Back to Journals » Infection and Drug Resistance » Volume 16
The Characteristics of Extended-Spectrum β-Lactamases (ESBLs)-Producing Escherichia coli in Bloodstream Infection
Authors Li R, Xu H, Tang H, Shen J , Xu Y
Received 13 December 2022
Accepted for publication 16 March 2023
Published 6 April 2023 Volume 2023:16 Pages 2043—2060
DOI https://doi.org/10.2147/IDR.S400170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Rongrong Li,1,2 Huaming Xu,3 Hao Tang,4 Jilu Shen,1,5 Yuanhong Xu1
1Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China; 2Department of Pathogen Biology and Provincial Laboratories of Pathogen Biology and Zoonoses, Anhui Medical University, Hefei, People’s Republic of China; 3The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, People’s Republic of China; 4Department of Clinical Laboratory, The Second Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 5Anhui Public Health Clinical Center, Hefei, People’s Republic of China
Correspondence: Yuanhong Xu, Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China, Tel +86 13505694447, Email [email protected]
Background: Bloodstream infection (BSI) is a common type of infection frequently diagnosed in clinics. The emergence and spread of ESBLs-producing Escherichia coli (E. coli) has emerged as one of the biggest challenges in global community health.
Methods: The production of ESBLs was determined by the composite disk diffusion method. The expression of the various resistance and virulence genes were detected by PCR and sequencing. Multi-locus sequence typing (MLST) and phylogenetic groups were used for the classification. The transfer of resistant plasmids was determined by conjugation assay. The statistical differences were analyzed using Statistical Product and Service Solutions (SPSS) version 23.0.
Results: A total of 60 strains of ESBLs-producing E. coli were collected. The resistance genes that were identified included blaCTX-M, blaTEM, blaSHV, blaOXA-1 and mcr-1. The most common one was the blaCTX-M including blaCTX-M-27 (n = 16), blaCTX-M-14 (n = 15), blaCTX-M-15 (n = 11), blaCTX-M-55 (n = 14) and blaCTX-M-65 (n = 5). A total of 31 STs were detected, and the most abundant among which was ST131 (n = 16, 26.7%). Most of the E. coli (n = 46, 76.7%) belonged to the groups B2 and D. And some virulence genes were related to the classification of the E. coli. Among them, the detection rates of hek/hra, kpsMII and papGII-III in groups B2 and D were higher than those in groups A and B1. The detection rates of cnf1, iucC and papGII-III in ST131 were higher than those in non-ST131. And the distributions of hek/hra, iroN, iucC, kpsMII and papGII-III were related to the blaCTX-M subtypes. Finally, most bacterial (n = 32, 53.3%) resistance genes could be transferred between the bacteria by plasmids, especially IncFIB.
Conclusion: ESBLs-producing E. coli in BSI exhibited had high resistance rates and carried a variety of virulence factors (VFs). This is necessary to strengthen the monitoring of ESBLs-producing isolates in the medical environment.
Keywords: Escherichia coli, sepsis, beta-lactamases, blaCTX-M
Introduction
A variety of pathogens, including E. coli, Klebsiella pneumoniae (K. pneumoniae), Staphylococcus, Streptococcus, and Enterococcus, constitute an important class of pathogenic bacteria that can cause BSI. Among them, E. coli has been recognized as the most common pathogen.1 Clinically, common E. coli includes five kinds of enteropathic E. coli and extraintestinal pathogenic E. coli (ExPEC). The pathogenicity of these E. coli strains was different. Therefore, rapid and simple identification of the type of E. coli could be very useful both for the clinical diagnosis and treatment. According to the literature, DNA microarray technology could be potentially used to detect and identify different pathogenic E. coli strains, especially urogenic E. coli (UPEC), by designing a series of effective and suitable long oligonucleotide microarray probes.2 This could significantly improve the detection speed of pathogenic bacteria typing and aid in facilitating better hospital infection treatment.
At present, the clinical treatment drugs for E. coli mainly include trimethoprim-sulfamethoxazole (sulfonamide), ciprofloxacin (fluoroquinolones), levofloxacin (fluoroquinolones), penicillin and cephalosporins (β-Lactam).3 However, E. coli has been found to be resistant to many different kinds of antibiotics including cephalosporins, quinolones and so on.4,5 The most common was ESBLs-producing E. coli, which could effectively hydrolyze the various antibiotics to make them inactive, including penicillin, cephalosporins and monocyclic. Interestingly, 350 different ESBL variants have been identified so far. According to amino acid sequence, they could be divided into 9 independent families including TEM, SHV, CTX-M, PER, VEB, GES, BES, TLA and OXA.6,7 The prevalence of blaCTX-M has increased rapidly.8,9 And the ESBLs-producing E. coli has spread all over the world in recent years.10,11 In the United States, the ESBLs-producing bacteria were reported to cause about 26,000 drug-resistant infections and 1700 deaths every year. Moreover, in 2019 antibiotic resistance threat report of the Centers for Disease Control and Prevention (CDC), it was classified as an increasingly serious threat.12 Among the hospitalized patients, ESBL producers accounted for 11.6% and 16.1% of E. coli primarily causing UTI and BSI, respectively.13 In 2021, the bacterial resistance monitoring in China showed that the population of ESBLs-producing E. coli had reached up to 52.6%.14 Besides, the role of Metallo-β-lactamases (MBLs) in mediating the drug resistance of E. coli could not be ignored. At present, there are more than 2000 β-lactamases (BLs) reported in the literature.15 The BLs have been divided into four distinct categories: A, B, C and D according to different amino acid sequences. Among these, catalytic activities of the categories A, C and D mainly depended on the expression of serine at the active site of the enzyme, so they were termed as serine BLs. However, distinct from the classes A, C and D, the catalytic activity of class B relied primarily on Zn2+ of the active site, so it was named as MBLs.16 MBLs have been identified in a variety of pathogenic bacteria since their discovery, including Pseudomonas aeruginosa, K. pneumoniae, E. coli, Acinetobacter baumannii and so on. The most widely disseminated were NDMs, IMPs, and VIMs.17,18 MBLs have been found to be connected with the β-lactam ring in the process of amide bond hydrolysis to resist the activity of the different antibiotics,19,20 and hydrolyzed a wide range of BLs, including carbapenems, cephalosporins, penicillin and so on.3 This condition can make it more difficult for the patients to get effective and optimal treatments.
The pathogenesis of invasive diseases caused by ExPEC could be largely determined by the VFs. However, it is necessary to deeply examine the role of VFs in the specific clinical syndromes and host populations for preventing ExPEC infection and its related incidence rate, mortality, as well as increased health care costs.21E. coli contains a variety of VFs, including adhesins, toxins, protectors, iron absorption system, virulence islands and other virulence proteins.22,23 The commonly found VFs include Cnf1, FimH, Sfa/Foc, IbeA, IroN, OmpA, HlyC, which can play distinct roles in the pathogenesis. For instance, Cnf1 can exert a key role in the regulation of invasion, dysfunction and apoptosis of human urinary tract epithelial cells. The toxin protein of HlyC can cause both adhesion and the growth of bacteria and promote the inflammation, dissolution and hemolysis of human urinary tract epithelial cells, whereas IbeA is involved in bacterial invasion.24,25 These VFs can lead to various dangerous conditions in patients, such as bacteremia, septicemia, urinary sepsis, and even death, which can result in considerable difficulties in the treatment of clinical infection. In addition, a specific relationship between bacterial resistance and toxicity has been reported previously in the literature.26–28 For example, Lee et al compared the distribution of VFs in E. coli and found that ɑ-Haemolysin, yersiniabactin receptor, serum resistance-associated outer membrane protein (TraT), and aerobactin receptor (IutA) could serve as independent predictors of pathogenicity. IutA and TraT were significantly more commonly found in groups blaCTX-M-1 and blaCTX-M-9, respectively.28 These virulence and resistance genes might possibly be transmitted to other strains together to generate the virulent strains that could be more difficult to treat. Therefore, it is necessary to reveal the molecular relationship between the bacterial virulence and resistance to manage the transmission and treatment of infectious diseases. Based on this, we have collected ESBLs-producing E. coli from BSI and studied its molecular characteristics and the relationship between the bacterial virulence and resistance, which could provide a sound reference for the clinical infection control.
Methods
Bacteria and Identification
We collected E. coli from the First Affiliated Hospital of Anhui Medical University in BSI from January 2020 to January 2021 randomly and eliminated the duplicate strains from the same patient. All the bacteria were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, BioMerieux Inc, France).
Phenotypic Detection of ESBLs Production
The phenotypic detection of ESBL production was conducted by a combined disc test. Briefly, the discs of cefotaxime (30μg, OXOID, UK), cefotaxime/clavulanic acid (30μg/10μg, OXOID, UK) and ceftazidime (30μg/10μg, OXOID, UK), cefotaxime/clavulanic acid (30μg/10μg, OXOID, UK) were used for the test. The positive control strain was taken from the characteristic strain of the laboratory, and the negative control strain was ATCC25922. The production results of ESBLs were thereafter analyzed according to the Clinical and Laboratory Standards Institute (CLSI 2022) guidelines.
AST
23 kinds of antibacterial drugs were tested by VITEK@2 compact system (BioMerieux Inc., France) and the K-B method. These included, amtreonam, ertapenem, gentamicin, tobramycin, compound simoxazole, cefotetan, amikacin, ampicillin, ampicillin/sulbactam, cefepime, ceftriaxone, ciprofloxacin, imipenem, levofloxacin, piperacillin/tazobactam, ceftazidime, cefazolin, cefotaxime (30μg, OXOID, UK), cefuroxime (30μg, OXOID, UK), meropenem (10μg, OXOID, UK), minocycline (30μg, OXOID, UK), piperacillin (100μg, OXOID, UK) and tetracycline (15μg, OXOID, UK). The results of all antibiotics except tetracycline were interpreted according to the guidelines of CLSI 2022, and the results of tetracycline were interpreted according to the break point recommended by the Food and Drug Administration (FDA) in 2022.
Detection of ESBLs Genes and the Virulence Genes
The encoding genes of ESBLs were as follows: blaTEM, blaSHV, blaCTX-M (variant), blaGES, blaCARB, blaPER, blaVEB and blaOXA, as well as carbapenemase genes (blaKPC and blaNDM-1) and colistin resistance gene mcr-1. The virulence genes included cnf1, fimH, hek/hra, hlyC, ibeA, iroN, iucC, kpsMII, nlpI, papGII-III, sfa/foc and ompA. The standard PCR method was used to detect the expression of all the above genes, and the specific primers used have been listed in Table 1. The PCR reaction was carried out under the following conditions: 30 cycles of 10s of denaturation at 98°C, 10s of annealing at specific temperature (Table 1), 20s of extension at 72°C, and a final additional 5 min at 72°C. The amplified products were observed by electrophoresis in 2% agarose gel and then sequenced (Shanghai Biotechnology, China) commercially. Finally, DNA sequences were analyzed by NCBI-BLAST program.
 |
Table 1 The Sequences of Primers and Corresponding PCR Programs Used in This Study |
MLST
The STs of ESBLs-producing E. coli were determined by MLST (Achtman). The internal parts of seven different housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, recA) of E. coli were amplified with specific primers (the primer sequences have been shown in Table 1) after extracting E. coli DNA by the thermal lysis. Similarly, the amplification products of these genes were observed by electrophoresis in 2% agarose gel and sequenced (Shanghai Sangong Biotechnology, China).
DRY LAB
The dry lab annotated the bioinformatics port of the nuclear acid-based technology.32 Bioinformatics-related information was often obtained through online and/or offline tools, software, databases as well as other network services. In this study, the determination of STs was primarily based on the genetic variation of seven housekeeper genes. Seven housekeeper genes were amplified and then sequenced by special primers and compared with the data in the online database (https://pubmlst.org/organisms/escherichia-spp) to obtain the specific sites of each gene. The STs of bacteria were determined by combining the sites of the seven housekeeping genes. Finally, all STs were imported into goEBURST software to analyze the cloning relationship.
Phylogenetic Group Detection
The main phylogenetic groups of all ESBLs-producing E. coli were detected by the multiple PCR as described previously by Clermont et al.33 Briefly, the existence or deletion of three DNA marker genes (chuA, yjaA and TspE4. C2) were used to group the isolates into distinct phylogenetic groups. It was specifically divided into the following four groups: group A (chuA −, TspE4. C2 −), group B2 (chuA +, yjaA +), group D (chuA +, yjaA −) and group B1 (chuA −, TspE4. C2 +). The primer pairs used were chuA.F (5’-GACGAACCAACGGTCAGGAT-3’) and chuA.R (5’-TGCCGCCAGTACCAAAGACA-3’), YjaA.F (5’-TGAAGTGTCAGGAGACGCTG-3’) and YjaA.R (5’-ATGGAGAATGCGTTCCTCAAC-3’), and TspE4.C2.F (5’-GAGTAATGTCGGGGCATTCA-3’) and TspE4.C2.R (5’-CGCGCCAACAAAGTATTACG-3’), which generate 279-, 211-, and 152-bp fragments, respectively.
Plasmid Conjugation Assay
Sodium azide-resistant E. coli J53 was used as a recipient, and ESBLs-producing E. coli was employed as the donor. We used liquid mating test to select the transfer complex on Luria and Bertani agar containing 100 (µg/mL) sodium azide and 100 (µg/mL) ampicillin, respectively.34 The presence of ESBLs genes including blaCTX-M, blaTEM, blaSHV and blaOXA-1 were then detected by PCR and sequencing (Shanghai Sangong Biotechnology, China).
PCR-Based Replicon Typing
PCR-based replicon typing was carried out for the plasmids isolated from the transconjugant isolates by using the plasmid extraction kit (Accurate Biology, China). The determination of the incompatibility (Inc) group was carried out by using the specific primers (the primer sequences have been shown in Table 2) as previously described by Caratoli et al in 2005.35 Five multiplex- and three simplex-PCRs were performed with 18 pairs of primers to identify FIA, FIB, FIC, HI1, HI2, I1-Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F, and FIIA replicons. All amplification products were then commercially sequenced (Shanghai Sangong Biotechnology, China).
 |
Table 2 Primers Used for Amplifying 18 Major Replicons from Plasmids |
Statistical Analysis
Statistical significance was carried out by the chi-square test. Analyses were performed using SPSS version 23.0, and a p-value <0.05 was considered to be statistically significant.
Results
Antimicrobial Susceptibility
In this study, 60 distinct strains of ESBLs-producing E. coli of BSI were randomly collected, including 26 males and 34 females, with an average age of 65.84 ± 13.386 years old. The fingerprints of two strains of E. coli by MALDI-TOF MS have been depicted in Figure 1. These 60 strains of E. coli were resistant to more than two different types of antibiotics, namely, multidrug-resistant E. coli. which were all resistant to ampicillin, ceftriaxone, cefotaxime, cefazolin, cefuroxime and piperacillin, but were all sensitive to tegacyclin, minocycline, ertapenem, meropenem and imipenem. The non-sensitivity rates of the remaining 12 antibiotics ranged from 6.7% to 98.3% (Table 3). As shown in Table 3, the non-sensitivity rates of E. coli to 13 antibiotics were observed to exceed 50%. In addition, the non-sensitivity rates of quinolones (levofloxacin and ciprofloxacin) were found to exceed 90%, whereas the non-sensitivity rates of piperacillin/tazobactam, cefotetan and amikacin were lower than 20%.
 |
Table 3 Non-Sensitivity Rates of 60 E. coli Strains to 23 Antibiotics |
Drug Resistance Genes
Multiple resistance genes were detected in 60 different strains of E. coli, including blaCTX-M, blaTEM, blaSHV, blaOXA-1 and mcr-1 (Table 4), and no blaPER, blaGES, blaVEB, blaKPC, blaNDM-1, blaOXA-2, blaOXA-10. Among them, blaCTX-M was the most common one and found in all E. coli. However, we were able to only detect the groups blaCTX-M-1 and blaCTX-M-9 but not groups blaCTX-M-2, blaCTX-M-8 and blaCTX-M-25. Figure 2A shows the partial electrophoretic results of 2% agarose gel of the different resistance genes. The sequencing results indicated that the blaCTX-M was distributed in five distinct subtypes, namely blaCTX-M-27, blaCTX-M-14, blaCTX-M-15, blaCTX-M-55 and blaCTX-M-65. Among them, blaCTX-M-27, blaCTX-M-14 and blaCTX-M-55 were relatively common (n = 45, 75%), whereas blaCTX-M-15 and blaCTX-M-65 were relatively rare (n = 16, 26.7%).
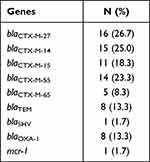 |
Table 4 Distribution of Resistance Genes in 60 Strains of E. coli |
Phylogenetic Group and MLST
The four phylogenetic groups were B2 (n = 27), D (n = 19), A (n = 11) and B1 (n = 3). The results of phylogenetic grouping depicted that the highly toxic groups B2 and D (n = 46, 76.7%) were more prevalent than the weakly toxic groups A and B1 (n = 14, 23.3%). In addition, we observed that the phylogenetic distributions of five blaCTX-M subtypes were significantly different (Table 5). And the result of blaCTX-M-55 exhibited statistical difference. In addition, after grouping the bacteria according to their virulence, blaCTX-M-55 still displayed unique difference, which were more common in groups A and B1 with weak toxicity, but the distributions of other subtypes were not different (Table 6). A total of 31 STs were found by MLST, among which the most common was ST131 (n = 16), and each other ST type was less than 3 strains (Table 7). The PCR results of seven housekeeper genes have been shown in Figure 2B. The results of the single local variants (SLVs) of goEBURST (Figure 3) indicated that 31 STs were divided into 23 different groups, among which 10 STs were distributed in 3 clone groups, whereas the other 21 STs were distributed in a single group, which suggested that the cloning relationship of these 60 strains was far apart. The statistical analysis of the relationships between eight positive resistance genes and ST131 indicated that there were significant differences in the distribution of blaCTX-M-27 and blaCTX-M-55 between the two types (P < 0.05, Table 8). The blaCTX-M-27 was mostly found in ST131 strains, but blaCTX-M-55 was only found in non-ST131 strains. In addition, statistical differences were observed between blaTEM, blaOXA-1 and blaCTX-M subtypes after classifying E. coli according to blaCTX-M subtypes (Table 9). blaTEM and blaOXA-1 could only be found in blaCTX-M-27 and blaCTX-M-55 strains, respectively.
 |
Table 5 Distribution of Five blaCTX-M Subtypes in Phylogenetic Groups |
 |
Table 6 Distribution of Five blaCTX-M Subtypes in Groups A+B1 and B2+D |
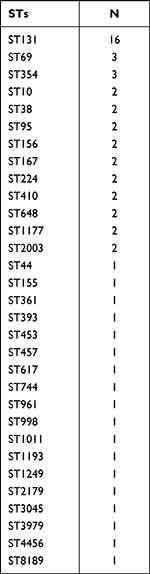 |
Table 7 ST Distribution of 60 Strains of E. coli |
 |
Table 8 Distribution of 8 Resistance Genes in ST131 and Non-ST131 E. coli |
 |
Table 9 Distribution of 3 Resistance Genes in Three blaCTX-M Subtypes of ST131 |
Distribution of VFs
The detection rates of 12 VFs in E. coli ranged from 1.7% to 100%, among which fimH, nlpI and ompA were detected in all the strains (Table 10). The electrophoretic results of partial VFs have been shown in Figure 2C. Interestingly, the phylogenetic grouping suggested that groups B2 and D were more virulent in comparison to the groups A and B1. In addition, statistical analysis revealed that the three virulence genes (hek-hra, kpsM and papGII-III) in the groups B2 and D were substantially more than that in groups A and B1 (Table 11). Moreover, we found that four virulence genes were different between ST131 and non-ST131 strains (P < 0.05, Table 12). Among them, cnf1, iucC, papGII-III were mostly seen in ST131 strains, whereas iroN was predominantly observed in non-ST131 strains. Finally, we analyzed the relationship between the bacterial toxicity and resistance and found that there were no statistical differences in the distribution of VFs between the groups blaCTX-M-1 and blaCTX-M-9 (P >0.05, data not shown). However, it was found that the distribution of five virulence genes in the five blaCTX-M subtype strains exhibited statistical differences after classifying them according to the blaCTX-M subtypes (P < 0.05, Table 13), which were hek/hra, iroN, iucC, kpsMII and papGII-III.
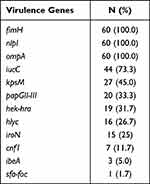 |
Table 10 Distribution of 12 Virulence Genes in E. coli |
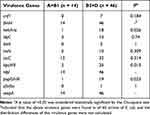 |
Table 11 Distribution of 12 Virulence Genes in Groups A+B1 and B2+D |
 |
Table 12 Distribution of 12 Virulence Genes in ST131 and Non-ST131 E. coli |
 |
Table 13 Distribution of 12 Virulence Genes in Five blaCTX-M Subtypes of E. coli |
Plasmid Conjugation Assay
The results of the conjugative assays showed that 32 transconjugates grew on the plates containing sodium azide and ampicillin, whereas the donor and recipient bacteria were unable to grow. The plasmids of these transconjugates were found to contain resistance genes including blaCTX-M and part of blaOXA-1 after PCR amplification and sequencing. The transconjugates of the remaining 28 strains of E. coli did not grow well on the dual-resistant plates. The results of PCR-based replicon typing showed that only one replicon was found in 18 among 32 transconjugates, namely, IncFIB (n = 12), IncFI1 (n = 5) or IncFHI2 (n = 1). In addition, 9 of 32 contained two replicons, and 5 of 32 contained three replicons. The partial PCR results of replicons have been shown in Figure 2D.
Discussion
E. coli is a common pathogen found in hospital-acquired infections. In 2021, statistical data of bacterial drug resistance monitoring in China revealed that the detection rate of E. coli ranked first among the various detected pathogens.14 The various drugs used to treat E. coli including penicillin and cephalosporin (β-Lactam), ciprofloxacin and levofloxacin (fluoroquinolones) and so on.3 In addition, the resistance rates of E. coli to six diverse kinds of antibiotics had exceeded 50%, including ESBLs-producing E. coli (52.4%) in bacterial drug resistance monitoring in China in 2021.14 And the monitoring of BSI pathogens in the hospital in 2020 showed that 60.9% of E. coli produced ESBLs.36 The emergence of ESBLs has led to the inactivation of several types of antibiotics, including penicillins, cephalosporins, and monocyclics. This high drug resistance rate has made it difficult for the current clinical infection control. Therefore, ESBLs-producing E. coli has emerged as an important target for the treatment of BSI. In this study, the non-sensitivity rates of 60 E. coli strains to 13 antibiotics exceeded 50%, whereas the non-sensitivity rates of quinolones (91.7% for levofloxacin and 98.3% for ciprofloxacin) exceeded 90%. The high drug resistance rate effectively reduced the number of antibiotics that can be used for management of infection. Therefore, the high drug resistance of ESBLs-producing E. coli constitutes a major challenge in the treatment of BSI. In addition, E. coli was found to be sensitive to carbapenems (imipenem, meropenem, ertapenem), minocycline and tegacyclin, but these antibiotics were high-grade antibiotics, which are not commonly used as the first choice for treatment, while the non-sensitivity rates of piperacillin/tazobactam, cefotetan and amikacin were less than 20%. Thus, it could be recommended that the clinicians should carefully choose these three highly sensitive antibiotics for the treatment of ESBLs-producing E. coli. The sensitivity of bacteria to the drugs could be affected by many factors, including irrational use of antibiotics, transmission of the resistant plasmids and so on.37,38 Therefore, it could be recommended that the doctors should use antibiotics reasonably to reduce the occurrence of drug-resistant bacteria and, at the same time, effectively aim to strengthen the control of hospital infection to reduce the spread of drug-resistant bacteria.
ESBLs, including TEM, SHV, CTX-M and OXA enzymes, have been identified as the main resistance mechanism to β-lactam antibiotics.39 Among them, The CTX-M first discovered by Bauernfeind et al in 1990 which could preferentially hydrolyze cefotaxime compared to the TEM and SHV enzymes.40 At present, there are more than 150 varieties of CTX-M (www.lahey.org/studies), which have emerged as the most common ESBL. blaCTX-M has been reported to spread worldwide and could be divided into different groups: blaCTX-M-1, blaCTX-M-2, blaCTX-M-8, blaCTX-M-9 and blaCTX-M-25. Among these, the most common were groups blaCTX-M-1 and blaCTX-M-9, in which blaCTX-M-14 and blaCTX-M-15 were the most common subtypes in recent years.41 In this study, we detected a variety of drug resistance genes, and the most common was blaCTX-M, which was mainly distributed in five subtypes. These included blaCTX-M-27 (n = 16), blaCTX-M-14 (n = 15), blaCTX-M-15 (n = 11), blaCTX-M-55 (n = 14) and blaCTX-M-65 (n = 5). It could be observed that at this stage, blaCTX-M-27 had a high prevalence among the patients with BSI in the hospital. In addition, the SENTRY antibacterial monitoring study in 2016 showed that, compared with blaCTX-M-15 (55.5%),13 blaCTX-M-27 (17.3%) was also important in E. coli-induced UTI and BSI. However, studies conducted 10 years ago indicated that blaCTX-M-14 was the most common gene in China, followed by blaCTX-M-3.42 blaCTX-M-3 and blaCTX-M-15 were two common genes in Europe.43 blaCTX-M-14 and blaCTX-M-15 were also considered to be two dominant genes in ESBLs-producing E. coli in Asia.44 Therefore, the epidemiology of blaCTX-M might change according to place of origin. In addition, we found that the bacterium carried both blaCTX-M and mcr-1, mcr-1 primarily mediated the resistance to colistin, which was the last line of defense in the treatment of E. coli.45 The co-existence of the multiple resistance genes could make it more difficult to treat for infection. The spread of the bacterial resistance primarily depends on the transfer of the resistant plasmids, including the conjugated plasmids and non-conjugated plasmids. These plasmids could be transferred by means of separate transfer, co-transfer with the conjugated plasmids, and formation of the hybrid plasmids or homologous recombinant plasmids, which ultimately can lead to difficulties in treatment.46 Interestingly, prior reports have indicated that blaCTX-M appeared on a variety of plasmids, including IncF, IncI, IncA/C, IncN and IncH, etc.47 We found that 53% of the plasmids could be successfully combined and transferred, especially IncFIB. Thus, it was concluded that the IncFIB plasmid was an important cause of blaCTX-M transmission.
Clermont et al33 proposed a simple and rapid phylogenetic grouping technique based on the triple PCR earlier in 2000. According to this method, E. coli was divided into four major phylogenetic groups (A, B1, B2 and D). The extraintestinal virulent strains mainly belonged to groups B2 and D, whereas the commercial strains primarily belonged to groups A and B1. However, the emergence of complicated scientific research technology has shed light on additional MLST data and revealed novel strain nucleotide sequences. Therefore, Clermont et al were able to improve the above-described method in 2013 and further divided E. coli into distinct groups (A, B1, B2, C, D, E, and F) and class I.48 In this study, we have classified E. coli according to the toxicity and discussed the relationship between VFs and toxicity, as well as the relationship between bacterial toxicity and resistance. Hence, the old Clermont typing method was more suitable for our study. The phylogenetic groups indicated that most E. coli belonged to the highly toxic groups B2 and D (n = 46, 76.7%), which was consistent with the previous reports that most of the E. coli strains that could cause human UTI and other parenteral infections belonged to group B2, or to a lesser extent, group D, and only a few belonged to groups A and B1.49 The results of the statistical analysis showed that the phylogenetic grouping of blaCTX-M-55 was statistically different, and it was found to be more common in groups A and B1. However, the distributions of other subtypes were not significantly different. This observation suggested that the strains containing blaCTX-M-55 were generally less toxic. The toxicity of E. coli was controlled by VFs, and their roles in regulating toxicity have been discussed in the next section. In addition, previous studies have indicated that the population structure of E. coli harbouring blaCTX-M was mainly dominated by the high-risk clone ST131. The success of this clone could be attributed to the acquisition of antimicrobial resistance, especially the resistance to fluoroquinolones.50 Similarly, the highest detection rate of ST131 in this study was 26.7% (16/60). However, other important epidemic clones, including ST38, ST69, ST410 and ST1193, ST10, were also detected in this report. These clones were usually resistant to the broad-spectrum cephalosporins and quinolones. The distribution of eight drug resistance genes in ST131 and non-ST131 indicated that blaCTX-M-27 was mostly found in ST131, but blaCTX-M-55 was only detected in non-ST131. Overall, combined with the above phylogenetic results, we were able to conclude that blaCTX-M-27 and blaCTX-M-55 were mostly found in highly toxic ST131 and weakly toxic non-ST131 strains, respectively.
E. coli possesses a variety of VFs to make it resist different defense mechanisms, including fimbriae, flagella, and toxins. Different VFs and hosts can effectively form complex interactions. And understanding the difference of VFs could aid in understanding the pathogenic mechanism of E. coli. In this study, 12 virulence genes were identified, and these VFs participated in the process of extraintestinal pathogenesis, of which FimH, Hek/hra, PapGII-II, and Sfa/foc belonged to adhesins. They could enable the bacteria to adhere to a variety of cells and mucosal surfaces and play important roles in the development of bacterial toxicity.51 Iron absorption factors iroN and iucC were the typical VFs of ExPEC, which were essential for E. coli to survive in the urinary tract, because the iron availability in the urinary tract was extremely deficient, and all the bacteria must absorb iron from the host proteins (such as heme). They also participated in the biosynthesis and virulence formation of the bacteria. In addition, prior studies have found that UPEC could more easily breakthrough the urethral mucosal barrier, leading to urinary tract infection and even septicemia when Aerobacin was highly expressed.52 The protectin kpsM encoded proteins are related to the capsular polysaccharide output. The capsular was identified as a VF related to immune escape of bacteria, which could be used by the bacteria to resist adverse external environment and adhere to the specific cell surfaces to attack the target cells.53 OmpA was a transmembrane protein that could effectively combine with the N-glucosamine epitope of the glycoprotein Gp96 on the surface of human microvascular endothelial cells to effectively enhance the antiserum effect of E. coli and increase the invasion of the blood–brain barrier.54 Finally, NlpI was involved in regulation of cell adhesion, invasion and division and can aid the bacteria escape the immune killing effect of the host.55 The function of Cnf1, HlyC and IbeA has been described in the introduction section.
In this study, the detection rates of 12 VFs varied from 1.7% to 100%. Interestingly, similar to our results, Johnson et al reported13,56 that the detection rates of VFs varied from 0.4% to 98%. and the high-risk epidemic ST131 isolates carried more virulence genes in comparison to the non-ST131 isolates, especially iha, hlyD, sat, iutA, fyuA, malX, ompT and traT, tsh and espC.57 In this report, cnf1, iroN, iucC, papGII-III were also related to ST131 (P < 0.05). Among them, cnf1, iucC, papGII-III were mostly found in ST131, which suggested that these VFs might play significant roles in the toxicity of ST131. In addition, compared with the groups B1 and A, groups B2 and D with stronger toxicity possessed more VFs, and hek-hra, kpsM, papGII-III showed statistical differences between the two groups. Hence, these might play stronger roles in controlling the toxicity of E. coli. The potential relationship between the bacterial virulence and resistance remains a hotspot in pathogen research.49 There are differences found in the resistance of bacteria with different toxicity. It was reported that the virulence genes appeared more frequently in carbapenemase producers than in the non-producers, and fimA, iutA and cnf1 exhibited significant differences.13,56 In this study, we found that hek/hra, iroN, iucC, kpsM, and papGII-III showed statistical differences among blaCTX-M subtypes. This finding suggested that there might be a possible relationship between the bacterial virulence and resistance. However, some limitations of our study include that the results might not be universal enough, and further studies were needed to evaluate a greater number of hospitals. The emergence of ESBL-producing strains encoding various VFs was a focus of infection treatment. Therefore, appropriate implementation of the monitoring plans could be crucial to limit their transmission.
Conclusion
In conclusion, ESBLs-producing E. coli emerged as a common pathogen of BSI and was found to be resistant to many antibiotics. The common resistance genes included blaCTX-M, blaTEM, blaSHV and blaOXA-1. Among them, blaCTX-M was the main resistance gene. The blaCTX-M subtypes mainly included blaCTX-M-27, blaCTX-M-14, blaCTX-M-15,blaCTX-M-55 and blaCTX-M-65. The pathogenicity of blaCTX-M had changed to a certain extent over time. Compared with the results of ten years ago in China, in which blaCTX-M-14 and blaCTX-M-3 accounted for the majority, in recent years, blaCTX-M-27, blaCTX-M-14 and blaCTX-M-55 have been identified as novel epidemic genes. The transmission of these resistant genes mostly depended on the transfer of the resistant plasmids. A variety of ESBLs-producing E. coli clones were prevalent in the hospitals, including ST131, as well as other common popular clones. VFs were pathogenic factors of E. coli, with the detection rates ranging from 1.7% to 100%. In addition, their distributions showed clear statistical differences among distinct types of E. coli. The emergence of highly resistant and highly virulent pathogens could lead to serious infection. Hence, real-time monitoring and limiting its transmission were essential for management of infection. At the same time, further research is needed to explore the specific role of VFs as pathogenic factors.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval and Consent to Participate
This study has been approved by the ethics review committee of the First Affiliated Hospital of Anhui Medical University and was conducted in accordance with the Declaration of Helsinki. And the committee agreed to waive the informed consent of the participants (Quick-PJ 2023-01-25).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Anhui Provincial Natural Fund (Project’s number: 9021138203) and the Subject Construction Fund of the First Affiliated Hospital of Anhui Medical University (Project’s number: 9001001855).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kobayashi T, Ikeda M, Okada Y, et al. Clinical and microbiological characteristics of recurrent Escherichia coli bacteremia. Microbiol Spectr. 2021;9(3):e0139921. doi:10.1128/Spectrum.01399-21
2. Behzadi P, Najafi A, Behzadi E, Ranjbar R. UMicroarray long oligo probe designing for Escherichia coli: an in-silico DNA marker extraction. Cent Eur J Urol. 2016;69(1):105–111. doi:10.5173/ceju.2016.654
3. Issakhanian L, Behzadi P. Antimicrobial agents and urinary tract infections. Curr Pharm Des. 2019;25(12):1409–1423. doi:10.2174/1381612825999190619130216
4. Kondo T, Sakamoto K, Morinaga Y, et al. Escherichia coli ST131 isolated from urological patients can acquire plasmid-mediated extended spectrum β-lactamase from other bacteria with high frequency. Int J Urol. 2022;29(6):587–594. doi:10.1111/iju.14845
5. Mostafa HH, Cameron A, Taffner SM, et al. Genomic surveillance of ceftriaxone-resistant Escherichia coli in Western New York suggests the extended-spectrum β-lactamase blaCTX-M-27 is emerging on distinct plasmids in ST38. Front Microbiol. 2020;11:1747. doi:10.3389/fmicb.2020.01747
6. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi:10.1128/CMR.18.4.657-686.2005
7. Sharma J, Sharma M, Ray P. Detection of TEM & SHV genes in Escherichia coli & Klebsiella pneumoniae isolates in a tertiary care hospital from India. Indian J Med Res. 2010;132:332–336.
8. Gomi R, Yamamoto M, Tanaka M, Matsumura Y. Chromosomal integration of blaCTX-M genes in diverse Escherichia coli isolates recovered from river water in Japan. Curr Res Microb Sci. 2022;3:100144. doi:10.1016/j.crmicr.2022.100144
9. Pourmohsen M, Shakib P, Zolfaghari MR. The prevalence of bla VIM, bla KPC, bla NDM, bla IMP, bla SHV, bla TEM, bla CTX-M, and class I and II integrons genes in Aeromonas hydrophila isolated from clinical specimens of Qom, Iran. Clin Lab. 2023;69(1). doi:10.7754/Clin.Lab.2022.220314
10. Martischang R, François P, Cherkaoui A, et al. Epidemiology of ESBL-producing Escherichia coli from repeated prevalence studies over 11 years in a long-term-care facility. Antimicrob Resist Infect Control. 2021;10(1):148. doi:10.1186/s13756-021-01013-7
11. Hertz FB, Jansåker F, Okon KO, et al. ESBL-production in Escherichia coli and Klebsiella pneumoniae isolates from Nigeria. Microbiologyopen. 2019;8(9):e00816. doi:10.1002/mbo3.816
12. CDC. Antibiotic resistance threats in the United States; 2019. Available from: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
13. Mendes RE, Jones RN, Woosley LN, et al. Application of next-generation sequencing for characterization of surveillance and clinical trial isolates: analysis of the distribution of β-lactamase resistance genes and lineage background in the United States. Open Forum Infect Dis. 2019;6(Suppl 1):S69–S78. doi:10.1093/ofid/ofz004
14. Hu F, Guo Y, Zhu D, et al. Surveillance of bacterial drug resistance in China in 2021. J Chin Infect Chemother. 2022;22(05):521–530. doi:10.16718/j.1009-7708.2022.05.001
15. Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018;62(10):e01076–18. doi:10.1128/AAC.01076-18
16. Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–976. doi:10.1128/AAC.01009-09
17. Pongchaikul P, Mongkolsuk P. Comprehensive analysis of imipenemase (IMP)-type metallo-β-lactamase: a global distribution threatening Asia. Antibiotics. 2022;11(2):236. doi:10.3390/antibiotics11020236
18. Behzadi P, García-Perdomo HA, Karpiński TM, Issakhanian L. Metallo-ß-lactamases: a review. Mol Biol Rep. 2020;47(8):6281–6294. doi:10.1007/s11033-020-05651-9
19. Palacios AR, Mojica MF, Giannini E, et al. The reaction mechanism of metallo-β-lactamases is tuned by the conformation of an active-site mobile loop. Antimicrob Agents Chemother. 2018;63(1):e01754–18. doi:10.1128/AAC.01754-18
20. Ju LC, Cheng Z, Fast W, Bonomo RA, Crowder MW. The continuing challenge of metallo-β-lactamase inhibition: mechanism matters. Trends Pharmacol Sci. 2018;39(7):635–647. doi:10.1016/j.tips.2018.03.007
21. Sianipar O, Asmara W, Dwiprahasto I, Mulyono B. Mortality risk of bloodstream infection caused by either Escherichia coli or Klebsiella pneumoniae producing extended-spectrum β-lactamase: a prospective cohort study. BMC Res Notes. 2019;12(1):719. doi:10.1186/s13104-019-4751-9
22. Behzadi P. Classical chaperone-usher (CU) adhesive fimbriome: uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol (Praha). 2020;65(1):45–65. doi:10.1007/s12223-019-00719-x
23. Lüthje P, Brauner A. Virulence factors of uropathogenic E. coli and their interaction with the host. Adv Microb Physiol. 2014;65:337–372. doi:10.1016/bs.ampbs.2014.08.006
24. Tabasi M, Asadi Karam MR, Habibi M, Yekaninejad MS, Bouzari S. Phenotypic assays to determine virulence factors of uropathogenic Escherichia coli (UPEC) isolates and their correlation with antibiotic resistance pattern. Osong Public Health Res Perspect. 2015;6(4):261–268. doi:10.1016/j.phrp.2015.08.002
25. Hozzari A, Behzadi P, Kerishchi Khiabani P, Sholeh M, Sabokroo N. Clinical cases, drug resistance, and virulence genes profiling in uropathogenic Escherichia coli. J Appl Genet. 2020;61(2):265–273. doi:10.1007/s13353-020-00542-y
26. Da Silva GJ, Mendonça N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence. 2012;3(1):18–28. doi:10.4161/viru.3.1.18382
27. Biggel M, Moons P, Nguyen MN, Goossens H, Van Puyvelde S. Convergence of virulence and antimicrobial resistance in increasingly prevalent Escherichia coli ST131 papGII+ sublineages. Commun Biol. 2022;5(1):752. doi:10.1038/s42003-022-03660-x
28. Lee S, Yu JK, Park K, Oh EJ, Kim SY, Park YJ. Phylogenetic groups and virulence factors in pathogenic and commensal strains of Escherichia coli and their association with blaCTX-M. Ann Clin Lab Sci. 2010;40(4):361–367.
29. Wang S, Zhao SY, Xiao SZ, et al. Antimicrobial resistance and molecular epidemiology of escherichia coli causing bloodstream infections in three hospitals in Shanghai, China. PLoS One. 2016;11(1):e0147740. doi:10.1371/journal.pone.0147740
30. Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181(1):261–272. doi:10.1086/315217
31. Tartof SY, Solberg OD, Manges AR, Riley LW. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J Clin Microbiol. 2005;43(12):5860–5864. doi:10.1128/JCM.43.12.5860-5864.2005
32. Behzadi P, Ranjbar R. DNA microarray technology and bioinformatic web services. Acta Microbiol Immunol Hung. 2019;66(1):19–30. doi:10.1556/030.65.2018.028
33. Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66(10):4555–4558. doi:10.1128/AEM.66.10.4555-4558.2000
34. Paul D, Dhar D, Chakravarty A, et al. Transcriptional analysis of IncF repB-mediated bla OXA-48-positive plasmid characterized from Escherichia coli ST448. Microb Drug Resist. 2021;27(5):596–601. doi:10.1089/mdr.2019.0486
35. Carattoli A, Bertini A, Villa L, et al. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–228. doi:10.1016/j.mimet.2005.03.018
36. Kondore N. Identification of pathogenic bacteria in bloodstream infection by mass spectrometry, drug resistance monitoring and clinical characteristics. Chin Modern Doctors. 2021;59(21):114–119.
37. Phan MD, Peters KM, Alvarez Fraga L, et al. Plasmid-mediated ciprofloxacin resistance imparts a selective advantage on Escherichia coli ST131. Antimicrob Agents Chemother. 2022;66(1):e0214621. doi:10.1128/AAC.02146-21
38. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241–251. doi:10.1016/S1473-3099(20)30477-1
39. Ibrahim ME, Algak TB, Abbas M, et al. Emergence of bla TEM, bla CTX‑M, bla SHV and bla OXA genes in multidrug‑resistant Enterobacteriaceae and Acinetobacter baumannii in Saudi Arabia. Exp Ther Med. 2021;22(6):1450. doi:10.3892/etm.2021.10885
40. Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18(5):294–298. doi:10.1007/BF01647010
41. Rana C, Rajput S, Behera M, et al. Global epidemiology of CTX-M-type β-lactam resistance in human and animal. Comp Immunol Microbiol Infect Dis. 2022;86:101815. doi:10.1016/j.cimid.2022.101815
42. Liu W, Chen L, Li H, et al. Novel CTX-M {beta}-lactamase genotype distribution and spread into multiple species of Enterobacteriaceae in Changsha, Southern China. J Antimicrob Chemother. 2009;63(5):895–900. doi:10.1093/jac/dkp068
43. Livermore DM, Canton R, Gniadkowski M, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007;59(2):165–174. doi:10.1093/jac/dkl483
44. Song W, Lee H, Lee K, et al. CTX-M-14 and CTX-M-15 enzymes are the dominant type of extended-spectrum beta-lactamase in clinical isolates of Escherichia coli from Korea. J Med Microbiol. 2009;58(Pt2):261–266. doi:10.1099/jmm.0.004507-0
45. Wang R, van Dorp L, Shaw LP, et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun. 2018;9(1):1179. doi:10.1038/s41467-018-03205-z
46. Yang X, Wai-Chi Chan E, Zhang R, et al. A conjugative plasmid that augments virulence in Klebsiella pneumoniae. Nat Microbiol. 2019;4(12):2039–2043. doi:10.1038/s41564-019-0566-7
47. Rozwandowicz M, Brouwer MSM, Fischer J, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73(5):1121–1137. doi:10.1093/jac/dkx488
48. Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58–65. doi:10.1111/1758-2229.12019
49. Millán Y, Araque M, Ramírez A. Distribution of phylogenetic groups, virulence factors and antimicrobial susceptibility in strains of uropathogenic Escherichia coli. Rev Chilena Infectol. 2020;37(2):117–123. doi:10.4067/s0716-10182020000200117
50. Zeng S, Luo J, Li X, et al. Molecular epidemiology and characteristics of CTX-M-55 extended-spectrum β-lactamase-producing Escherichia coli from Guangzhou, China. Front Microbiol. 2021;12:730012. doi:10.3389/fmicb.2021.730012
51. Maciel JF, Matter LB, Trindade MM, et al. Virulence factors and antimicrobial susceptibility profile of extraintestinal Escherichia coli isolated from an avian colisepticemia outbreak. Microb Pathog. 2017;103:119–122. doi:10.1016/j.micpath.2016.12.020
52. Aluta RP, Borges CA, Beraldo LG, et al. Frequencies of virulence genes and pulse field gel electrophoresis fingerprints in Escherichia coli isolates from canine pyometra. Vet J. 2014;202(2):393–395. doi:10.1016/j.tvjl.2014.08.016
53. Johnson JR, O’Bryan TT. Detection of the Escherichia coli group 2 polysaccharide capsule synthesis Gene kpsM by a rapid and specific PCR-based assay. J Clin Microbiol. 2004;42(4):1773–1776. doi:10.1128/JCM.42.4.1773-1776.2004
54. Wang Y. The function of OmpA in Escherichia coli. Biochem Biophys Res Commun. 2002;292(2):396–401. doi:10.1006/bbrc.2002.6657
55. Barnich N, Bringer MA, Claret L, Darfeuille-Michaud A. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn’s disease. Infect Immun. 2004;72(5):2484–2493. doi:10.1128/IAI.72.5.2484-2493.2004
56. Pinheiro JJ, Gazal LES, de Araujo GO, et al. Occurrence of genes associated with virulence in Escherichia coli isolates from chicken carcasses at different stages of processing at a slaughterhouse. Braz J Microbiol. 2021;52(4):2413–2420. doi:10.1007/s42770-021-00549-5
57. Hung W-T, Cheng M-F, Tseng F-C. Bloodstream infection with extended-spectrum beta-lactamase-producing Escherichia coli: the role of virulence genes. J Microbiol Immunol Infect. 2019;52(6):947–955. doi:10.1016/j.jmii.2019.03.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.