Back to Journals » Drug, Healthcare and Patient Safety » Volume 15
The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection – A Systematic Review
Authors Knudsen MJS , Rubin IMC , Petersen AM
Received 8 November 2022
Accepted for publication 11 March 2023
Published 21 March 2023 Volume 2023:15 Pages 63—71
DOI https://doi.org/10.2147/DHPS.S348501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Rajender R Aparasu
Maja Johanne Søndergaard Knudsen,1,* Ingrid Maria Cecilia Rubin,1,* Andreas Munk Petersen1– 3
1Department of Clinical Microbiology, Copenhagen University Hospital – Amager and Hvidovre, Hvidovre, Denmark; 2Department of Gastroenterology, Copenhagen University Hospital – Amager and Hvidovre, Hvidovre, Denmark; 3Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
*These authors contributed equally to this work
Correspondence: Maja Johanne Søndergaard Knudsen, Email [email protected]
Introduction: The aim of this systematic review of randomized clinical trials (RCTs) was to examine the efficacy, safety, and tolerability of vancomycin for treatment of recurrent Clostridioides difficile infection (rCDI).
Methods: The PubMed database was searched from inception to August 23, 2022. An initial screening was performed followed by a full-text evaluation of the papers. Inclusion criteria were RCTs investigating vancomycin for treatment of rCDI.
Results: A total of six studies and 269 patients were included in the review. Three studies used a fixed dose regimen of vancomycin, one study used pulse regimen, one study used a taper-and-pulse regimen, and one study used a taper-and-pulse regimen for the participants with two or more recurrences. The resolution of infection varied from 19% to 58.3% in five of six studies reporting this as an outcome. Four out of six studies reported new episodes of rCDI as an intervention outcome, in those studies 50– 63% of participants experienced rCDI. Regarding the safety and tolerability of vancomycin treatment for rCDI, one study described several adverse events regarding gastrointestinal discomfort along with fatigue and skin rash. There were no records of serious adverse events in the included studies.
Conclusion: While oral vancomycin is mostly safe and well tolerated in the RCTs reviewed here, the efficacy for treating rCDI varies greatly from 19– 58.3%, and 50– 63% of participants experienced new episodes of rCDI.
Keywords: Clostridioides difficile, infection, diarrhoea, recurrent, vancomycin
Introduction
Clostridioides difficile infection (CDI) is an important cause of nosocomial diarrhoea associated with the use of antibiotics.1–3 Particularly geriatric patients with comorbidities and recent treatment with antibiotics are at risk of contracting CDI.4,5 Infection with C. difficile can also be community-acquired. The clinical presentation of CDI varies between patients, from cases of mild diarrhoea to severe diarrhoea, sometimes further complicated by pseudomembranous colitis, sepsis or toxic megacolon resulting in an increased mortality.6–8
Although most patients with CDI are treated successfully after the first episode, recurrent Clostridioides difficile infection (rCDI) occurs in 20–25% of the patients.2,9,10 The percentage of patients with rCDI increases with the number of recurrences, thus 40% of patients with one recurrence get another recurrence, and for patients with two or more recurrences the number is as high as 60%.9 Also, the mortality rate is higher for patients who develop recurrence, increasing from 26% for primary CDI to 36% for rCDI.11 Risk factors for contracting rCDI are antacid medication, older age, longer hospital stays, and continuous use of non-CDI antibiotic treatment.12,13 Regarding antibiotic treatment, third-generation cephalosporins pose the greatest risk for developing hospital acquired CDI.14 Recurrent CDI also has a high negative impact on the health-care system, increasing average length of hospital stay and treatment costs.15 Taken together, efficient treatments for rCDI are needed.
Vancomycin has been a cornerstone in the treatment of CDI, both the first episode as well as recurrences. Recently both the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the American counterpart, the Society for Healthcare Epidemiology of America, and the Infectious Diseases Society of America (SHEA/IDSA), have published updated treatment recommendations for rCDI, where the role of vancomycin for recurrent CDI has been modified and in some cases replaced by other treatment modalities.16,17 Nevertheless, a fixed dose of vancomycin can still be used for the initial CDI, and for treatment of rCDI a taper-and-pulse regimen of vancomycin can be used. However, these are not the first-in-line treatments. A taper regimen of vancomycin is an extended period of treatment with progressive decrease of the dose. A pulse regimen of vancomycin is also an extended period of treatment with some skipped days between doses. A taper-and-pulse regimen usually starts with a taper regimen and continues with a pulse regimen.18
Recurrent CDI can be due to either reinfection or relapse. Regarding reinfection, this is a new episode of CDI, whereas relapse is a continuation of a passed episode. A method of examining whether an episode of CDI is due to reinfection or relapse, would be to perform whole genome sequencing (WGS). If WGS is not available or not performed routinely, it can be investigated if one or both samples have toxins A/B, or they have different ribotypes. If they differ on those parameters, they are likely to represent a reinfection. One of the natural causes of relapse of CDI is remaining C. difficile spores in the intestine. A study by Housman et al investigated the efficacy of vancomycin and fidaxomicin in reduction of spore counts and found that fidaxomicin had greater efficacy than vancomycin in clearing C. difficile spores.19
In this study, we aim to perform a systematic review of randomized clinical trials (RCT) on treatment of rCDI, where vancomycin in monotherapy was used in one of the treatment arms. The objective of this review is to investigate the efficacy, safety, and tolerability of vancomycin in monotherapy for treatment of rCDI. Although a widely used treatment, there are only a few studies examining vancomycin in monotherapy for rCDI, and there is a lack of evidence on this topic.
Methods
Search Strategy
We searched PubMed database from inception to August 23, 2022. The decision to perform the search in PubMed only was made based on a study by Halladay et al.20 Vancomycin was defined as vancomycin (text word), vancocin (text word), and vancomycin (MeSH terms). Clostridioides difficile infection was defined as Clostridioides difficile infection (text word), Clostridium difficile infection (text word), C. difficile infection (text word) and Clostridium infection (MeSH terms). Recurrent was defined as recurrent (text word). For the full search strategy, please refer to Supplementary Document 1.
Two authors independently selected the studies in a two-stage process, first a screening of titles and abstracts was performed, whereafter a final decision of inclusion was performed after a full-text evaluation (MJSK and IMCR). All disagreements were settled by a third author (AMP).
Inclusion/exclusion of studies
We included RCT in which only participants with recurrent C. difficile infection were included. The RCT ought to clearly state the efficacy of vancomycin as a treatment in one of the study arms.
During the initial screening of records, we excluded reviews, guidance/recommendations, case reports, non-English articles (Hungarian, Spanish, French, German, Czech, Serbian, Chinese, Hebrew, Finnish, Portuguese), retrospective/prospective cohort studies, and records unrelated to study subject. Furthermore, studies concerning primary C. difficile infection were excluded. The remaining records were included if they met the inclusion criteria during the full text eligibility assessment.
Quality Assessment
We adapted the Quality Assessment Tool of the National Institutes of Health for randomized clinical trials to assess the risk of bias of the individual studies with 14 different criteria. Criteria 9, 13 and 14 were adapted to include compliancy and cross-over trials, respectively. (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). For each study and criteria, the bias was reported as, high risk, low risk or unclear risk. Additionally, the table used in the bias assessment process is found in Supplementary Document 2.
Assessment of Safety and Tolerability
The safety of vancomycin was evaluated based on the description of serious adverse events in the included studies. The tolerability of vancomycin was assessed according to non-serious adverse events.
Results
Study Selection
A total of 448 records were identified through the literature search. During the initial screening, 433 records were excluded, and 15 records proceeded to screening for eligibility. A total of seven records were excluded due to study design other than randomized clinical trials, studies not stating the effect of vancomycin treatment, or randomized clinical trials investigating the effect of vancomycin on primary C. difficile. In addition, one record was not retrieved, and another record was a protocol for a future clinical trial. Therefore, six articles1–3,6,22,23 with a total number of 269 participants were included and analysed in the study. An overview of the screening and inclusion of records can be found in Figure 1 adapted from the PRISMA statement.21
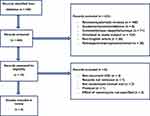 |
Figure 1 Shows the flow chart of inclusion and exclusion of records. Note: The flow chart is adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. Creative Commons.21 |
Description of Studies
Six randomized clinical trials were included in this review – a summary of each study is shown in Table 1. Two out of six were two-armed trials investigating the efficacy of FMT versus vancomycin. Three trials were three-armed, all of which were designed with FMT in one arm, vancomycin in another arm, and rectal bacteriotherapy, fidaxomicin or bowel lavage, respectively, in the third arm. Generally, in all these trials, a treatment with vancomycin of varying duration was administered prior to the faecal microbiota transplantation (FMT), rectal bacteriotherapy and bowel lavage interventions. The remaining trial investigated the efficacy of vancomycin in combination with Saccharomyces boulardii versus vancomycin in combination with placebo. The trials were relatively small and included each 12–31 participants in the vancomycin-only group. Four trials were conducted in Europe and two in North America. In two of the trials a total of 2g of vancomycin was administered daily, whereas four of the trials administered 500mg of vancomycin as the total daily dose. Three studies used a fixed dose regimen of vancomycin, one study used a pulse regimen, one study used a taper-and-pulse regimen, and one study used a taper-and-pulse regimen for the participants with two or more recurrences. The participants included in studies had between 2.4 and 4.4 recurrences of CDI. One study clustered the number of recurrences, in this study 42% of participants had two or more recurrences.6
 |
Table 1 Shows the results for All Included Studies |
Clinical Efficacy of Vancomycin
Five of the six studies reported resolution of infection as an intervention outcome. The number of participants cured varied between 19% and 58.3% in those studies. Four studies reported recurrence of CDI as an intervention outcome. In these studies, between 37% and 50% had no recurrences of CDI during the study period.
Tolerability and Safety
Four out of the six studies reported no adverse events related to vancomycin. One study reported no significant difference between the two intervention groups without further description. The last study described several adverse events regarding gastrointestinal discomfort along with fatigue and skin rash.
Risk of Bias
The result of the bias assessment is presented in Figure 2. Overall, the studies represented a low risk of bias on most criteria. A high risk of bias was seen in all studies but one, regarding blinding of participants and providers and the people assessing the outcome. Another criterion with high risk of bias or unclear risk of bias in all studies was power and sample size.
 |
Figure 2 Shows the risk of bias assessment on criteria 1–14 (refer to Supplementary Document 2). For each study, criteria with low risk of bias are colored green, high risk of bias are colored red, and unclear risk of bias are colored yellow. |
Discussion
Prevention of recurrences of CDI is a challenge and patients with one recurrence are at increased risk of further recurrences.24 Risk factors associated with oral vancomycin treatment are among others a perturbation of the gut microbiome and colonization with vancomycin-resistant Enterococcus faecium (VRE).25,26
According to the latest guidance documents from ESCMID, and SHEA/IDSA, fidaxomicin is now the first-choice treatment for primary CDI, and vancomycin remains second choice. Nonetheless, fidaxomicin is not available in all countries. Regarding the first rCDI adjunctive treatment with Bezlotoxumab (a monoclonal antibody against C. difficile toxin B) can be administered along with fidaxomicin or vancomycin. Concerning the second rCDI, FMT is recommended.16,17 This is after many clinical trials have shown inferiority of vancomycin monotherapy to fidaxomicin, FMT or to Bezlotoxumab.27–29
In four of the six studies included in this review, investigating the effect of vancomycin plus FMT versus vancomycin, vancomycin plus FMT showed superiority to vancomycin alone.2,3,6,23 In one study, vancomycin and placebo were compared to vancomycin and S. boulardii, where treatment with S. boulardii was initiated on the 7th day of vancomycin treatment and continued for 28 days.1 The combination of vancomycin and S. boulardii exhibited a significant decrease in recurrences of CDI compared to vancomycin alone. Thus, in five out of six studies, the intervention arm with vancomycin as monotherapy turned out to be inferior in comparison with the competing treatment arm. In one study, a taper-and-pulse regime of vancomycin proved non-inferior to vancomycin followed by FMT.22 In all six studies, participants receiving S. boulardii, FMT, rectal bacteriotherapy or bowel lavage also received vancomycin treatment for a various duration of time prior to the other intervention. Therefore, vancomycin is expected to have an effect in combination with another treatment for rCDI.
A limitation to evaluate the efficacy of vancomycin monotherapy in this review, is that three of the studies used a fixed dose vancomycin regimen of 10–14 days,1,2,23 instead of the recommended 10 days plus a taper-and-pulse regimen,3,6,22 therefore underestimating the actual efficacy of monotherapy vancomycin, as recommended by the guidelines above. Two of the three studies with a fixed vancomycin dose, had the lowest clinical resolution of 19% and 31%, respectively.2,23 The third study using a fixed high dose of vancomycin did not list clinical resolution as an endpoint, instead they used recurrence, and 50% of their participants had recurrence.1 One study used a pulse regimen of vancomycin, resulting in a clinical cure rate at 26%.3 The two studies with a taper-and-pulse regimen are those reporting the highest clinical cure rate of 45% and 58.5%, respectively.6,22 A systematic review and meta-analysis by Sehgal et al also found taper-and-pulse regimens of vancomycin to be superior to either pulse or taper regimens alone.18
Two of the six studies used a higher dose of vancomycin; 2g/day or 500mg four times a day.1,23 The remaining four studies used the standard dose of 125mg four times a day. A study by Gonzales et al showed that the concentration of vancomycin in faeces increased with a higher dose of vancomycin orally administered.30 Nonetheless, another study by Thabit et al propose that there is no association between the concentration of vancomycin in faeces and the clinical outcome.31 Therefore, the difference in dose of vancomycin administered in the six studies is not contradictory to a comparison of them.
Our review is limited by the number of only six included studies with high bias concerning study population and power. Another limitation is the different endpoints, where five studies used resolution rate, and one study used recurrence rate. Additionally, half of the studies used a fixed dose of vancomycin, whereas the other half used a pulse/taper-and-pulse regime. Finally, the time to follow-up in the six studies varied from 8 weeks to 120 days. We call for future studies, specifically examining treatments for rCDI, with adequate power and good study designs. This includes RCT, looking at non-antimicrobial options, such as S. boulardii, FMT or combinations of pro- and prebiotics, either as stand-alone treatments or in combination with vancomycin. With the advent of microbiota targeting-or preserving strategies, treatment for both CDI and rCDI are likely to change over to the coming years.
As there is still a lack of data on effective treatment for rCDI, prevention of primary CDI is crucial. As with other nosocomial infections, adherence to strict infection control guidelines, as well as antibiotic stewardships to reduce unnecessary antibiotic use play a very important role.32
Conclusion
While oral vancomycin is mostly safe and well tolerated in the RCTs reviewed here, the efficacy is low in treating recurrent CDI. The two studies reporting the lowest resolution rate had used a fixed dose of vancomycin as opposed to a taper-and-pulse regime. Our findings show a tendency of the taper-and-pulse regimen being superior to a fixed dose regimen or a pulse regimen. Vancomycin is also expected to have an effect in combination with another treatment for rCDI. To obtain more knowledge on treatment of rCDI with vancomycin in monotherapy of taper-and-pulse regimen, more studies with adequate power need to be conducted with comparison to other recommended treatments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent clostridium difficile disease: use of high‐dose vancomycin combined with saccharomyces boulardii. Clin Infect Dis. 2000;31(4):1012–1017. doi:10.1086/318130
2. Hvas CL, Dahl Jørgensen SM, Jørgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology. 2019;156(5):1324–1332.e3. doi:10.1053/j.gastro.2018.12.019
3. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843. doi:10.1111/apt.13144
4. Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi:10.1016/S0140-6736(10)61266-4
5. Garcia PG, Chebli LA, da Rocha Ribeiro TC, et al. Impact of superimposed Clostridium difficile infection in Crohn’s or ulcerative colitis flares in the outpatient setting. Int J Colorectal Dis. 2018;33(9):1285–1294. doi:10.1007/s00384-018-3105-8
6. Rode AA, Chehri M, Krogsgaard LR, et al. Randomised clinical trial: a 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Aliment Pharmacol Ther. 2021;53(9):999–1009. doi:10.1111/APT.16309
7. Guery B, Berger P, Gauzit R, et al. A prospective, observational study of fidaxomicin use for Clostridioides difficile infection in France. J Int Med Res. 2021;49(6):030006052110212. doi:10.1177/03000605211021278
8. Majors D, Ellis P. Risk factors for recurrent clostridium difficile infections and strategies to decrease readmissions in a community hospital. Hosp Pharm. 2015;50(11):1003–1010. doi:10.1310/hpj5011-1003
9. Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359(18):1932–1940. doi:10.1056/NEJMRA0707500
10. Du C, Luo Y, Walsh S, Grinspan A. Oral fecal microbiota transplant capsules are safe and effective for recurrent clostridioides difficile infection: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55(4):300–308. doi:10.1097/MCG.0000000000001495
11. Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21:2. doi:10.1016/j.cmi.2014.08.017
12. Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304. doi:10.1016/J.JHIN.2008.08.012
13. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:6. doi:10.1016/j.jinf.2009.03.010
14. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(4):881–891. doi:10.1093/jac/dkt477
15. Spencer RC. Clinical impact and associated costs of Clostridium difficile-associated disease. J Antimicrob Chemother. 1998;41(SUPPL.C):5–12. doi:10.1093/jac/41.suppl_3.5
16. Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–e1044. doi:10.1093/cid/ciab549
17. van Prehn J, Reigadas E, Vogelzang EH, et al. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27. doi:10.1016/j.cmi.2021.09.038
18. Sehgal K, Zandvakili I, Tariq R, Pardi DS, Khanna S. Systematic review and meta-analysis: efficacy of vancomycin taper and pulse regimens in clostridioides difficile infection. Expert Rev Anti Infect Ther. 2022;20(4):577–583. doi:10.1080/14787210.2022.1997588
19. Housman ST, Thabit AK, Kuti JL, Quintiliani R, Nicolau DP. Assessment of clostridium difficile burden in patients over time with first episode infection following fidaxomicin or vancomycin. Infect Control Hosp Epidemiol. 2016;37(2):215–218. doi:10.1017/ice.2015.270
20. Halladay CW, Trikalinos TA, Schmid IT, Schmid CH, Dahabreh IJ. Using data sources beyond PubMed has a modest impact on the results of systematic reviews of therapeutic interventions. J Clin Epidemiol. 2015;68(9):1076–1084. doi:10.1016/j.jclinepi.2014.12.017
21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. doi:10.1136/bmj.n71
22. Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis. 2017;64(3):265–271. doi:10.1093/cid/ciw731
23. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi:10.1056/NEJMoa1205037
24. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:7. doi:10.1016/S0002-9270(02)04195-3
25. Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008;52:7. doi:10.1128/AAC.00090-08
26. Sethi AK, Al-Nassir WN, Nerandzic MM, Donskey CJ. Skin and environmental contamination with vancomycin-resistant enterococci in patients receiving oral metronidazole or oral vancomycin treatment for clostridium difficile –associated disease. Infect Control Hosp Epidemiol. 2009;30:1. doi:10.1086/592710
27. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. N Engl J Med. 2011;364:5. doi:10.1056/nejmoa0910812
28. Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection a randomized trial. Ann Intern Med. 2016;165:9. doi:10.7326/M16-0271
29. Deeks ED. Bezlotoxumab: a Review in Preventing Clostridium difficile Infection Recurrence. Drugs. 2017;77:15. doi:10.1007/s40265-017-0809-y
30. Gonzales M, Pepin J, Frost EH, et al. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis. 2010;10(1):363. doi:10.1186/1471-2334-10-363
31. Thabit AK, Nicolau DP. Impact of vancomycin faecal concentrations on clinical and microbiological outcomes in Clostridium difficile infection. Int J Antimicrob Agents. 2015;46(2):205–208. doi:10.1016/j.ijantimicag.2015.03.016
32. Dubberke E. Strategies for prevention of Clostridium difficile infection. J Hosp Med. 2012;7(SUPPL. 3). doi:10.1002/jhm.1908
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
