Back to Journals » Biologics: Targets and Therapy » Volume 17
The Cancer Driver Genes IDH1 and IDH2 and CD204 in WHO-Grade 4 Astrocytoma: Crosstalk Between Cancer Metabolism and Tumour Associated Macrophage Recruitment in Tumour Microenvironment
Authors Kurdi M , Mulla N, Katib Y, Alsinani T, Hakamy S, MJ Addas B, Malibary H , Halawa TF, S Farhan M, Faizo E, Baeesa S
Received 23 October 2022
Accepted for publication 1 February 2023
Published 5 February 2023 Volume 2023:17 Pages 15—22
DOI https://doi.org/10.2147/BTT.S394556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Maher Kurdi,1,2 Nasser Mulla,3 Yousef Katib,4 Taghreed Alsinani,5 Sahar Hakamy,2 Bassam MJ Addas,6 Husam Malibary,7 Taher F Halawa,8 Marwa S Farhan,9 Eyad Faizo,10 Saleh Baeesa6
1Department of Pathology, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia; 2Brain Tumour Unit, KFMRC, King Abdulaziz University, Jeddah, Saudi Arabia; 3Department of Internal Medicine, Faculty of Medicine, Taibah University, Medina, Saudi Arabia; 4Department of Radiology, Faculty of Medicine, Taibah University, Madinah, Saudi Arabia; 5Department of Surgery, King Fahad General Hospital, Jeddah, Saudi Arabia; 6Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 7Department of Internal Medicine, Faculty of Medicine, King Abdul Aziz University, Jeddah, Saudi Arabia; 8Pediatric Hematology and Oncology Consultant, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia; 9Clinical Pathology Department, Cairo University, Cairo, Egypt; 10Division of Neurosurgery, Department of Surgery, Faculty of Medicine, Tabuk University, Tabuk, Saudi Arabia
Correspondence: Maher Kurdi, Department of Pathology, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia, Tel +966 556655467, Email [email protected]
Purpose: IDH1 and IDH2 are hotspot mutations commonly identified in WHO-grade 4 astrocytomas. Their association with TAMs has never been investigated. We aim to explore the crosstalk between the IDH1/2 mutation metabolic effect and TAMs in tumour microenvironment and how this relationship affects the tumour recurrence.
Patients and Methods: The study included 20 samples of patients with WHO-grade 4 astrocytoma. The alteration hotspot in codon IDH1R132 and IDH2R172 was examined using direct sequencing. The protein expression of CD204 on TAM was detected through immunohistochemistry.
Results: IDH1R132 and IDH2R172 were symmetrically identified as wildtype in 18/20 tumours (90%) and the remaining 2 tumours (10%) showed synonymous mutations on both codons. Tumours with IDH1/2-wildtype showed high expression of CD204+TAMs in 10 cases and low expression in 8 cases. Typical expression was seen equally in IDH1/2 mutant tumours. There was no significant association between IDH1/2 and CD204+TAM expression (p= 0.999). The association between the two groups was significantly observed among IDH-wildtype tumours (p=0.027). Highly expressed CD204 in IDH-wildtype tumours showed a median recurrence at 10 months compared to low CD204 expression, showed a median recurrence interval at 24 months.
Conclusion: IDH1R132 or IDHR172 has the same impact on the classification and prognosis of WHO-grade 4 astrocytoma. There was no crosstalk between IDH1/2 metabolic effect and CD204+TAM. However, IDH-wildtype glioblastomas with dense CD204+TAM are associated with early recurrence. Because the sample size is small, a larger study is recommended to determine the impact of IDH1/2 on TAMs.
Keywords: WHO-grade 4 astrocytoma, IDH1/2 mutation, TAM, CD204, recurrence
A Letter to the Editor has been published for this article.
Introduction
WHO-grade 4 astrocytoma is the most common primary malignant brain tumor in adults.1 The current 5th edition of the world health organization (WHO) of central nervous system (CNS) tumours has adapted a new classification based on IDH1/2 mutation, in which the terminology IDH-mutant glioblastoma was omitted and defined as IDH-mutant WHO-grade 4 astrocytoma. Glioblastoma is referred to as IDH-wildtype glioblastoma.1,2 This new segregation will increase the understanding of biological behavior of the tumour and their response to different multimodal treatments. The current treatment for WHO-grade 4 astrocytoma is surgical resection and radiotherapy followed by adjuvant chemotherapy. Nevertheless, the outcome of the disease remains fatal, and the overall survival (OS) is 15 months within 5 years.3
Isocitrate dehydrogenase (IDH) genes encode for the IDH1 and IDH2 isoenzymes that catalyze the oxidative decarboxylation of isocitrate to alpha-ketoglutarate and are essential for cellular metabolism.4 Both genes are located on the chromosome 2q34 and 15q26 respectively. IDH1/2 mutations comprise genetic modification in cellular function.5 The modifications involve variants in missense, causing a substitution of arginine in the IDH1/2 genes. The mutations occur in the early phase of tumorigenesis and can determine the cell fate and differentiation. Hotspot mutations at R132 in IDH1 and R172 in IDH2 cause high enzymatic activity resulting in an excessive production of D-2-hydroxyglutarate. This oncometabolite alters the cellular metabolism and the DNA histone methylation.5 Up to date, there is no clear evidence if IDH1/2 oncometabolites interact with the cells in tumour microenvironment.
In tumour microenvironment, the main dominant cells are tumour associated macrophages (TAMs) and tumour infiltrating lymphocytes (TILs). They interact with glial tumour cells to accelerate the progression of the tumor. Recent studies provided an insight about the influence of IDH mutations on glioma microenvironment. Interferon (IFN-γ) and CD8 cytotoxic T-cells-associated genes were expressed at low levels in IDH-mutant tumors compared to IDH-wildtype tumors.6 The high frequency of TAMs was proven to be associated with elevated expressions of CD163, CD204, and CD206 receptors on TAMs.7 However, their role in gliomagenesis remain unclear. Few studies have discovered the expression of CD204, macrophage scavenger receptor 1, in several cancers.8–10 Kurdi et al revealed that CD204 is an independent predictive marker for glioblastoma.11 Although the interaction between TAMs and TILs has not been well studied, they may work in concert with CD204+TAMs to act as immunological checkpoint regulators that suppress T-cells.11 The association between CD204+TAMs and IDH1 mutation in tumour microenvironment of glioblastoma were not significant according to Kurdi et al.12
In our study, we explored the crosstalk between the metabolic effect of IDH1/IDH2 and CD204+TAM recruitment in tumour microenvironment, and how this relationship can affect tumour recurrence.
Materials and Methods
The study included the clinical information of 20 patients diagnosed with WHO-grade 4 astrocytoma, in the period between 2017 and 2019. The study was approved by the National Biomedical Ethics Committee at King Abdulaziz University (HA-02-J-008) under a general ethical report. All patients involved in the study have been consented to release their medical information and using their clinical samples before starting the study. All patients had total resection of the tumor followed by chemoradiotherapy. The histological diagnosis followed the recent 5th edition of the WHO classification of CNS tumours. Clinical data included patient age at diagnosis, gender, location of the tumour, received adjuvant therapies and recurrence interval time (Table 1).
 |
Table 1 Clinical and Biological Information of All Patients in the Study Group (n=20 |
Formalin-fixed paraffin embedded tissue (FFPE) of each tumour was collected for immunohistochemistry of anti-CD204 antibody and IDH1/2 DNA sequencing through polymerase chain reaction (PCR). The following procedures were performed:
Immunohistochemistry of Anti-CD204 Antibody
The anti-CD204 antibody (Rabbit polyclonal antibody, Abcam, Cat# 217843), was used in the IHC. The procedure was performed by using the ultraView detection Kit on a GX stainer from Ventana (Tuscon, AZ, USA). The entire assay consisted of deparaffinization with EZ Prep at 75°C and antigen unmasking for sixty minutes followed by primary incubation for sixteen minutes at 37°C. The antibody was optimized using 1:300 dilution. Anti-CD204 stains the macrophages present in the tumour microenvironment and mainly TAMs. The assessment of CD204 expression on TAM receptors was described by Kurdi et al11,12 The expression is considered high when CD204+TAMs are present in more than 40% of the entire section, and the expression is considered low when the CD204+TAMs are positive in less than 40% of the entire section (Figure 1).
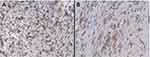 |
Figure 1 CD204+TAMs in WHO-grade 4 astrocytomas. (A) high expression, (B) low expression. Magnification x40. |
Assessment of IDH1/2 Mutation Through PCR Amplification and DNA Sequencing
DNA extraction was performed using QIAamp DNA extraction kit from Qiagen, (Germantown, MD, USA). PCR was initiated with a denaturation step at 95C followed by annealing at 45C for 40 seconds. The process used a Hot-Start Taq DNA polymerase to insert a dNTP set to the 30 ends of the primer annealed to the DNA template. The primers’ sequence used in this process was described by Alkhatabi et al13 (Table 2). After DNA amplification, the product was sequenced through cycle sequencing for verification. One µL of purified PCR product, 2.5 mol/L of each primer, and 2 µL of ABI PRISM sequencing kit, and 2 µL buffer to reach a total of 10 µL. The sequencing started from 96C for 1.5 min and followed by 25 cycles of denaturation, annealing, and extension (total temperature range between 50–90C and for 5 minutes). PCR amplicons were sequenced using ABI Prism kit (CA, USA) on an ABI3500 system according to manufacturer’s protocol. The mutational hotspots in codon 132 and 172 of IDH1 and IDH2 on exon 4 respectively were examined through Sanger sequencing.
 |
Table 2 IDH1/2 Primer Designed Sequence Used in the PCR Process |
Results
IDH1 and IDH2 Mutational Analysis
Sequencing of the 20 samples of WHO-grade 4 astrocytoma showed that IDH1 and IDH2 were wildtype in 18 cases (90%) and the remaining two cases (10%) were IDH-mutant on both codons (R132 and R172). There was no discrepancy in hotspot mutations in both IDH1/2 codons among all cases (Table 1) Synonymously, a point mutation of IDH1R132 occurred at position 325 C>T of exon 4 replaced a (CGT) with (TGT). Another mutation on IDH2R172 was positioned at 110 G>A of exon 4 replaced (CGT) with (CAT) (Figure 2).
Correlation of IDH1/2 with CD204
Tumours with IDH1/2-wildtype showed high expression of CD204+TAMs in 10 cases and low expression in 8 cases. Typical expression was seen equally in IDH1/2 mutant tumours. Thus, there was no statistically significant association between IDH1/2 mutational status and the CD204+TAM expression in tumour microenvironment (p= 0.999) (Table 3). The significance was observed when both groups are compared to recurrence interval (See no.3).
 |
Table 3 The Association Between IDH 1/2 and CD204+TAM Expression |
The Association Between of IDH1/2 and CD204+TAMs with Tumour Recurrence
The association between CD204+TAM and IDH1/2 mutational status was significantly observed among IDH-wildtype cases in terms of tumour recurrence interval (p=0.027), the most aggressive type. When CD204 was highly expressed in IDH-wildtype tumours (n=10), the median recurrence interval was observed at 10 months, while in tumours with low CD204 expression (n=8), the median recurrence interval was observed at 24 months (Figure 3A). The recurrence probability for tumours with IDH-wildtype and CD204 high expression was 40% at 12 months and 10% at 24 months. On the other hand, tumours with IDH-wildtype and CD204 low expression, the recurrence probability was 62% at 12 months and 25% at 36 months. Regardless of the IDH1/2 and CD204 status, the use of chemotherapy has improved the survival and reduced the chance of tumour recurrence (Figure 3B).
Discussion
Glioma microenvironment is a crucial area for the crosstalk between tumour cells and other immune-related cells. The most common signaling cells interacting with tumour cells are TAMs and TILs. When TAMs encircle tumour cells, the T-cells stop communication, and this allow tumour cells to evade immunity through sequencing of signaling pathways, which results in tumour growth and proliferation. The immune evasion can be preventable if the receptors connecting TAM with tumour cell are blocked. One of these newly identified receptors is CD204 on M2-polarized TAM. Few studies have discovered the expression of CD204 in several cancers.10
Kurdi et al explored the association between CD204 and TILs in microenvironment.11 The association between CD204+TAMs and IDH1 mutation in tumour microenvironment of WHO-grade 4 were found insignificant based on a previous study conducted by Kurdi et al.12 Even though IDH1/2 are major prognostic classifiers in WHO-grade 4 astrocytoma, their impact on tumour microenvironment is still under debate. There is no clear evidence if the histone demethylation in tumour cells have signaling interaction with all the receptors in TAMs or TILs. The Cancer Genome Atlas (TCGA) demonstrated a low expression of CD8 cytotoxic T-cells and IFN in IDH-mutant tumors compared with IDH-wildtype tumors.7
In our study, we investigated the relationship between IDH1 and IDH2 mutations and CD204+TAMs and we found no significant relationship between the groups. The two IDH-mutant tumours in this study showed variable CD204 expressions. However, the significance was found in tumour cases with IDH-wildtype. When CD204 is overexpressed in IDH-wildtype tumours, the tumours recur faster than IDH-wildtype tumours with low CD204 expression (Figure 3A). Moreover, the combined chemoradiotherapy used on those patients have improved the patient survival (Figure 3B). Our results explain two possible mechanisms: (1) the oncometabolite and histone demethylation product of IDH1/2 in tumour cells may not usually prevent tumour-macrophage interaction, and (2) the IDH-wildtype tumours with dense CD204 expression in microenvironment let tumour cells evade the immunity and suppress T-cells.
Conclusion
Our study concluded that (a) IDH1R132 or IDHR172 have the same impact on WHO-grade 4 astrocytoma classification and prognosis, (b) There is no crosstalk between the metabolic effect of IDH1/2 on and CD204+TAM, (c) IDH-wildtype glioblastoma with dense CD204+TAM is associated with early tumour recurrence. We recommend researchers to test the relationship between IDH1/2 mutation and other types of TAM receptors.
Data Sharing Statement
Available upon request.
Ethics Approval
Ethical approval for this study was granted by the National Biomedical Ethics Committee at King Abdulaziz University (HA-02-J-008). All patients involved in this study have provided informed consent. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients involved in the study have been consented to release their medical information and using their clinical samples before starting the study.
Acknowledgments
The research article is original, has not already been published in any other journal (medical, or otherwise) or is not currently under consideration for publication by another journal, and does not infringe any existing copyright or any other rights prescribed by law; The article contains nothing that is unlawful, defamatory, or which would, if published, constitute a breach of contract or of confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work”.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi:10.1093/neuonc/noab106
2. Kurdi M, Moshref RH, Katib Y, et al. Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification. World J Clin Oncol. 2022;13(7):567–576. doi:10.5306/wjco.v13.i7.567
3. Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi:10.1158/0008-5472.CAN-04-1337
4. Yang H, Ye D, Guan KL, et al. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–5571. doi:10.1158/1078-0432.CCR-12-1773
5. Yen K, Bittinger M, Su S, et al. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29:6409–6417. doi:10.1038/onc.2010.444
6. Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi:10.1158/0008-5472.CAN-10-1666
7. Kaminska B, Czapski B, Guzik R, et al. Consequences of IDH1/2 mutations in gliomas and an assessment of inhibitors targeting mutated IDH proteins. Molecules. 2019;24(5):968. doi:10.3390/molecules24050968
8. Fridman WH, Zitvogel L, Sautes-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi:10.1038/nrclinonc.2017.101
9. Ichimura T, Morikawa T, Kawai T, et al. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann Surg Oncol. 2014;21:2105–2112. doi:10.1245/s10434-014-3503-2
10. Yuan Y, Zhao Q, Zhao S, et al. Characterization of transcriptome profile and clinical features of a novel immunotherapy target CD204 in diffuse glioma. Cancer Med. 2019;8:3811–3812. doi:10.1002/cam4.2312
11. Kurdi M, Alghamdi B, Butt NS, et al. The relationship between CD204 (M2)-polarized tumour-associated macrophages (TAMs), tumour-infiltrating lymphocytes (TILs), and microglial activation in glioblastoma microenvironment: a novel immune checkpoint receptor target. Discov Oncol. 2021;12(1):28. doi:10.1007/s12672-021-00423-8
12. Kurdi M, Katib Y, Faizo E, et al. Association between CD204-expressed tumor-associated macrophages and MGMT-promoter methylation in the microenvironment of grade 4 astrocytomas. World J Oncol. 2022;13(3):117–125. doi:10.14740/wjon1473
13. Alkhatabi H, Bin Saddeq HA, Alyamani L, et al. Investigation of isocitrate dehydrogenase 1 and 2 mutations in acute leukemia patients in Saudi Arabia. Genes. 2021;12:1963. doi:10.3390/genes12121963
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


