Back to Journals » International Journal of General Medicine » Volume 13
The Clinical Approach on Receipt of an Unexpected Laboratory Test Result
Received 25 June 2020
Accepted for publication 28 September 2020
Published 29 October 2020 Volume 2020:13 Pages 969—976
DOI https://doi.org/10.2147/IJGM.S269299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Amina Masood,1 Mohammed Yousuf Karim2
1Locum General Practitioner, The Cambridge Practice, Aldershot, UK; 2Immunology Laboratory, Department of Pathology, Sidra Medicine, Doha, Qatar
Correspondence: Mohammed Yousuf Karim Department of Pathology, Sidra Medicine, Al Luqta St, Doha 26999, Qatar
Tel +974 4003 2968
Email [email protected]
Abstract: Approximately 70% of all healthcare decisions affecting diagnosis and treatment involve the use of tests performed within pathology laboratories. The utilisation of diagnostic laboratory services continues to increase, with growth both in volume of tests requested, as well as in the breadth of test repertoire. Every year in the United Kingdom, approximately 1 billion tests are run in hospital laboratories, equivalent to 14 tests per person. Fifty million tests are requested in primary care. Accordingly, there is an inevitable increase in the number of unexpected laboratory results which clinicians review. This is an important, and potentially time-consuming, issue, which we considered to merit a more detailed discussion. Unexpected laboratory results may be critical or non-critical in nature. They may be absolutely genuine, reflecting a clinical change in the patient’s condition, a differential diagnosis not previously considered, or an additional test specifically added by the laboratory. However, such results may also occur due to a variety of different circumstances, including much more rarely laboratory error. As there is little published evidence or guidance available, herein we discuss aspects of the clinical approach for physicians after receiving an unexpected laboratory test result.
Keywords: pathology, critical, non-critical, error, communication, diagnosis, assay
Introduction
It has been reported that approximately 70% of all healthcare decisions affecting diagnosis and treatment involve the use of tests performed within Pathology laboratories, though the exact figure is difficult to determine.1,2 The utilisation of diagnostic laboratory services continues to increase, with growth both in volume of tests requested, as well as in the breadth of test repertoire. Every year in the United Kingdom, approximately 1 billion tests are run in hospital laboratories within the National Health Service, equivalent to 14 tests per person. Fifty million reports are sent from laboratories to general practitioners.3 Accordingly, there is an inevitable increase in the number of unexpected laboratory results which physicians will receive, and need to act upon. This is an important, and potentially time-consuming, issue, which we considered to merit more detailed discussion as there is little published guidance available.4,5 In this article, we discuss aspects of the clinical approach after physicians receive an unexpected laboratory test result.
Causes of Unexpected Laboratory Results
Unexpected laboratory results can be either “critical”, ie test results that are significantly outside the normal (reference) range and which indicate an immediate dangerous or life-threatening state, or “non-critical”.6 This is the major factor which influences the nature and urgency of the clinician’s response. Unexpected test results may be absolutely genuine, reflecting a clinical change in the patient’s condition, a differential diagnosis not previously considered, or an additional test specifically added by the laboratory. However, such results may also occur due to a variety of different circumstances. The explanation can be related to the sample collection or the sample itself, related to the sensitivity/specificity of the particular test method, due to laboratory error, or from application of inappropriate age/sex reference ranges.7–11 Examples of a range of different causes of unexpected results are provided in Table 1. We have provided instances from the different laboratory disciplines, but of course this list is far from comprehensive.12–14
 |
Table 1 Causes and Clinical Examples of Unexpected Laboratory Results |
There are various clinical scenarios in which an unexpected laboratory result may occur, including:
- for an analyte with a normal distribution of values, 2.5% of normal individuals will have values above and below the reference range respectively.15
- a negative result in a patient with classic presenting features of a clinical diagnosis.
- one of the parameters in a group test is markedly abnormal, eg one parameter in a full blood count.15
- a major change in an in-patient test over a short time period, eg drop in haemoglobin but no obvious clinical evidence of bleeding.4
- in a clinically stable out-patient having regular bloods for monitoring a chronic condition, or for drug monitoring in primary care.
- results of tissue biopsy out of keeping with clinical presentation, and/or with other laboratory tests.
- false positive or false negative testing for infectious disease such as Hepatitis C or HIV.5,14
- there are abnormal results on the patient sample which were not ordered by the doctor.16
Reflex Testing
This is the “but I didn’t order this test” situation. Reflex tests may be specifically added by laboratory physicians or clinical scientists after reviewing initial results and clinical details.16
For some tests (subject to certain healthcare provider contractual arrangements), there are laboratory algorithms which generate “reflex” requesting of additional tests. For example, in the Immunology laboratory, the finding of fluorescent staining in the cytoplasm of an anti-nuclear antibody request might initiate testing of anti-mitochondrial antibodies.17 Thus, despite the nucleus itself being negative, the presence of cytoplasmic staining may lead to identification of “M2” anti-mitochondrial antibodies. These M2 antibodies have very strong association with present or future diagnosis of primary biliary cirrhosis (PBC).18 Indeed, laboratory-initiated protocols have also led to PBC diagnosis from finding unexplained high alkaline phosphatase activity, or elevated IgM concentrations.17
Repeat Testing
Repeated testing at frequent intervals in in-patients may sometimes produce what appear to be different results. There may also be relevance in primary care for patients on long-term blood monitoring. However, it is important to note that these differences are not always clinically significant, particularly over short periods. There may be acceptable biological variation, the patient may be in a different physical condition (eg hydration status, nutritional intake), and there is known imprecision in analyser performance.4,19 The last-mentioned relates to the concept of “uncertainty of measurement” which refers to a parameter associated with the result of a measurement, that characterises the dispersion of the values that could be reasonably attributed to the measurand.19 According to the ISO15189 accreditation standards, sources that contribute to uncertainty may include sampling, sample preparation, sample portion selection, calibrators, reference materials, input quantities, equipment used, environmental conditions, condition of the sample, and changes of operator.19,20 For example, in a patient with hyponatraemia, a sodium level of 129 mmol/L repeated 3 days later as 131 mmol/L may not necessarily indicate clinical improvement, but could reflect the expected clinical variability as well as the uncertainty of measurement. Assessing the trends of repeated results may be more informative to the clinician.
Marked changes in biochemical and haematological parameters such as haemoglobin or coagulation profiles can often arise due to incorrectly performed sampling procedures from intravenous lines or heparinised lines. Spuriously low platelet counts (pseudothromboctyopenia) may be caused by development of platelet clumps.21 This can relate to the collection method, more commonly seen with capillary venous blood collections and collections from lines. Inadequate mixing of the sample with anticoagulant can lead to microthrombi, and overfilling of the collection tube can lead to pseudothrombocytopenia. Platelet clumping may also be induced by viral infections and medications, including chemotherapeutic agents. In such cases, the laboratory will review the blood film and comment on the presence of platelet clumps, and may request a repeat sample if clinically indicated. There can be other explanations: for example, EDTA-induced pseudothrombocytopenia occurs in up to 0.1% of all full blood count samples and is usually caused by an EDTA-dependent platelet agglutinating antibody. This can be diagnosed by using an alternative sample collection tube such as sodium citrate or heparin, whereby the platelet count is normal.21
Laboratory Error
It is important for clinicians to keep in mind the possibility of laboratory error, though this is infrequent (approximately 0.33% of all tests). Over recent years, the quality of laboratory testing has improved significantly. For example, pre-analytical processes have benefited from electronic ordering, barcoding of primary samples, robot-assisted aliquoting, temperature monitoring of reagents, and automatic checks for clots, haemolysis, lipaemia, and hyperbilirubinaemia.7 The improvement has been most marked in analyser performance with highly standardised and low imprecision assays to such an extent that analytic errors are very rare.22 This overall improvement in the laboratory has also resulted from implementation of quality management systems, requirement for laboratory inspection and accreditation, subscription to external quality assurance schemes, and improvements in laboratory information management systems. Laboratory equipment is calibrated, with regular preventive maintenance, and corrective technical assistance in case of a fault. Quality controls (commercial and/or in-house) are used in each assay run, and validation between instruments to maintain reproducibility. Despite these measures, errors still do occur. Laboratory errors can be divided into pre-analytic, analytic, or post-analytic, depending on the stage of the process in which they occur: a range of examples is provided in Table 2. The vast majority of errors are now pre-analytic and post-analytic.22,23
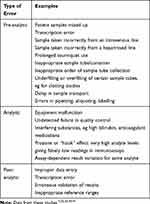 |
Table 2 Classification of Laboratory Errors |
There are important caveats regarding the ordering of certain tests. For example, testing for red cell disorders such as sickle cell disease, hereditary spherocytosis, or paroxysmal nocturnal haemoglobinuria should not be conducted soon after a blood transfusion (ideally not for 90 days).
Adequate training is essential for those collecting samples, including physicians and physician assistants, nursing staff, and phlebotomists. Common practical issues relate to taking blood from peripheral and central venous lines, and there are local protocols and international guidelines which should be followed for sample collection.24,25 There are important pre-analytic issues for other sample types, eg when collecting a bone marrow aspirate it is important not to collect excess volume, as the potential haemodilution impairs morphologic and flow cytometric evaluation.26
In the event of analytic error, many are detected before final result validation. However, according to the “Swiss cheese” model of errors, there remain a tiny number of incorrect analyser results which evade detection (variously estimated at 0.05–0.1% of all tests performed). This may lead to adverse events/patient harm in 0.001–0.01% of cases where such results are released to the clinician.23 All laboratory errors are documented and investigated using incident reporting systems, and corrective and preventive actions implemented to reduce risk of similar recurrence.
Communicating the Result
The Royal College of Pathologists of the UK published guidance in 2017 for its members on communication of critical and unexpected pathology results. This identifies which results need to be urgently communicated to clinicians, and the best routes for such interactions.27 Generally, laboratories have standard operating procedures (SOPs) whereby, in the event of a critical result, scientific or medical staff will immediately contact the requesting physician or appropriate clinical personnel responsible for the patient’s care. If they are unable to make contact, the SOP will indicate whom next to try to contact. Unfortunately, in around 5% of cases, the laboratory personnel are unable to communicate a critical result to a physician or appropriate healthcare professional, which can result in inappropriate or delayed treatment decisions.28 This is a particular issue for out-patients, and for primary care outside of working hours. Difficulties may also arise at the transition between in-patient and primary care, particularly when results are released after the patient’s discharge from hospital.29 For communication of unexpected results from out-patient departments, local agreements should be put in place which address the nature of the result, whom to contact, and how quickly. Although telephone communication remains the method of choice for transmitting critical results for both in- and out-patients, electronic alerts and acknowledgements are being adopted increasingly for non-critical results.30,31
Acting on the Result
In Box 1, we have summarised the steps which the physician could consider on receipt of an unexpected test result. Of course, assessing and deciding on management of a critical result is an urgent priority, as to whether the patient requires immediate intervention or treatment, and a flowchart is provided in Figure 1. Despite this, a retrospective study of critical laboratory in-patient test results reported median time from result availability to appropriate treatment was 150 minutes, with more than 1/4 of treatments delayed by ≥5 hrs.32 The major factor in such delays was in communicating the result to the primary decision maker – the physician caring for the patient – who did not always receive the information in a timely manner. Another factor was the presence of multiple disease processes, where secondary but serious conditions might not be treated until the primary problem had been controlled.32
 |
Box 1 Acting on an Unexpected Laboratory Test Result |
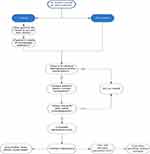 |
Figure 1 Flowchart illustrating clinical approach to an unexpected laboratory result. |
For both critical and non-critical unexpected results, it is often the case that the clinician receiving or viewing the result is not the same as the ordering clinician. This can occur when a patient has tests ordered by a physician in A&E, and then is admitted to the ward. It may affect junior doctors at changes of shift or out-of-hours, or covering for annual leave. Hence the physician may not be familiar with the clinical background, and may need to review this. Even for the ordering clinician viewing the result, an updated clinical assessment may be required. This may not only involve history and clinical examination, but exercising careful clinical judgement about the result. The clinician needs to consider whether the unexpected result aligns with those of other investigations, or whether further tests could help, including repeat testing. Consideration of sampling error or sample transport delay should not be overlooked.33,34 In some instances, discussing the unexpected result with the testing laboratory can be productive in reaching an explanation.
Abnormal electrolyte results are a common illustration of unexpected test reports, eg spurious hypercalcaemia may result due to prolonged tourniquet use.8 Another well-known example is the elevated potassium result, while all the other components of the renal profile are normal. In many cases, this is a spurious increase due to delay in sample transport, and can be identified as such in the laboratory, and this invalid result is not released. In cases where doubt remains and/or the abnormal result is released, it is essential for the clinician to act immediately, whether by considering the age of the sample, repeating the test, or considering whether the elevated potassium needs immediate treatment.34 Sample degradation resulting in haemolysis can also cause falsely elevated aspartate transaminase (AST) and phosphate levels. Hence the finding of raised potassium, AST, and phosphate in the same patient sample could be a pointer to consider haemolysis. Pre-analytical error can also arise from the conditions employed for sample transport, which may lead to false positive critical values. In a study from Boston, there was a 20–40% reduction in critical potassium values (>6.0 mmol/L) from out-patient clinics, after laboratory staff noted samples were being sent on ice rather than at room temperature.35,36
Good Communication is Essential
The laboratory handbook should be easily searchable, ideally web-based, and provide accurate, regularly updated, information regarding sample requirements and test interpretation. The handbook should inform the clinician of local laboratory policies, including for repeat testing, reflex testing, and the communication of critical and non-critical results. We cannot emphasise enough the importance of encouraging clear and easy communication channels between clinicians and laboratory consultant physicians/clinical scientists. There should be well publicised channels, eg telephone hotlines, laboratory emails which are checked 2–3 times daily, interactive websites. If necessary, early communication with the laboratory can often save time by either finding an explanation for an unexpected result, or by assisting in the management plan.33,34
Furthermore, after discussion with the clinician, the laboratory can review the result to consider the rare possibility of error, whether to repeat the test if the sample remains in the laboratory (by the same or a different method). The specimen can even be physically re-inspected to ensure no obvious changes might have been overlooked, eg gross haemolysis or hyperlipidaemia – though physical inspection is part of the laboratory SOP in any case.
Conclusion
With the paucity of guidance in the literature, here we have described the possible clinical approach to the receipt of such a result. Unexpected laboratory test results may be genuine, but in rare instances, they can result from laboratory error. They may be critical, or non-critical in nature, which is a main determinant of the physician response. The clinician should consider the context of the result in terms of the clinical background of the patient, and the results of other investigations. It is important to consider sampling error, or sample transport delay as potential explanations.33,34 We emphasise that early communication with the laboratory can be important, and may save clinician, laboratory, and patient time and concern in certain cases. Discussion with the laboratory can address the possibility of error, whether further tests could help, and whether there is a need for repeat testing.
Acknowledgments
Open Access funding provided by the Qatar National Library.
The authors would like to acknowledge Dr Diane Roscoe and Dr Basma Basha for their kind comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996;42(5):813–816. doi:10.1093/clinchem/42.5.813
2. Hallworth MJ. The ‘70% claim’: what is the evidence base? Ann Clin Biochem. 2011;48:487–488. doi:10.1258/acb.2011.011177
3. https://www.england.nhs.uk/wp-content/uploads/2014/02/pathol-dig-first.pdf. accessed June 9 2020.
4. Brigden ML, Heathcote JC. Problems in interpreting laboratory tests. What do unexpected results mean? Postgrad Med. 2000;107:145–146. doi:10.3810/pgm.2000.06.1127
5. Dickey L. Confronted by an unexpected laboratory result. Am Fam Physician. 2001;64:1281–1282.
6. Lundberg G. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA. 1990;263(5):709. doi:10.1001/jama.1990.03440050103044
7. Lippi G, Banfi G, Church S, et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for clinical chemistry and laboratory medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med. 2015;53:357–370.
8. Joshi D, Center JR, Eisman JA. Investigation of incidental hypercalcaemia. BMJ. 2009;339:b4613.
9. https://bpac.org.nz/BT/2015/April/laboratory-investigations.aspx.
10. Premawardhana LD. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300–307. doi:10.11613/BM.2017.033
11. Kilickaya O, Schmickl C, Ahmed A, et al. Customized reference ranges for laboratory values decrease false positive alerts in intensive care unit patients. PLoS One. 2014;9(9):e10793. doi:10.1371/journal.pone.0107930
12. Sankaralingam A, Karim Y, Swaminathan R. Phenindione interferes with measurement of creatinine. Clin Biochem. 2013;46(18):1912–1913. doi:10.1016/j.clinbiochem.2013.08.017
13. Bright P, Lock RJ, Unsworth DJ. Immunoglobulin A deficiency on serological coeliac screening: an opportunity for early diagnosis of hypogammaglobulinaemia. Ann Clin Biochem. 2012;49(5):503–504. doi:10.1258/acb.2012.012011
14. Taylor D, Durigon M, Davis H, et al. Probability of a false-negative HIV antibody test result during the window period: a tool for pre- and post-test counselling. Int J STD AIDS. 2015;26(4):215–224. doi:10.1177/0956462414542987
15. Harvey Y. Abnormal full blood count in the asymptomatic patient. Common sense pathology. Royal College of Pathologists of Australia. Clin Biochem. 2019;1–12.
16. Misbah SA, Kokkinou V, Jeffery K, et al. The role of the physician in laboratory medicine: a European perspective. J Clin Pathol. 2013;66(5):432–437. doi:10.1136/jclinpath-2012-201042
17. Sinclair D, Spedding A, Young R. Can the laboratory affect the investigation and diagnosis of primary biliary cirrhosis? J Clin Pathol. 2006;59(4):360–362. doi:10.1136/jcp.2005.028936
18. Byrne EA, Katbamna R, Scott C, et al. Patients with m2 antibodies: what diagnosis and follow-up are they given? Gut. 2010;59(6):859–860. doi:10.1136/gut.2009.190769
19. White GH, Farrance I. AACB Uncertainty of Measurement Working Group. Uncertainty of measurement in quantitative medical testing: a laboratory implementation guide. Clin Biochem Rev. 2004;25:S1–S24.
20. Beck SC, Lock RJ. Uncertainty of measurement: an immunology laboratory perspective. Ann Clin Biochem. 2015;52(1):7–17. doi:10.1177/0004563214551066
21. Qureshi BH. EDTA-Dependent Pseudothrombocytopenia. Lab Med. 1998;29(8):471–473. doi:10.1093/labmed/29.8.471
22. Plebani M. Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med. 2016;4:1881–1891.
23. Plebani M. Laboratory-associated and diagnostic errors: a neglected link. Diagnosis. 2014;1:89–94. doi:10.1515/dx-2013-0030
24. Clinical Laboratory Standards Institute. CLSI H3-A6 Document.
25. Simundic AM, Bölenius K, Cadamuro J, et al. Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Ann Biol Clin (Paris). 2019;77:131–154.
26. Bain BJ. Bone marrow aspiration. J Clin Pathol. 2001;54(9):657–663. doi:10.1136/jcp.54.9.657
27. https://www.rcpath.org/uploads/assets/bb86b370-1545-4c5a-b5826a2c431934f5/The-communication-of-critical-and-unexpected-pathology-results.pdf.
28. Howanitz P, Steindel S, Heard N. Laboratory critical values policies and procedures. Arch Pathol Lab Med. 2002;126:663–669.
29. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128. doi:10.7326/0003-4819-143-2-200507190-00011
30. Campbell CA, Horvath AR. Towards Harmonisation of Critical Laboratory Result Management - Review of the Literature and Survey of Australasian Practices. Clin Biochem Rev. 2012;33:149–160.
31. Kuperman GJ, Teich JM, Tanasijevic MJ, et al. Improving response to critical laboratory results with automation: results of a randomized controlled trial. J Am Med Inform Assoc. 1999;6(6):512–522. doi:10.1136/jamia.1999.0060512
32. Kuperman GJ, Boyle D, Jha A, et al. How promptly are inpatients treated for critical laboratory results? J Am Med Inform Assoc. 1998;5(1):112–119. doi:10.1136/jamia.1998.0050112
33. Ridley JW. Essentials of Clinical Laboratory Science.
34. Price CP, Christenson RH. Evidence-Based Laboratory Medicine: Principles, Practice, and Outcomes.
35. Dighe A, Jones J, Parham S. Survey of critical value reporting and reduction of false positive critical value reports. Arch Pathol Lab Med. 2008;132:1666–1671.
36. Dighe AS, Rao A, Coakley AB, Lewandrowski KB. Analysis of laboratory critical value reporting at a large academic medical center. Am J Clin Pathol. 2006;125(5):758–764. doi:10.1309/R53XVC2U5CH6TNG8
37. Lippi G, Favaloro EJ. Preanalytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol. 2017;1646:29–42.
38. Ang EY, Soh JY, Liew WK, et al. Reliability of acute illness dihydrorhodamine-123 testing for chronic granulomatous disease [published correction appears in Clin Lab. Clin Lab. 2013;59:203–206.
39. Egner W, Swallow K, Lock RJ, Patel D. Falsely low immunoglobulin (Ig)G4 in routine analysis: how not to miss IgG4 disease. Clin Exp Immunol. 2016;186(1):57–63. doi:10.1111/cei.12805
40. Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48(5):418–432. doi:10.1258/acb.2011.011073
41. De Silva DA, Halstead AC, Côté AM, Sabr Y, von Dadelszen P, Magee LA. Unexpected random urinary protein: creatinine ratio results-limitations of the pyrocatechol violet-dye method. BMC Pregnancy Childbirth. 2013;13(1):152. doi:10.1186/1471-2393-13-152
42. Sickle cell tests. Lab Tests online. AACC. https://labtestsonline.org.uk/tests/sickle-cell-test. Accessed September 25, 2020.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
