Back to Journals » Nature and Science of Sleep » Volume 14
The Characteristics of Sleep Apnea in Tibetans and Han Long-Term High Altitude Residents
Authors Tan L, Li T, Luo L, Xue X, Lei F, Ren R, Zhang Y, He J, Bloch KE , Tang X
Received 2 May 2022
Accepted for publication 23 August 2022
Published 1 September 2022 Volume 2022:14 Pages 1533—1544
DOI https://doi.org/10.2147/NSS.S371388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sarah L Appleton
Lu Tan,1 Taomei Li,1 Lian Luo,1 Xiaofang Xue,2,3 Fei Lei,1 Rong Ren,1 Ye Zhang,1 Jiaming He,2,3 Konrad E Bloch,4 Xiangdong Tang1,5– 7
1Sleep Medicine Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Emergency, Diqing Tibetan Autonomous Prefectural People’s Hospital, Shangri-La, People’s Republic of China; 3Department of Intensive Care Unit, Diqing Tibetan Autonomous Prefectural People’s Hospital, Shangri-La, People’s Republic of China; 4Department of Respiratory Medicine, Sleep Disorders Center, University Hospital of Zurich, Zurich, Switzerland; 5Mental Health Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 7State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Xiangdong Tang, Sleep Medicine Center, Mental Health Center, Department of Respiratory and Critical Care Medicine, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, 28 Dian Xin Nan Jie, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-28-85422733, Fax +86-28-85422632, Email [email protected]
Purpose: Obstructive sleep apnea (OSA) is common both at low and high altitude. Since adaptations to high altitude and respiratory control may differ among Tibetans and Hans, we compared characteristics of sleep-disordered breathing in the two ethnic groups at high altitude.
Materials and Methods: This was a prospective observational study including 86 Tibetan and Han long-term (> 5 years) high altitude residents with chief complaints of snoring and/or witnessed apnea underwent clinical evaluation and polysomnography at 3200 meters in Shangri-La, China.
Results: In 42 Tibetans, 38 men, median (quartiles) age was 50.0 (41.0; 56.0)y, total apnea/hypopnea index (AHI) 53.9 (32.0; 77.5)/h, obstructive AHI 51.0 (28.0; 72.2)/h and central AHI 1.5 (0.2; 3.1)/h. In 44 Hans, 32 men, median (quartiles) age was 47.0 (43.5; 51.0)y, total AHI 22.2 (12.8; 39.2)/h, obstructive AHI 17.7 (12.0; 33.0)/h and central AHI 2.4 (0.5; 3.4)/h (p < 0.001 total and obstructive AHI vs Tibetans). In Tibetans, mean nocturnal oxygen saturation was lower [median 85.0 (83.0; 88.0)% vs 88.5 (87.0; 90.0)%] and obstructive apnea and hypopnea duration was longer [22.0 (19.6; 24.8) sec vs 18.3 (16.7; 20.6) sec] than in Hans (all p < 0.001). In regression analysis, Tibetan ethnicity, neck circumference and high-altitude living duration were the predictors of total AHI. We also found that with every 10/h increase in total AHI, there were an approximately 0.9 beat/min and 0.8 beat/min increase in mean heart rate during rapid eye movement (REM) and non-REM sleep and 1.9 mmHg and 2.0 mmHg increase in evening and morning systolic blood pressure.
Conclusion: Our data suggest that Tibetans presented more severe obstructive sleep apnea, hypoxemia and longer apnea duration compared to Hans at 3200 meters, which was correlated with higher heart rate and blood pressure suggesting a greater cardiovascular risk.
Keywords: obstructive sleep apnea, high altitude, Tibetan, long-term Han resident
Introduction
Obstructive sleep apnea (OSA) is one of the most common sleep disorders characterized by recurrent upper airway collapse that results in intermittent oxygen desaturations and fragmented sleep.1 The prevalence of moderate-to-severe sleep-disordered breathing (apnea–hypopnea index ≥15/h) was 10% and 3% among 30–49 years old men and women in a cohort study including 1520 participants.2 In a Swiss sample of the general population including 2121 participants, the prevalence was 49.7% in men and 23.4% in women aged 35–75 years.3 Major consequences of OSA included excessive daytime sleepiness,4 hypertension, cardiovascular and cerebrovascular disease5,6 and traffic or work accidents.7
The Qinghai-Tibetan Plateau, located in the eastern part of China, is the highest plateau in the world with an average altitude of over 4000 meters.8 Hypobaric hypoxia is one of the main factors that influences the living of human beings at high altitude.9 Hypobaric hypoxia may also contribute to the higher prevalence of OSA at high altitude. Questionnaire studies conducted in Peru found a higher prevalence of observed apnea at 3825 m compared to lower altitude.10 Another study included 170 age-, body mass index-, and sex-matched participants living at sea-level (Lima) and high-altitude (Puno, 3825 m) found that the prevalence of sleep apnea, defined by an apnea–hypopnea index (AHI) over 5/h, was 54% and 77%, respectively (p < 0.001).11 Corresponding data were also reported from Kyrgyz highlanders.12 Further studies also found higher AHI in patients with chronic mountain sickness compared to healthy high-altitude dwellers.13
Tibetans as the permanent residents of the Qinghai-Tibetan Plateau have lived there for over 25,000 years,14 while lowland Hans have immigrated and lived at high altitude since the late 19th and early 20th century.15 Due to the difference in the duration of high-altitude exposure and genetics between Tibetans and Hans, the two ethnic groups differ in their adaptation to high altitude. Thus, previous studies found that Tibetans presented higher oxygen saturation, larger vital and total lung capacity compared to Hans living at the same altitude.16–18 Tibetans also have better performance during exercise, greater minute ventilation and oxygen uptake at maximal effort,19 and sustained increase in flow velocity and cerebral oxygen delivery at peak exercise20 compared to Han. In other studies, high-altitude living Hans were found to have a blunted hypoxic ventilation response (HVR),21 while Tibetans presented a similar HVR compared to low altitude residents.22 The cited observations may suggest differences in control of breathing and in susceptibility and clinical manifestations of OSA among Tibetans and Hans, although this has not been conclusively studied.
Therefore, the purpose of this study was to compare the characteristics of sleep apnea and clinical outcomes among Tibetan and Han OSA patients living permanently or long term at 3200 meters in Shangri-La area. We hypothesized that Tibetan highland OSA patients may have a greater number of sleep apnea, lower nocturnal oxygen saturation and more severe clinical outcomes than Hans at the same altitude due to different adaptations to hypobaric hypoxia environment in the two ethnic groups.
Materials and Methods
Study Design and Setting
This was a prospective observational study carried out in Shangri-La, Yunnan Province of China at an altitude of 3200 m from August 2017 to December 2017 to evaluate the difference of sleep apnea, oxygen saturation and clinical outcomes in Tibetan and Han long-term high-altitude residents. The study was approved by the West China Hospital of Sichuan University Biomedical Research Ethics Committee [2016(90)] and complied with the Declaration of Helsinki. Informed consent was obtained from all participants when they underwent polysomnography (PSG).
Patients
Native Tibetan and Han adults (>18 years old, both sexes) with a chief complaint of snoring and/or witnessed apnea referred for evaluation of suspected OSA living in Shangri-La, Yunnan Province of China at altitude of 3200 meters for over 5 years were included in the study. We excluded patients who had uncontrolled hypertension (systolic blood pressure >160 mmHg and diastolic blood pressure >100 mmHg), angina, atrial fibrillation or diabetes (fasting blood glucose >6.5 mmol/L), previous stroke, severe chronic obstructive pulmonary disease (FEV1/FVC <0.7) or obesity hypoventilation syndrome (PaCO2 >6kPa), a chronic sleep-disrupting medical condition, a current major psychiatric condition (ie, anxiety or depression) and evidence of other comorbid sleep disorder. Patients with current use of hypnotics, anxiolytics, antidepressants, and any other antipsychotics during the past 3 months, who were exposed to low altitude (<1500 m) or higher altitude (>4000 m) in the previous 6 months for over 2 weeks were also excluded.
Overnight PSG
Overnight PSG was performed according to standard procedures (Alice 5; Respironics Inc., Murrysville, USA). Briefly, it consisted of continuous recordings from six electroencephalographic (EEG) leads (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, O2-A1), two electrooculographic leads (ROC-A1, LOC-A2), four electromyographic leads (two submental and bilateral tibialis anterior), thermistors for nasal and oral airflow, nasal pressure swing, strain gauges for thoracic and abdominal excursion, finger pulse oximetry, electrocardiography, body position and audio-visual recordings. Sleep and respiratory events were scored according to American Academy of Sleep Medicine guidelines (Version 2.3).23 An apnea was defined as more than 90% reduction in airflow for at least 10 seconds. Obstructive or central events were scored based on whether or not it was associated with persistent or increased respiratory effort throughout the entire period of events. Mixed apneas were scored as obstructive events in this study to be consistent with previous study.24 Hypopnea was defined as 30% or more reduction of airflow for at least 10 seconds associated with at least 3% reduction in oxygen saturation or an arousal. Obstructive hypopnea was scored if there was snoring during the event or there was increased inspiratory flattening of the nasal pressure compared to baseline breathing or there was an associated thoracoabdominal paradox that occurs during the event but not during pre-event breathing. Central hypopnea was scored if not met any criteria of obstructive hypopnea. Total AHI was computed as the sum of apnea and hypopnea events divided by total sleep time (TST). Obstructive or central AHI was computed as the sum of obstructive or central apneas and hypopneas per hour. The oxygen desaturation index (ODI) was computed as the number of cyclic desaturations >3% divided by TST. Nadir oxygen saturation was the lowest oxygen saturation during sleep.
Clinical and Morning Evaluations
A medical history and clinical examination were obtained including weight, height, neck, waist and hip circumference, smoking habits, alcoholic intake, blood pressure, pulse rate and spirometry. The assessment of tonsil and tongue hypertrophy, and Mallampati score was also performed. Berlin questionnaire was used to evaluate the risk of OSA,25 the generalized anxiety disorder-7 and patient health questionnaire-9 were used to exclude patients with moderate-to-severe anxiety and depression,26,27 and the Chinese version of the Epworth sleepiness scale (ESS) was used to evaluate daytime sleepiness.28 Venous blood was withdrawn to assess hemoglobin, erythrocyte parameters, fasting blood glucose and blood lipids to exclude patients with uncontrolled diabetes or other severe medical diseases. Spirometry was performed together with clinical examination according to international guidelines including variables such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1% predicted and FEV1/FVC.
Arterial blood gas analysis was carried out at night before overnight PSG. Morning evaluations included blood pressure, sleep quality evaluated by morning questionnaire, and vigilance evaluated by psychomotor vigilance test (PVT). During the PVT test, patients were sitting comfortably on a chair in a quiet room. They had to press the “space” on the keyboard in response to the appearance of a red dot on the computer screen at random intervals. A PVT response was regarded valid if response time (RT) ≥100ms. Failing to press the button for 3 s or longer was counted as error and excluded from the analyses. The following PVT outcome metrics were assessed and included in our analysis: mean RT, mean 1/RT, slowest 10% 1/RT and number of lapses.29
Statistical Analysis
Results were shown as median and quartile range for continuous variables and numbers and percent for categorical variables. Comparisons of continuous variables and proportions between two groups were performed using Mann–Whitney U-test and chi-square test (X2 test), respectively.
Generalized least square regression was used to assess the effect of ethnicity on AHI and mean obstructive apnea/hypopnea (OAH) duration when controlling for various potential confounders. AHI was square root transformed and mean OAH duration was inverse transformed to obtain a normal distribution. Independent variables with p < 0.2 in the univariable analysis were included in the multivariable analysis. Multiple linear regression analysis was used to assess the effect of AHI on clinical outcomes such as heart rate during sleep and evening or morning blood pressure in all patients.
As age, body mass index (BMI) and OSA symptoms such as daytime sleepiness were risk factors of OSA severity, data were compared between groups matched for age within ±5 years, BMI within ±2 kg/m2 and ESS score within ±2 scores to control for the above risk factors between the two ethnic groups. Moreover, based on our previous study that found longer apnea duration in Tibetans than in Han OSA patients, and longer apnea duration was correlated with higher blood pressure and prevalence of hypertension in other studies, data were also compared between groups matched for age within ±5 years, BMI within ±2 kg/m2 and AHI within ±5 /h, respectively, to explore the difference of apnea duration between groups with similar AHI. The gender in the two matched groups was all male due to the low number of female OSA patients in two ethnic groups.
Stata 15.0 was used for all statistical analysis, and statistical significance was defined as p < 0.05.
Results
A total of 164 participants were assessed for eligibility and 86 were available for the final analysis, including 42 Tibetans and 44 Hans. A patient flow chart is illustrated in Figure 1. Demographic characteristics are shown in Table 1. The proportion of men was slightly higher in Tibetans than in Hans. The median age in the two groups was 50 years and 47 years (p = 0.156), and the percentage of patients living over 30 years at high altitude was higher in Tibetans than Hans (76.2% vs 25%, p < 0.001). Despite similar BMI, Tibetan OSA patients were higher and heavier compared to Hans. Tibetans also had larger neck and waist circumference and higher waist-to-hip ratio (all p < 0.05). There was a higher percentage of participants with tongue hypertrophy (p = 0.001) and a trend of higher percentage of participants with higher degree of Mallampati score in Tibetans (p = 0.081). Tibetan OSA patients presented a higher percentage of hypertension, smoking and drinking tea with butter. The percentage of patients with high risk of OSA evaluated by Berlin Questionnaire was 57.1% in Tibetans and 22.7% in Hans (p = 0.001).
 |
Table 1 Demographic Characteristics in Tibetan and Han Highland OSA Patients |
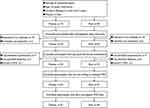 |
Figure 1 Patient flowchart. Abbreviations: COPD, chronic obstructive pulmonary disease; PSG, polysomnography. |
The polysomnography parameters are shown in Table 2. Tibetan OSA patients had higher AHI compared to Han counterparts (53.9/h vs 22.2/h, p < 0.001), with predominately higher obstructive AHI (51.0/h vs 17.7/h, p < 0.001) and lower central hypopnea index (0.1/h in Tibetans vs 0.8/h in Hans, p = 0.016). The periodic breathing duration and index showed no significant difference between the two groups (p > 0.05). Besides higher AHI, Tibetans also had longer mean and longest OAH duration and lower mean and nadir oxygen saturation than Hans (all p < 0.001). The median percentage of time with oxygen saturation below 85% was 36.6% in Tibetans and only 3.7% in Hans (p < 0.001). With regard to sleep structure, Tibetans presented higher sleep efficiency with longer total sleep time and shorter wake after sleep onset time. Moreover, higher percentage of non-rapid eye movement (NREM) sleep stage 1 and arousal index were found in Tibetans that was correlated with higher AHI. Both groups had hardly any stage N3 sleep. The mean heart rate during rapid eye movement (REM) sleep and NREM sleep showed no significant difference between two groups.
 |
Table 2 Polysomnography Parameters in Tibetan and Han Highland OSA Patients |
Clinical assessment, morning questionnaire and psychomotor vigilance test are shown in Table 3. The FVC and FEV1 were larger in Tibetans, while FVC, FEV1% predicted and FEV1/FVC showed no significant difference between two groups. Arterial blood gas analysis before PSG revealed mild hypoxemia and hypocapnia, while Tibetans showed lower partial pressure of oxygen than Hans (p = 0.017). Evening and morning systolic and diastolic blood pressure were all significantly higher in Tibetan OSA patients. However, the subjective sleep quality evaluated by morning questionnaire and mean reaction time (RT), 1/RT, slowest 10% 1/RT and lapse evaluated by PVT showed no differences between the two ethnic groups.
 |
Table 3 Clinical Assessments, Morning Questionnaire and Psychomotor Vigilance Test in Tibetan and Han Highland OSA Patients |
To investigate the determinants of AHI and mean OAH duration, generalized least square regression analysis was performed. The results confirmed that Tibetan ethnicity, neck circumference and high-altitude living duration were positive predictors of AHI. As for mean OAH, the results revealed that Tibetan ethnicity, BMI and high-altitude living duration were positive predictors of mean OAH duration (Table 4).
 |
Table 4 Generalized Least Square Regression Analysis of the Effect of Ethnicity on Apnea/Hypopnea Index and Mean OAH Duration |
Further comparisons among groups in males matched for age, BMI and ESS score are performed in eTable 1. Tibetans also had higher AHI, obstructive AHI and longer mean or longest OAH duration compared to Hans, while no difference in ESS score and percentage of patient with high risk of OSA. Another comparison matched for age, BMI and AHI, Tibetan OSA patients consistently presented longer mean (22.0 seconds vs 19.1 seconds, p = 0.002) and longest OAH duration (58.5 seconds vs 45.5 seconds, p = 0.031) with lower nadir oxygen saturation despite similar AHI compared to Han counterparts (eTable 1). The summary of respiratory and oxygen saturation parameters in three separate comparisons is shown in Figure 2.
The multivariable linear regression analysis to assess the association among OSA severity, heart rate and blood pressure is shown in eTable 2. There was an approximately 0.9 beat/min and 0.8 beat/min increase in mean heart rate during REM (p = 0.017) and NREM sleep (p = 0.024) with every 10/h increase in total AHI, respectively (all p < 0.05). The evening and morning systolic blood pressure also presented a 2.0 mmHg increase with every 10/h increase in total AHI (p < 0.05).
Discussion
This was the first prospective observational study using polysomnography to explore the characteristics of sleep apnea and clinical outcomes in Tibetans and Hans with snoring and/or witnessed apnea referred for evaluation of suspected OSA living at an altitude of 3200 meters. We found a significantly higher AHI, longer mean and longest apnea duration and lower oxygen saturation in Tibetans than Hans. Similar results were found in further comparisons matched for age, BMI and ESS score and in comparisons matched for age, BMI and AHI. Further analysis found that Tibetan ethnicity and high-altitude living duration were positive predictors of AHI and mean OAH duration. Linear regression analysis revealed an approximately 1 beat/min and 2 mmHg increase in mean heart rate during sleep and systolic blood pressure with 10/h increase in AHI. These findings suggest that Tibetan OSA patients had more severe OSA and worse clinical outcomes at an altitude of 3200 meters compared to Hans.
Previous studies have found a higher prevalence of OSA or OSA-related symptoms at higher altitudes.10,11 The prevalence was much higher in patients with chronic diseases, such as pulmonary hypertension, cardiovascular disease, and chronic mountain sickness compared to those without at high altitude.12,13,30 One hospital-based study including patients who underwent PSG in Lhasa (altitude of 3658 m) also evaluated the OSA severity in Tibetans and Hans with a similar age and higher BMI compared to our study. The study in Lhasa found a trend of lower AHI in Tibetans (27.3/h vs 34.5/h, p = 0.054) compared to Han counterparts.31 However, in our study, we found Tibetan OSA patients had higher AHI with predominantly higher obstructive AHI than Han counterparts at 3200 meters even after controlling for confounding factors. There are two potential explanations for this. First, Tibetans had larger neck circumference, higher waist–hip ratio and higher percentage of participants with tongue hypertrophy compared to Hans that were associated with higher AHI.32,33 Correspondingly, regression analysis confirmed that the neck circumference was a predictor of AHI. The larger neck circumference and higher waist–hip ratio may be related to the high intake of salt, meat, fat and alcohol and low intake of fruit and vegetables in Tibetans,34–36 reflected in the higher percentage of patients taking tea with butter in our study. Second, one study found that Tibetans presented more periodic breathing at a simulated altitude of 5000 meters which indicated that they have instability of ventilation control during sleep37 although no significant difference in periodic breathing was found in our study. Ventilation control instability was a risk factor of OSA,38 which may contribute to the higher AHI in Tibetan OSA patients in our study; however, this needs to be confirmed in further studies.
Besides the higher AHI, Tibetans also presented longer apnea duration at 3200 meters even after controlling for OSA severity and other factors. In a previous study, we also found longer apnea duration in Tibetan highlanders than Han highlanders or Han lowlanders at low altitude.39 There were three possible mechanisms to explain this that need to be confirmed in further studies. First, Tibetans have a better tolerance to hypoxemia due to the longer living duration at high altitude or genetic differences.37,40 Therefore, once having OSA, the time needed to reach the arterial oxygen saturation threshold that triggers arousal may be prolonged in Tibetans, thus causing longer apnea duration. Second, the association between longer apnea duration and larger neck circumference was found in previous studies consistent with the longer apnea duration in Tibetans in our study. Third, previous studies found that reduced gas stores reflected in smaller lung volume were associated with a more rapid fall in SpO2. Conversely, the larger lung volume (larger FVC and FEV1) therefore results in a slower fall in the SpO2 compared to Hans, thus contributing to a longer apnea duration. Moreover, the mean and lowest oxygen saturation was lower in Tibetans in line with longer apnea duration, and this was consistent with another study conducted in Tibetan and Han OSA patients at 3658 m.31
Although Tibetans presented more severe OSA than Hans at high altitude, they had longer sleep time, higher sleep efficiency and shorter wake time after sleep onset. Our study was partially consistent with a previous study that found shorter wake time after sleep onset in Tibetans vs Hans at 2261 meters and a longer sleep time and higher sleep efficiency at simulated 5000 meters in Tibetans.37 However, the mechanism was not clear and genetic difference may explain this and need to be confirmed in future studies. Moreover, we also found less central events in both Tibetans and Han OSA patients that were consistent with our previous study.24 We speculated that this may be due to the inclusion criteria for our study that selected participants with clear symptoms of OSA. Although the absolute value for central events was low, the central hypopnea index was higher in Hans compared to Tibetans [median (quartile range) of 0.8 (0.0; 2.8) vs 0.1 (0.0; 0.8), p = 0.016]. Differences in respiratory control and the adaptation to high altitude in the two groups of participants may explain this.
The higher AHI, longer apnea duration and more severe hypoxemia in Tibetan OSA patients may result in clinical consequences such as higher blood pressure, heart rate and longer QT interval.41–43 Consistent with these previous reports, we found a positive relationship between the total AHI and heart rate during REM and NREM sleep, and evening and morning systolic blood pressure. Since the prevalence of hypertension was higher at high altitude, especially in Tibetans,44 the combination of more severe OSA and longer apnea duration may result in higher risk of presenting hypertension as well as other cardiovascular diseases in Tibetan OSA patients at high altitude. Conversely, cardiovascular disease may lead to a high loop gain of the respiratory control system that may have contributed to the severity of OSA.
Our study had some limitations that should be considered in its interpretation. First, the Tibetan OSA patients we included in the study presented more risk factors than Hans. Although multiple linear regression analysis was controlling for confounding factors and comparisons in groups matched for ESS, BMI and AHI found the same results, we could not avoid a potential selection bias. Second, the different diet, such as higher intake of fat in Tibetan OSA that may be related to hypertension, may also be risk factors of OSA. Patients matched for potential factors of OSA should be included in the future studies, and the prevalence of OSA in general populations of Tibetans and Hans should be investigated. Third, we could only speculate that the difference in the severity of OSA in these two ethnic groups was related to differences in control of ventilation during sleep since the arterial blood gas analysis was similar during wake time between two groups. Further studies are needed to evaluate the specific mechanism regarding the different severity of OSA in Tibetans and Hans at high altitude. Fourth, we found increased mean heart rate and blood pressure with higher AHI in the total sample. However, the relatively small sample size, the observational design and lack of follow-up data prevent firm conclusions on the relative prevalence of cardiovascular disease among Tibetans and Hans, and further studies on this topic are warranted.
Conclusions
In the current prospective observational study among long-term high-altitude residents, Tibetan OSA patients presented higher AHI, especially higher obstructive AHI, more severe hypoxemia and longer apnea duration compared to Hans at 3200 meters. We speculated that these differences are likely due to the better adaptation to hypoxic environment in Tibetans, thus resulting in different respiratory control during sleep as reflected in longer apnea events in Tibetans than Hans, while the awake PaCO2 was the same in the two groups. Moreover, higher AHI was correlated with worse clinical outcomes such as higher heart rate and blood pressure, which suggested a greater cardiovascular risk. These implicated that more attention should be paid to highland OSA patients, especially Tibetans.
Acknowledgments
The authors wanted to thank all the patients for their participation in this study. The abstract of this paper was presented at the ERS International Congress 2018 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in European Respiratory Journal name “Sleep apnea in Tibetans and Han long-term high altitude residents”: https://erj.ersjournals.com/content/52/suppl_62/PA4359 (DOI: 10.1183/13993003.congress-2018.PA435).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (U21A20335, 82100107).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi:10.1152/physrev.00043.2008
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
3. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi:10.1016/S2213-2600(15)00043-0
4. Black J. Sleepiness and residual sleepiness in adults with obstructive sleep apnea. Respir Physiol Neurobiol. 2003;136(2–3):211–220. doi:10.1016/S1569-9048(03)00083-1
5. Chan W, Coutts SB, Hanly P. Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke. 2010;41(12):2973–2975. doi:10.1161/STROKEAHA.110.596759
6. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi:10.1136/bmj.320.7233.479
7. Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and Meta-analysis. Sleep. 2016;39(6):1211–1218. doi:10.5665/sleep.5834
8. Wu T. The Qinghai-Tibetan plateau: how high do Tibetans live? High Alt Med Biol. 2001;2(4):489–499. doi:10.1089/152702901753397054
9. Simancas-Racines D, Arevalo-Rodriguez I, Osorio D, Franco JV, Xu Y, Hidalgo R. Interventions for treating acute high altitude illness. Cochrane Database Syst Rev. 2018;6:CD009567. doi:10.1002/14651858.CD009567.pub2
10. Schwartz NG, Rattner A, Schwartz AR, et al. Sleep disordered breathing in four resource-limited settings in Peru: prevalence, risk factors, and association with chronic diseases. Sleep. 2015;38(9):1451–1459. doi:10.5665/sleep.4988
11. Pham LV, Meinzen C, Arias RS, et al. Cross-sectional comparison of sleep-disordered breathing in Native Peruvian highlanders and lowlanders. High Alt Med Biol. 2017;18(1):11–19. doi:10.1089/ham.2016.0102
12. Latshang TD, Furian M, Aeschbacher SS, et al. Association between sleep apnoea and pulmonary hypertension in Kyrgyz highlanders. Eur Respir J. 2017;49(2):2. doi:10.1183/13993003.01530-2016
13. Rexhaj E, Rimoldi SF, Pratali L, et al. Sleep-disordered breathing and vascular function in patients with chronic mountain sickness and healthy high-altitude dwellers. Chest. 2016;149(4):991–998. doi:10.1378/chest.15-1450
14. Lorenzo FR, Huff C, Myllymaki M, et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46(9):951–956. doi:10.1038/ng.3067
15. Weitz CA, Liu JC, He X, Chin CT, Garruto RM. Responses of Han migrants compared to Tibetans at high altitude. Am J Hum Biol. 2013;25(2):169–178. doi:10.1002/ajhb.22368
16. Weitz CA, Garruto RM. A comparative analysis of arterial oxygen saturation among Tibetans and Han born and raised at high altitude. High Alt Med Biol. 2007;8(1):13–26. doi:10.1089/ham.2006.1043
17. Weitz CA, Garruto RM, Chin CT, Larger FVC. FEV1 among Tibetans compared to Han born and raised at high altitude. Am J Phys Anthropol. 2016;159(2):244–255. doi:10.1002/ajpa.22873
18. Droma T, McCullough RG, McCullough RE, et al. Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3658 m). Am J Phys Anthropol. 1991;86(3):341–351. doi:10.1002/ajpa.1330860303
19. Sun SF, Droma TS, Zhang JG, et al. Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol. 1990;79(2):151–161. doi:10.1016/0034-5687(90)90015-Q
20. Huang SY, Sun S, Droma T, et al. Internal carotid arterial flow velocity during exercise in Tibetan and Han residents of Lhasa (3658 m). J Appl Physiol. 1992;73(6):2638–2642. doi:10.1152/jappl.1992.73.6.2638
21. Zhuang J, Droma T, Sun S, et al. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3658 m. J Appl Physiol. 1993;74(1):303–311. doi:10.1152/jappl.1993.74.1.303
22. Beall CM, Strohl KP, Blangero J, et al. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol. 1997;104(4):427–447. doi:10.1002/(SICI)1096-8644(199712)104:4<427::AID-AJPA1>3.0.CO;2-P
23. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
24. Tan L, Li T, Zhang Y, et al. Effect of one night of nocturnal oxygen supplementation on highland patients with OSA: a randomized, crossover trial. Chest. 2021;160(2):690–700. doi:10.1016/j.chest.2021.02.046
25. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi:10.7326/0003-4819-131-7-199910050-00002
26. Tong X, An D, McGonigal A, Park SP, Zhou D. Validation of the Generalized Anxiety Disorder-7 (GAD-7) among Chinese people with epilepsy. Epilepsy Res. 2016;120:31–36. doi:10.1016/j.eplepsyres.2015.11.019
27. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
28. Zhang JN, Peng B, Zhao TT, Xiang M, Fu W, Peng Y. Modification of the Epworth Sleepiness Scale in Central China. Qual Life Res. 2011;20(10):1721–1726. doi:10.1007/s11136-011-9898-3
29. Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. doi:10.1093/sleep/34.5.581
30. Otero L, Hidalgo P, Gonzalez R, Morillo CA. Association of cardiovascular disease and sleep apnea at different altitudes. High Alt Med Biol. 2016;17(4):336–341. doi:10.1089/ham.2016.0027
31. Yang Y, Li R. 西藏藏汉民族阻塞性睡眠呼吸暂停综合征睡眠监测及临床特点分析 [Polysomnography and clinical characteristics of Tibetan and Han residents with obstructive sleep apnea syndrome in Tibet]. 中华结核和呼吸杂志. 2019;42(6):413–418. Chinese. doi:10.3760/cma.j.issn.1001-0939.2019.06.003
32. Subramanian S, Jayaraman G, Majid H, Aguilar R, Surani S. Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath. 2012;16(4):1091–1095. doi:10.1007/s11325-011-0607-9
33. Fischer MK, Martinez D, Cassol CM, Rahmeier L, Vieira LR. Immediate and overnight recumbence-dependent changes of neck circumference: relationship with OSA severity in obese and nonobese subjects. Sleep Med. 2012;13(6):650–655. doi:10.1016/j.sleep.2012.02.007
34. Sehgal AK, Krishan I, Malhotra RP, Gupta HD. Observations on the blood pressure of Tibetans. Circulation. 1968;37(1):36–44. doi:10.1161/01.CIR.37.1.36
35. Sun SF. Epidemiology of hypertension on the Tibetan Plateau. Hum Biol. 1986;58(4):507–515.
36. Zhao X, Li S, Ba S, et al. Prevalence, awareness, treatment, and control of hypertension among herdsmen living at 4300 m in Tibet. Am J Hypertens. 2012;25(5):583–589. doi:10.1038/ajh.2012.9
37. Plywaczewski R, Wu TY, Wang XQ, Cheng HW, Sliwinski PS, Zielinski J. Sleep structure and periodic breathing in Tibetans and Han at simulated altitude of 5000 m. Respir Physiol Neurobiol. 2003;136(2–3):187–197. doi:10.1016/S1569-9048(03)00081-8
38. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. doi:10.1016/S0140-6736(13)60734-5
39. Tan L, Li T, Lei F, et al. Longer apnea duration at low altitude in Tibetan and Han highlanders compared with Han lowlanders: a retrospective study. J Sleep Res. 2019;29:e12934. doi:10.1111/jsr.12934
40. Kong F, Liu S, Li Q, Wang L. Sleep architecture in partially acclimatized lowlanders and native Tibetans at 3800 meter altitude: what are the differences? High Alt Med Biol. 2015;16(3):223–229. doi:10.1089/ham.2014.1058
41. Ali NJ, Davies RJ, Fleetham JA, Stradling JR. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101(6):1526–1532. doi:10.1378/chest.101.6.1526
42. Baumert M, Smith J, Catcheside P, et al. Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. Sleep. 2008;31(7):959–966.
43. Cicek D, Lakadamyali H, Gokay S, Sapmaz I, Muderrisoglu H. Effect of obstructive sleep apnea on heart rate, heart rate recovery and QTc and P-wave dispersion in newly diagnosed untreated patients. Am J Med Sci. 2012;344(3):180–185. doi:10.1097/MAJ.0b013e318239a67f
44. Mingji C, Onakpoya IJ, Perera R, Ward AM, Heneghan CJ. Relationship between altitude and the prevalence of hypertension in Tibet: a systematic review. Heart. 2015;101(13):1054–1060. doi:10.1136/heartjnl-2014-307158
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

