflammation response index (SIRI), which Qi et al introduced in 2016 as the absolute count of N × M/L, could predict the prognostic impact in pancreatic cancer.11 SIRI has also been recently found to be an independent prognosticator of overall survival in oral cancer.12 So far, the lymphocyte-related immune response to the tumor in the tumor micro-environment is attractive in the treatment of cancer in the era of immune oncology. Our study aimed to evaluate the clinical impacts of SIRI, NLR and the lymphocyte–monocyte ratio (LMR; L/M) among patients with laryngeal cancer/hypopharyngeal cancer treated by upfront CRT/RTO.
Patients and Methods
Patients and Hematological/Clinical Factors
Patients diagnosed with hypopharyngeal or laryngeal cancer without distant metastasis who underwent chemoradiation (CRT) as primary treatment between January 2012 and December 2014 at Kaohsiung Chang Gung Memorial Hospital were included in this retrospective study from an institutional cancer database. All these patients showed pathological proof of squamous cell carcinoma. Patients who had evidence of acute infection and/or hematologic disorders were excluded. The study ultimately included 141 cases. Pretreatment white blood cell count (WBC) was obtained within 2 weeks before radiotherapy or induction chemotherapy. LMR was calculated from this pretreatment WBC as the absolute count of lymphocytes (L) divided by the absolute count of monocytes (M). The NLR was calculated by dividing the absolute count of neutrophils (N) by the absolute count of lymphocytes (L). In addition, the pretreatment systemic inflammation response index (SIRI),10 which is calculated as absolute count of neutrophils (N) × absolute count of monocytes (M)/absolute count of lymphocytes (L), was also used for analysis. The clinical tumor staging system was based on the American Joint Committee on Cancer (AJCC) 8th edition. This study was approved by the Medical Ethics and Human Clinical Trial Committees at Chang Gung Memorial Hospital and was assigned the number 202000103B0. The requirement to obtain informed consent of every participant was waived by our ethics committee because it was a retrospective study, which was in accordance with the ethical standards of the Helsinki Declaration. All patient data accessed complied with relevant data protection and privacy regulations.
All the treatments were based mostly on the American National Comprehensive Cancer Network (NCCN) guidelines. Intensity-modulated radiation therapy (IMRT) was applied to all the patients in our study. The dose at the gross tumor and node was prescribed as a median dose of 70.0 Gy (range 66.0–72.0 Gy), a daily fraction size of 1.8 Gy–2.2 Gy and five fractions per week. During the IMRT courses, cisplatin was prescribed concurrently. Fifty-five patients (38.7%) also received two to three cycles of induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) regimens before starting IMRT. Modified doses of the regimens in induction chemotherapy were used to reduce severe bone marrow toxicity and increase tolerability in our patients, as reported in our previous publication.13 After CRT/RTO, the tumor response was assessed according to the criteria of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.14 If residual tumor was noted in images from positron emission tomography (PET) scan, CT scan or MRI around three months after CRT/RTO, salvage surgery was subsequently performed for operable patients.
Statistical Analysis
The duration of overall survival (OS) was measured from the date of diagnosis to the date of death (of any cause) or last follow-up date. Progression-free survival (PFS) was measured from the date of diagnosis to the date on which recurrence was found at follow-up. Cox regression analysis was performed univariately for the association of clinical factors and survival outcomes. The optimal cutoff levels for LMR and SIRI were determined by applying receiver operating curve (ROC) analysis. The Kaplan–Meier curves were applied to analyze the association between the clinical features and NLR, LMR and SIRI for OS and PFS. Survival curves of different NLR, LMR and SIRI levels were compared by the Log rank test. In the multivariate Cox regression analysis, the model was adjusted for any prognostic clinical factors significantly associated with OS and PFS in previous Kaplan–Meier analysis. All statistical analyses were performed using the Statistical Package for Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA). A two-sided p-value < 0.05 was considered to be statistically significant.
Results
Baseline patient characteristics and clinical factors are shown in Table 1. The median age at which patients were diagnosed with cancer was 56 years (range 28 to 87 years). The median follow-up time was 45.8 months (range 3 to 91 months). Sixty-one (43.3%) of the 141 patients had their tumors relapse, and 67 (47.5%) died in the follow-up period; of these, 54 (80.6%) died of the disease. The optimal cutoff value for LMR was found by applying ROC analysis to be 2.99 for 5-year PFS, and the area under the curve was 64.9% (Figure 1A). The optimal cutoff value for SIRI was found by applying ROC analysis to be 3.26 for 5-year OS, and the area under the curve was 61.3% (Figure 1B).
 |
Table 1 Baseline Patient Characteristics and Clinical Outcome (n=141) |
Since only pretreatment LMR and SIRI, and not NLR, were significantly associated with 5-year PFS and 5-year OS, we emphasized these two factors in our further investigation. We found that LMR had a significant association with T classification (p= 0.002), while SIRI was significantly associated with T classification (p= 0.002) and TNM stage (p= 0.028) (Table 2). Regarding the complete response rate to CRT/RTO in this cohort, those patients with advanced TNM (stage IVB vs stage I, II, III, IVA, p < 0.001), advanced T classification (T3, T4a/b vs T1, T2, p = 0.002), advanced N classification (N3b vs N0/1/2/3a, p = 0.001), higher SIRI (≥3.26 vs <3.26; p < 0.001) and lower LMR (≤2.99 vs >2.99; p = 0.006) showed a significantly less complete response rate to CRT/RTO (Table 3).
 |
Table 2 Analysis of LMR and SIRI Associated to Tumor Characteristics (n=141) |
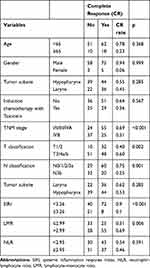 |
Table 3 Complete Response Rate for Classified Variables (n=141) |
In the univariate analysis of hematological and clinical factors associated with survival outcome (5-year OS and 5-year PFS), patients with higher pretreatment LMR (>2.99) were associated with significantly better 5-year PFS (p = 0.005; Table 4; Figure 2A) and 5-year OS (p = 0.01; Table 4; Figure 2B) than those with lower LMR (≤2.99) in Kaplan–Meier analysis. Moreover, patients with lower pretreatment SIRI (<3.26) were associated with significantly better 5-year PFS (p < 0.001; Table 4; Figure 3A) and 5-year OS (p < 0.001; Table 4; Figure 3B) rates than those with higher SIRI (≥3.26) in Kaplan–Meier analysis.
 |
Table 4 Kaplan–Meier Analysis of Prognostic Factors Associated with PFS and OS (n=141) |
Since SIRI has a more significant association than LMR with 5-year OS and PFS, SIRI, rather than LMR, was put into Cox regression analysis to enable us to analyze the independent prognosticators of 5-year OS and PFS in this cohort. The analysis showed that the hazard of 5-year PFS for patients with higher SIRI (≥3.26) was 2.217 times that of patients with lower SIRI (<3.26) (95% confidence interval (CI): 1.214–3.725, p = 0.008) after adjusting the T classification (T3/4a/b vs T1/2, hazard ratio (HR) = 2.18, 95% CI: 1.072–4.433, p = 0.031) and N classification (N3b vs N0/1/2/3a, HR = 2.329, 95% CI: 1.395–3.889, p = 0.001) (Table 5) according to Cox regression analysis. Furthermore, both SIRI (≥3.26 vs SIRI < 3.26) (HR = 1.941, 95% CI: 1.223–3.081, p = 0.005) and N classification (N3b vs N0/1/2/3a) (HR = 2.203, 95% CI: 1.327–3.657, p = 0.002) were independent prognosticators of 5-year OS in the Cox regression analysis (Table 6).
 |
Table 5 Cox Regression Analyses of SIRI Adjusted by Independent Prognostic Factors Associated with Progression-Free Survival (n=141) |
 |
Table 6 Cox Regression Analyses of SIRI Adjusted by Other Independent Prognostic Factors Associated with Overall Survival (n=141) |
Discussion
There are several reports about the prognostic value of LMR in a variety of malignancies, including lymphoma, colorectal cancer, lung cancer, hepatoma, renal cell carcinoma, esophageal cancer, cervical cancer, breast cancer and gastric cancer.15–24 Generally, patients with higher LMR before these cancer treatments showed better clinical outcomes. However, there are very few reports discussing the prognostic value of pretreatment LMR in head and neck cancer in the context of primary radiation in nasopharyngeal carcinoma (NPC)25 and HNSCC.26 These studies showed that patients with lower LMR before treatment tended to have worse OS. These tumors may reflect the advanced stage of lower LMR. Our data consistently showed that patients with lower pretreatment LMR had significantly more advanced T classifications, worse complete response rates in the CRT/RTO and worse 5-year PFS 5-OS than those patients with higher pretreatment LMR. The standard treatment modality of NPC is based on RTO/CRT. Our cohort also underwent these treatments. A study from China showed that those patients with NPC that had lower LMR, N2/N3 classification and lymphocytes had poorer 5-year OS.25 The data in our current cohort are consistent with these results of worse PFS and OS in patients with lower LMR and advanced nodal stage. Another meta-analysis study about prognostic impact of LMR in HNSCC is worth mentioning. The authors enrolled 4260 cases from seven cohorts. The pooled data demonstrated that elevated LMR was associated with significantly improved OS and DFS.27
Pretreatment SIRI is a recently proposed factor that has been used to evaluate the clinical outcomes of cancer treatment. Patients with lower SIRI before surgical treatment showed a better OS than those patients with higher one in esophageal cancer.28 Additionally, among patients with clear cell renal cell carcinoma (CCRCC), those with lower SIRI levels had better overall survival and cancer-specific survival compared to those with higher ones. SIRI might be a better prognostic predictor than NLR, LMR, and MSKCC score in patients with localized or locally advanced CCRCC.29 Recent published papers regarding the clinical impacts of SIRI in head and neck cancer have demonstrated the effectiveness of this variable.12,30,31 Valero et al showed that patients with preoperative lower SIRI level had better overall survival rates in cases of oral cancer. Moreover, this study was confirmed by the external validation.12 Lin et al showed that preoperative SIRI is valuable in the prediction of the survival of oral cancer patients who have undergone surgical intervention. Patients with lower SIRI levels had significantly decreased risk of mortality compared to those with higher ones.30 Valero et al also reported another study with 824 cases of HNSCC treated by either primary surgery or primary radiation. This study also showed the similar result that the disease-specific survival would increase if SIRI level decreased, and the authors concluded that SIRI was a significant predictor of local, regional, and distant recurrence-free survival.31
Pretreatment NLR is not significantly associated with tumor stage, N classification, T classification, 5-year OS or PFS in our cohort with treatment based on CRT/RTO, although some reports have shown that pretreatment NLR is significantly related to surgical outcomes in cases of oral cancer.8,9 NLR = N/L; SIRI = N x M/L. The NLR and SIRI are related variables that are confounding statistically. The prognostic significance of NLR among patients underwent radiation for HSNCC has previously been mentioned.32,33 Monocytes also play a role in the immune response in the tumor microenvironment. When they were incorporated with SIRI in current study, the complete response rate of laryngeal cancer or hypopharyngeal cancer to radiation/chemoradiation increased to 90%. Therefore, it would be reasonable to use SIRI as the variable for outcomes analyses.
The proportion of subtypes of both lymphocytes and monocytes may be important in the treatment response after radiation or chemoradiation. However, the subtypes of lymphocytes and monocytes were not available for this cohort because this was a retrospective study. More prospective studies are necessary to answer this question. Interestingly, patients’ chronic inflammatory status was likely to affect clinical outcomes, but there were no instances of immune-related nephritis, syphilis or rheumatoid arthritis in this cohort. However, there were 12 cases of hepatitis B carrier (HBV) and hepatitis C carrier (HCV) identified in this cohort. These 12 patients were used for outcomes analyses. We found there were no clinical impacts on 5-year OS (p=0.513), PFS (p=0.585) or complete response rates (p=0.271) among patients who were HCV or HBV carriers. SIRI could more precisely predict the treatment outcomes in this cohort than the use of LMR and NLR. So far, our study is the pioneer in investigating the clinical impacts of SIRI in hypopharyngeal cancer/laryngeal cancer treated by CRT/RTO and has novel findings. However, the drawback of our study is that it is retrospective, and selective bias may exist. More studies and larger series of patients are necessary to confirm our findings.
Conclusion
Pretreatment SIRI is superior to LMR and NLR in predicting treatment response and clinical outcomes among patients with laryngeal/hypopharyngeal cancer treated by CRT/RTO. Patients with lower pretreatment SIRI (<3.26) showed a significantly better clinical complete response rate (90% vs 10%) than those with higher SIRI (≥3.26). According to Cox regression analysis based on this cohort, pretreatment SIRI is also an independent prognostic factor that predicts 5-year OS and 5-year PFS. Interestingly, it may be possible to incorporate pretreatment SIRI into the treatment strategy for patients with hypopharyngeal cancer/laryngeal cancer undergoing CRT/RTO in the future.
Disclosure
The authors have no conflicts of interest to disclose for this work.
References
1. Friedman KM, Prieto PA, Devillier LE, et al. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35(5):400–408. doi:10.1097/CJI.0b013e31825898c5
2. Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4 (+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi:10.1084/jem.20091918
3. Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi:10.1136/bmj.i1585
4. Hutterer GC, Pichler M, Chromecki TF, et al. Tumour-associated macrophages might represent a favourable prognostic indicator in patients with papillary renal cell carcinoma. Histopathology. 2013;63(3):309–315. doi:10.1111/his.12163
5. He KF, Zhang L, Huang CF, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632. doi:10.1155/2014/838632
6. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi:10.1016/j.cell.2006.01.007
7. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi:10.1038/nrc1256
8. Fang HY, Huang XY, Chien HT, et al. Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope. 2013;123(11):2690–2699.
9. Yang Y, Liu R, Ren F, Guo R, Zhang P. Prognostic and clinicopathological significance of neutrophil-to-lymphocyte ratio in patients with oral cancer. Biosci Rep. 2018;38(6):BSR20181550. doi:10.1042/BSR20181550
10. Rassouli A, Saliba J, Castano R, Hier M, Zeitouni AG. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck. 2015;37(1):103–110. doi:10.1002/hed.23567
11. Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
12. Valero C, Zanoni DK, McGill MR, et al. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer. 2020;126(5):994–1003. doi:10.1002/cncr.32591
13. Li SH, Lin WC, Huang TL, et al. Significance of mammalian target of rapamycin in patients with locally advanced stage IV head and neck squamous cell carcinoma receiving induction chemotherapy with docetaxel, cisplatin, and fluorouracil. Head Neck. 2016;38(Suppl 1):E844–52. doi:10.1002/hed.24111
14. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
15. Prochazka V, Pytlik R, Janikova A, et al. A new prognostic score for elderly patients with diffuse large B-cell lymphoma treated with R-CHOP: the prognostic role of blood monocyte and lymphocyte counts is absent. PLoS One. 2014;9(7):e102594. doi:10.1371/journal.pone.0102594
16. Markovic O, Popovic L, Marisavljevic D, et al. Comparison of prognostic impact of absolute lymphocyte count, absolute monocyte count, absolute lymphocyte count/absolute monocyte count prognostic score and ratio in patients with diffuse large B cell lymphoma. Eur J Intern Med. 2014;25(3):296–302. doi:10.1016/j.ejim.2014.01.019
17. Neofytou K, Smyth EC, Giakoustidis A, et al. The preoperative lymphocyte-to- monocyte ratio is prognostic of clinical outcomes for patients with liver-only colorectal metastases in the neoadjuvant setting. Ann Surg Oncol. 2015;22(13):4353–4362. doi:10.1245/s10434-015-4481-8
18. Go SI, Kim RB, Song HN, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31(12):323. doi:10.1007/s12032-014-0323-y
19. Song W, Tian C, Wang K, Zhang RJ, Zou SB. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: a meta-analysis. Sci Rep. 2017;18(7):46601. doi:10.1038/srep46601
20. Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2014;32(7):1041–1048. doi:10.1016/j.urolonc.2014.04.001
21. Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8(8):137–145.
22. Chen L, Zhang F, Sheng XG, Zhang SQ. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8(8):1355–1362.
23. Ni XJ, Zhang XL, Ou-Yang QW, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9(11):e111886. doi:10.1371/journal.pone.0111886
24. Zhou X, Du Y, Xu J, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour Biol. 2014;35(11):11659–11666. doi:10.1007/s13277-014-2504-x
25. Jiang R, Cai XY, Yang ZH, et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015;34(6):237–246. doi:10.1186/s40880-015-0025-7
26. Kano S, Homma A, Hatakeyama H, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2017;39(2):247–253. doi:10.1002/hed.24576
27. Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(7):1663–1670.
28. Geng Y, Zhu D, Wu C, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018;65:503–510. doi:10.1016/j.intimp.2018.10.002
29. Chen Z, Wang K, Lu H, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2019;11:909–919. doi:10.2147/CMAR.S186976
30. Lin J, Chen L, Chen Q, et al. Prognostic value of preoperative systemic inflammation response index in patients with oral squamous cell carcinoma: propensity score-based analysis [published online ahead of print, 2020 Jul 18]. Head Neck. 2020. doi:10.1002/hed.26375
31. Valero C, Pardo L, Sansa A, et al. Prognostic capacity of systemic inflammation response index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck. 2020;42(2):336–343. doi:10.1002/hed.26010
32. Kim DY, Kim IS, Park SG, Kim H, Choi YJ, Seol YM. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx. 2017;44(2):199–204. doi:10.1016/j.anl.2016.05.013
33. Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol. 2016;118(2):330–334. doi:10.1016/j.radonc.2015.10.029
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



