Back to Journals » Vascular Health and Risk Management » Volume 15
The burden of critical limb ischemia: a review of recent literature
Authors Duff S , Mafilios MS , Bhounsule P, Hasegawa JT
Received 19 March 2019
Accepted for publication 7 June 2019
Published 1 July 2019 Volume 2019:15 Pages 187—208
DOI https://doi.org/10.2147/VHRM.S209241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Steve Duff,1 Michael S Mafilios,2 Prajakta Bhounsule,3 James T Hasegawa3
1Veritas Health Economics Consulting, Carlsbad, CA, USA; 2Health Economics Associates, San Diego, CA, USA; 3Health Economics and Reimbursement, Abbott Vascular, Santa Clara, CA, USA
Abstract: Peripheral arterial disease is a chronic vascular disease characterized by impaired circulation to the lower extremities. Its most severe stage, known as critical limb ischemia (CLI), puts patients at an increased risk of cardiovascular events, amputation, and death. The objective of this literature review is to describe the burden of disease across a comprehensive set of domains—epidemiologic, clinical, humanistic, and economic—focusing on key studies published in the last decade. CLI prevalence in the United States is estimated to be approximately 2 million and is likely to rise in the coming years given trends in important risk factors such as age, diabetes, and smoking. Hospitalization for CLI patients is common and up to 60% are readmitted within 6 months. Amputation rates are unacceptably high with a disproportionate risk for certain demographic and socioeconomic groups. In addition to limb loss, CLI patients also have reduced life expectancy with mortality typically exceeding 50% by 5 years. Given the poor clinical prognosis, it is unsurprising that the quality of life burden associated with CLI is significant. Studies assessing quality of life in CLI patients have used a variety of generic and disease-specific measures and all document a substantial impact of the disease on the patient’s physical, social, and emotional health status compared to population norms. Finally, the poor clinical outcomes and increased medical resource use lead to a considerable economic burden for national health care systems. However, published cost studies are not comprehensive and, therefore, likely underestimate the true economic impact of CLI. Our summary documents a sobering assessment of CLI burden—a poor clinical prognosis translating into diminished quality of life and high costs for millions of patients. Continued prevention efforts and improved treatment strategies are the key to ameliorating the substantial morbidity and mortality associated with this disease.
Keywords: critical limb ischemia, peripheral arterial disease, burden, amputation, quality of life, economics
Introduction
Peripheral arterial disease (PAD) is a chronic vascular disease characterized by impaired circulation to the lower extremities. While early stages of PAD may be asymptomatic, the hallmarks of its most severe stage, known as critical limb ischemia (CLI), are recurring lower extremity rest pain, ulceration, and gangrene as well as an increased risk of cardiovascular events, amputation, and death.1 Clinical guidelines focus on strategies to promote wound healing and limb salvage as well as medical therapy to prevent cardiovascular ischemic events.2 Revascularization (either surgical or endovascular) is attempted to provide sufficient blood flow to the extremities in at least 50% of the CLI patients and upwards of 90% of the patients in certain interventional centers. Minor or major amputation is utilized when less invasive treatments cannot be used or have failed.
Numerous published studies have provided informative CLI overviews focusing on intervention outcomes or emphasizing one or two elements of disease burden.3–16 However, despite these numerous reviews, a comprehensive landscape assessment of CLI burden describing contemporary studies is lacking. Therefore, the objective of this literature review is to describe the burden of disease across several domains—epidemiologic, clinical, humanistic, and economic—focusing on key studies published since 2007.
Methods
Literature searches of the PubMed and EMBASE databases were undertaken to identify original research studies published from January 2007 through October 2018 assessing the epidemiologic burden (ie, incidence and prevalence), quality of life burden, and economic burden of lower extremity CLI. Non-systematic review studies, editorials, letters, commentaries, or posters presented at scientific meetings were excluded. The 12-year time frame was selected to focus on the most contemporary studies published on the burden of lower extremity CLI. Each search was conducted using controlled vocabulary and limited to studies published in English and involving humans. The preliminary literature searches identified 3,254 potentially relevant studies. Two researchers independently appraised all of the abstracts from the literature searches for potential inclusion in the literature review. Additional studies were identified based on a review of the reference lists from the full-text studies and a grey search of the internet. The definition of CLI is not standardized and often varied across the studies reviewed. Since this was a “pragmatic” burden of illness review, all studies mentioning lower extremity CLI were considered for the review. Studies reporting on related topics such as trends in diagnostic or revascularization techniques or focusing exclusively on treatment outcomes associated with specific CLI treatments that did not contain any information on the burden of CLI were excluded. A total of 73 studies were selected for inclusion in this literature review.
Results
Epidemiologic and clinical burden
Prevalence and incidence
Disease prevalence and incidence are epidemiologic terms that define a population at a single point in time (prevalence) or as the number of new cases that develop in a specified time period (incidence). Together, these two building blocks of epidemiology help to characterize disease burden in terms of the total numbers of patients, establish whether that number (or rate of disease development) may be growing or diminishing, and provide useful comparative information across different populations, whether they be geographically or demographically diverse.
Several recent studies have reported CLI prevalence and incidence in the United States (Table 1). CLI prevalence in the US adult population aged 40 or older is estimated to be 1.28%.17 With approximately 156 million US citizens in this age category,18 this prevalence estimate translates to approximately 2 million total CLI patients in the United States. Prevalence estimates in studies of the US Medicare population range from 0.3% to 2.0%.17,19,20 With a current beneficiary population of approximately 60 million,21 there may be nearly 1.2 million total CLI patients in the US Medicare program.17
 |
Table 1 CLI prevalence and incidence |
CLI prevalence in commercial plans—likely being younger and healthier—are approximately half of Medicare prevalence.17 This is consistent with an observed age gradient and prevalence 2 to 3 times higher in older (age 85+) Medicare patients compared to younger (age 65–69) Medicare patients.19 Annual incidence estimates of total CLI in these same epidemiologic studies range from 0.26% to 0.48%.
Readmission rates
Given the poor prognosis and high morbidity and mortality associated with CLI, patients are commonly hospitalized. In 2016, Agarwal and colleagues quantified the number of CLI hospitalizations in the United States based on an analysis of the US Nationwide Inpatient Sample (NIS), a database that contained discharge data from approximately 8 million hospitalizations during each year of the study period (2003–2011).22 The number of CLI hospitalizations during this time period remained remarkably stable, varying between 325,000 and 375,000 annually.22 This equates to nearly 225 CLI admissions per 1,000,000 US persons aged 40 or older.
Minimizing hospital readmission is an important goal given the risk hospitalizations pose to patients and the increasing use of readmission rates as a metric on which hospitalization performance may be judged. Historically, readmission rates for CLI patients have been high and, therefore, clinical studies occasionally use readmission as an important outcome measure. Recent studies of CLI readmission rates in the United States (Table 2 and Figure 1) demonstrate that readmission is common within 30 days (approximately 15–30%) of the index hospitalization, with rates approaching 60% at 6 months. Furthermore, a study by Kolte and colleagues (2017) using the NIS database shows an increasing likelihood of readmission with increasing disease severity.23
 |
Table 2 CLI readmission rates |
 |
Figure 1 US CLI readmission rates. Abbreviation: CLI, critical limb ischemia. |
Amputation rates and mortality
One of the most concerning outcomes for CLI patients is amputation. Recent studies evaluating amputation rates or incidence (terminology varies) are presented in Table 3 and Figure S1. Reviewing the methodologic details for each of these studies demonstrates that there is much heterogeneity in the contemporary literature related to the analysis timeframe (annualized, during hospitalization, or longitudinal evaluation), amputation definition (all types, major amputation only, or unspecified), and populations studied (natural history/untreated, Medicare only, undergoing interventions, all CLI, etc.). Therefore, comparisons and conclusions must be made with caution. Nevertheless, it is clear that, across all studies, amputation rates are unacceptably high—typically exceeding 15–20% at 1 year. The study by Agarwal and colleagues (2016) suggests an association between an increasing focus on limb salvage, greater use of endovascular treatment, and other factors with a decreasing rate of major amputation since 2003 (through 2011 in their study).22 However, there remains ample evidence that there is much room for improvement in terms of reducing the risk of amputation.
 |
Table 3 CLI amputation rates |
 |
Table 3 (Continued). |
 |
Table 3 (Continued). |
Amputation rates vary primarily by disease severity. Luders et al (2016) demonstrated that CLI patients have a much higher risk of amputation relative to less severe PAD patients (Rutherford 1–3).24 Within CLI, a clear gradient of higher amputation risk with higher disease severity exists both at initial hospitalization (3.1%, 26.7%, and 55.0% for Rutherford 4, 5, and 6, respectively)25 and through longer-term (4 year) follow-up (12.1%, 35.3%, and 67.3% for Rutherford 4, 5, and 6, respectively).26
Amputation rates also vary by the presence of comorbid conditions. For example, diabetes is a major risk factor associated with higher amputation rates. Studies by Baser (2013) and Spreen (2016) suggest that the probability of amputation is at least 50% higher in CLI patients with diabetes versus those without this comorbidity.19,27
Other disparities in amputation risk are apparent that may have demographic and socioeconomic underpinnings. While race/ethnicity, income, and insurance status/type may have some association with diabetes status and/or disease severity, these factors have been shown to be important in amputation. For example, in a study by Henry and colleagues,28 statistically significantly higher odds of amputation during hospitalization were shown for:
- Black and Native American CLI patients (Odds ratios (OR)=2.15 and 2.00, respectively; reference = white patients);
- Low-income patients (ORs =1.12–1.34; reference = highest income quartile); and
- Medicaid patients (OR=1.26; reference = Medicare) [Note: patients with private insurance were shown to have lower odds of amputation—OR =0.74].
Similarly, the Baser 2013 study19 also evaluated the annual incidence of amputation in different races in the US Medicare program during the years 2007–2008. The researchers quantified an annual amputation incidence of 20.5% for black CLI patients, approximately double the incidence observed in white and Hispanic CLI patients. The finding of racial disparity in amputation frequency was corroborated in research conducted by Mustapha and colleagues using more recent (2011–2015) Medicare data. In their study, blacks more frequently had major amputation than whites (10% vs 4%; P<0.001), which, according to the authors, was only partially explained by differences in patient characteristics (eg, a higher prevalence of gangrene in blacks vs whites—36% vs 22%; P<0.001).20
Not only is amputation a major concern of CLI patients because of limb loss, but also because of the elevated mortality associated with amputation. Studies in the United States and Germany have explored this issue in patients hospitalized for CLI29 or PAD.30 Major amputation was a statistically significant risk factor for in-hospital mortality in both studies with odds ratios of 2.81 (relative to CLI patients without major amputation)29 and 6.69 (relative to any PAD patients without major amputation).30
Longer-term mortality also appears elevated in patients undergoing amputation. In a US Medicare population study, 1-year mortality post-CLI diagnosis was 30.3% in patients without nontraumatic amputation and 40.4% in patients with nontraumatic amputation.19 In a study of elderly (>70 years of age) Dutch CLI patients, mortality rates at 1, 3, and 5 years were 27%, 49%, and 61%, respectively, in those treated without amputation.31 However, in the elderly CLI patients undergoing amputation, mortality rates at the same timepoints were 44%, 66%, and 85%, respectively, indicating substantially worse survival outcomes.31
Long-term mortality rates
As discussed earlier, amputation is associated with poor survival outcomes; however, the long-term prognosis for all CLI patients also is unfavorable. Using a variety of methods and data sources, many researchers have quantified mortality in CLI patients (Table 4 and Figure 2). One-year mortality ranges from 15% to 40%, depending on a variety of factors. In addition to the aforementioned impact of amputation, increasing disease severity and diabetes are associated with increased mortality. Over time horizons of 4 to 5 years, mortality commonly exceeds 50%, especially in patients with more severe disease (Rutherford 5 [ulcer] or Rutherford 6 [gangrene]). However, the evidence suggests that the impact of diabetes is attenuated with longer-term follow-up. Studies by Spreen (2016) and Freisinger (2017) in the Netherlands and Germany, respectively, show no mortality difference by diabetes status32 or minor differences that are not statistically significant.27,32
 |
Table 4 Long-term CLI mortality rates |
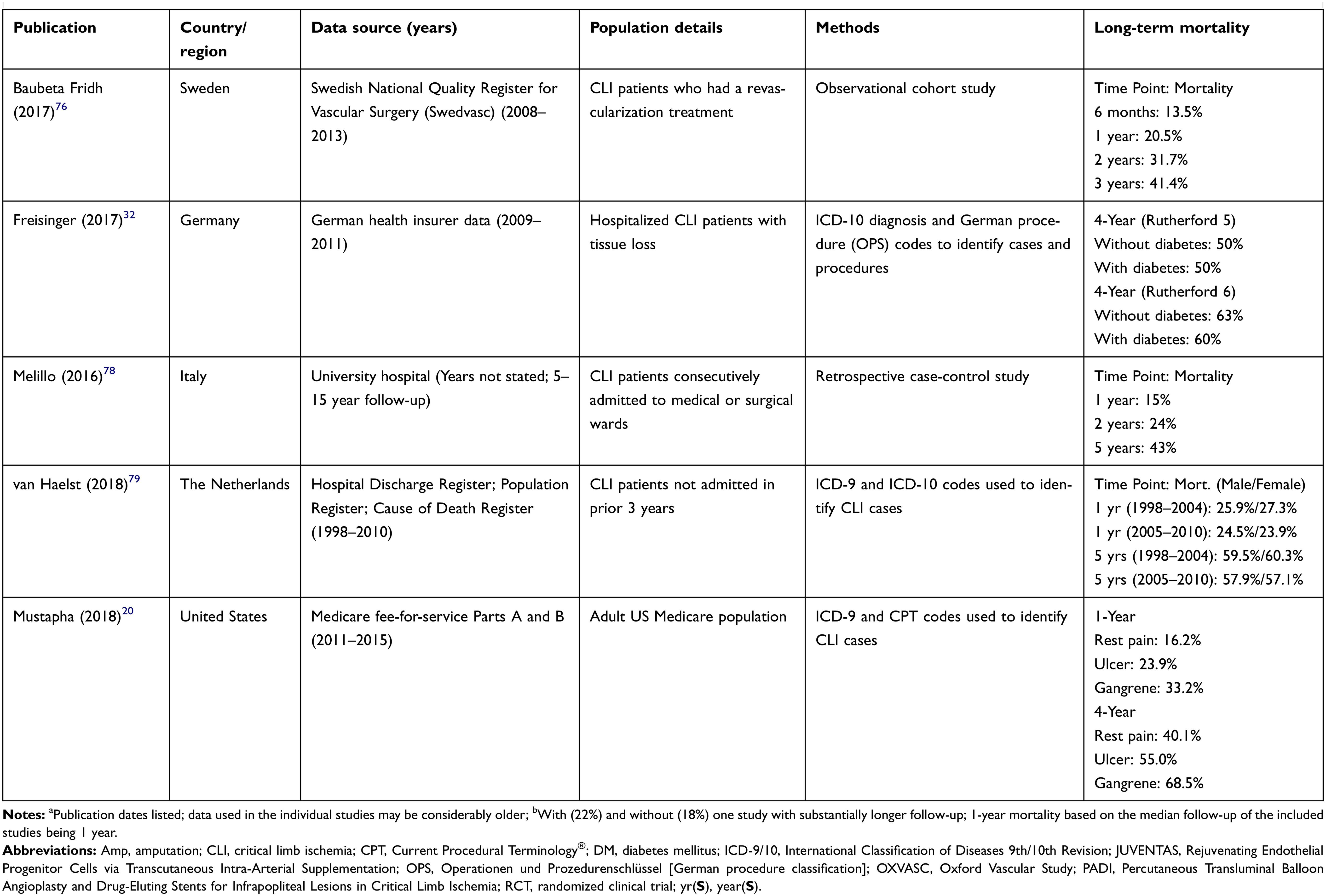 |
Table 4 Long-term CLI mortality rates |
 |
Figure 2 Long-term CLI mortality rates. Abbreviations: CLI, critical limb ischemia; OXVASC, Oxford Vascular Study. |
Quality of life burden
The quality of life burden in CLI patients is substantial. Patients with CLI suffer from ischemic rest pain, non-healing ulcers, and/or symptomatic gangrene.1,12 This debilitating disease causes dependency on caregivers for support, the need for permanent local wound treatment, and the chronic use of pain-relieving medications.11,33 Further, as discussed earlier, there is a significant risk (and fear) of major amputation and a high rate of mortality.11 Additionally, patients with CLI often suffer from one or more comorbidities such as coronary artery disease, dementia, cerebrovascular disease, and diabetes.15,34 All of these factors severely impair a patient’s quality of life.
Most of the studies published in the past twelve years assessing quality of life in CLI patients have examined the change in quality of life (ie, improvement) after an intervention (ie, endovascular procedures, surgical revascularization, etc.) in comparison to baseline quality of life scores. Only a few contemporary studies have directly assessed the quality of life burden of CLI in a non-interventional setting.14,35 Although these intervention studies were not designed to compare baseline quality of life scores in CLI patients to healthy people in the community, they may provide useful information on the magnitude of quality of life burden in CLI patients who are candidates for medical intervention. To determine the quality of life burden in CLI patients, this review primarily focused on comparing the baseline quality of life scores of CLI patients reported from interventional studies to the quality of life scores of healthy people reported from studies of community-based norms.
Instruments for assessing quality of life in CLI patients
Studies examining the quality of life in CLI patients have used a combination of generic quality of life questionnaires (ie, SF-36) and disease-specific measures (ie, VascuQOL) (Table 5). Generic instruments are applicable to CLI populations because they address multidimensional domains of quality of life relevant to CLI patients such as mental health, physical functioning, bodily pain, emotional health, commonly performed daily activities, and social functioning.11 The SF-36 is the most frequently used generic quality of life assessment tool in CLI studies. Other generic quality of life measures that have been used in CLI studies include the Nottingham Health Profile (NHP), the World Health Organization Quality of Life Assessment Instrument (WHOQOL-100 and WHOQOL BREF), and the EuroQol-5D. The EuroQol-5D has been used in CLI studies to derive health state utilities.
 |
Table 5 Commonly used generic and disease-specific quality of life instruments in CLI studies |
Disease-specific quality of life instruments are applicable to CLI populations because they address the specific limitations experienced by the patient, making them more sensitive to detect clinically relevant changes in health status in response to disease progression or treatment.36 However, a disease-specific quality of life measure for CLI has yet to be developed. Consequently, studies examining the quality of life in CLI patients have relied on disease-specific measures that have previously been developed for PAD patients. The Vascular Quality of Life Questionnaire (VascuQOL) is a PAD-specific measure that has been validated in CLI patients and used in several CLI studies.37 Ideally, studies examining quality of life in patients with CLI should include both generic and disease-specific measures.
Assessments of quality of life burden in CLI patients
Several studies have reported baseline SF-36 scores in CLI patients from clinical trials of various revascularization procedures (Table 6).14,38–48 In most of these studies, low quality of life baseline SF-36 scores were consistently observed in the physical health domains, including physical functioning, role physical functioning, and bodily pain compared to US community-based norms. The population norms in the United States for each of the SF-36 domains are as follows: physical functioning, 50.7; role physical, 49.5; bodily pain, 50.6; general health, 50.1; vitality, 53.7; social functioning, 51.4; role emotional, 51.4; mental health, 54.3; physical component summary, 49.2; and mental component summary, 53.8.49
 |
Table 6 Baseline SF-36/RAND-36 quality of life scores (0–100) for patients with CLI |
Sprengers and colleagues (2010) assessed quality of life using the SF-36 in 47 patients with “no-option” CLI using baseline data from the JUVENTAS trial, a randomized clinical trial examining the effects of bone marrow mononuclear cells in CLI patients.14 “No-option” CLI was defined as patients with no surgical or endovascular options for revascularization.14 The results of the study showed that patients with no-option CLI reported baseline SF-36 quality of life scores well below the general population quality of life scores on every health dimension of the SF-36 (Table 6).14 Similarly, Forbes and colleagues (2010) examined quality of life in 452 patients with CLI undergoing either angioplasty or bypass surgery from the BASIL trial and found that baseline SF-36 scores were well below the general population norms in both treatment groups for all the physical health domains, including physical functioning (22.7 and 23), role physical (10.3 and 13.1), and bodily pain (30.4 and 32) reflecting a substantial quality of life burden in these patients (Table 6).41
The EuroQol-5D and the EuroQol VAS (visual analog scale) have been used in several CLI studies to derive health state utilities (Table 7).35,41,47,50–53 The EuroQol-5D index population norm in the United States is 0.825 (on a 0 to 1 scale) and the EuroQol VAS population norm is 80 (on a 0 to 100 scale).54 A study by Pisa and colleagues (2012) was one of the few recently published studies which assessed the impact of CLI on a patient’s quality of life that was not derived from an interventional study.35 In this study, 200 patients with CLI were interviewed to assess the impact of CLI on health status using the EuroQol-5D, VAS, and other utility measures.35 The mean calculated EuroQol-5D and VAS scores for CLI patients were 0.56 and 56, respectively, indicating an impaired health status when compared to US population norms (Table 7).35
 |
Table 7 Baseline EuroQol-5D utility scores and VAS scores for patients with CLI |
More recently, Steunenberg and colleagues (2018) assessed quality of life in elderly CLI patients undergoing endovascular, surgical, or conservative therapy using the WHOQOL-BREF questionnaire.33 General population norms for the four WHOQOL-BREF domains are 73.5 for the physical health domain, 70.6 for psychological well-being domain, 71.5 for the social relationships domain, and 75.1 for the environment domain.55 The baseline WHOQOL-BREF scores for the CLI patients in the Steunenberg study were well below the general population norms for all four domains including physical health (10.9–11.6), psychological well-being (14.0–14.2), social relationships (15.4–15.5), and the environment (15.4–15.6) reflecting a substantial quality of life burden in these patients.33
Several studies have reported baseline VascuQOL in CLI patients from clinical trials of various revascularization procedures and medical therapies.37,41,44,56–60 In all of these CLI intervention studies, low baseline VascuQOL scores were consistently observed indicating that patients have an impaired quality of life at baseline prior to undergoing an intervention. For example, Forbes and colleagues (2010) evaluated quality of life using the VascuQOL-25 (scoring from 1 to 7) in 452 CLI patients enrolled in the BASIL trial and found low pre-operative baseline scores in both the angioplasty group (2.8) and bypass surgery group (2.9).41 Similarly, Landry and colleagues (2014) evaluated quality of life in 18 CLI patients undergoing lower extremity bypass surgery and found a low VascuQOL-25 score of 2.9 at baseline indicating a substantial quality of life burden in these patients.44
Economic burden
National CLI cost
A complete understanding of the burden of CLI would benefit from a comprehensive estimate of the national cost of the disease. This type of data is lacking in the contemporary CLI literature. Recent studies estimating the national economic burden of CLI have focused on the costs of hospitalized patients, which undoubtedly comprises a substantial portion of the CLI economic burden; however, no comprehensive accounting (beyond hospitalization) of national costs over a longer term has been published recently. An additional limitation of the data in the published literature is that estimates often have focused solely on the hospitalization costs of a subgroup of CLI patients (those undergoing surgical or endovascular revascularization) likely leaving a large number of CLI-related hospitalizations unexplored.
Despite these limitations, three studies do report national cost estimates for CLI.23,61,62 Sachs et al (2011) used the NIS (1999–2007) to estimate the costs of inpatient procedures (angioplasty or bypass) in CLI patients in the United States.61 The results of this study showed a 50% increase in the total inpatient costs from 2001 ($579 million) to 2007 ($870 million).61 An analysis by Kolte and colleagues (2017) using the Nationwide Readmissions Database (2013–2014) estimated the inpatient costs for open or endovascular treatment of CLI patients to be approximately $4.2 billion with an additional $625 million spent for 30-day unplanned readmissions.23 Finally, Malyar and colleagues (2013) examined all hospitalizations in Germany in 2007 and 2009 to estimate the inpatient costs for all CLI patients and found that total hospital reimbursement for CLI patients exceeded €1.1 billion in 2007 and €1.3 billion in 2009.62
A recent longitudinal analysis by Mustapha and colleagues (2018) of US Medicare data over the time period 2011 (for the index CLI diagnosis) to September 2015 offers a more comprehensive accounting of CLI costs than those studies focused solely on hospitalization.20 In unmatched and propensity-score-matched patient samples, Mustapha estimated a cost per CLI patient over the 4-year follow-up period of $93,800 and $117,800 for the 2 samples, respectively.20 The majority (~62–68%) of the cost was attributable to inpatient admissions with the remainder approximately equally split between hospital outpatient and physician/supplier costs. Although not an overall national estimate, the authors did calculate an annual cost to Medicare of ~$12 billion attributable to incident CLI cases. If considering other published epidemiologic data as well as non-Medicare patients, the total US cost of CLI likely would be several times higher than Mustapha’s conservative estimate.
Index CLI hospitalization cost
Since hospitalization comprises a large percentage of total CLI cost, research evaluating hospitalization cost at the individual patient level is particularly notable. US and German studies have documented the hospital costs of index CLI procedures using large national databases (Table 8). The US studies provide information on the hospital cost trajectory over a decade, whereas the German studies explore the impact of disease severity and co-morbidity on costs.
 |
Table 8 Index CLI hospitalization costs |
Agarwal (2016) and Dua (2016) conducted separate analyses using the NIS over similar time periods.22,63 The key difference in the studies was the inclusion criteria which yielded somewhat different patient populations. Agarwal included all adult hospitalizations with a CLI diagnosis whereas Dua excluded any emergent procedures or primary amputations without a prior revascularization, focusing only on patients with elective procedures. Over a 9-year period (2003–2011) with over 640,000 CLI admissions, Agarwal found that the mean hospitalization cost was consistent and unchanged—approximately $23,000 in each year.22 In patients treated electively for CLI, Dua reported a very different result—a 63% increase in the median hospitalization cost from 2001 ($12,568) to 2011 ($20,587).63 While the median cost would be expected to be lower than the mean, and the exclusion of costly emergent procedures and amputations also would explain a lower average cost in the Dua study, the difference in the cost trajectories between the two studies is difficult to reconcile.
More recently, a study by Martinez and colleagues (2018) extended the hospitalization cost findings to 2013/2014 using the Nationwide Readmissions Database (NRD), which included data from nearly 15 million admissions in 22 US states.64 The results of the study showed a mean index admission cost for CLI of $29,148, as well as a 30-day readmission cost of $17,681.64 This latter mean estimate is greater than the median 30-day readmission cost of $12,419 reported by Kolte et al (2017) from their analysis of the 2013/14 NRD.23
German researchers have employed the BARMER GEK database (the largest public health insurer in the country) to quantify the index hospitalization cost for CLI patients. In two studies, both using data from 2009 to 2011, results documented the higher admission cost associated with increasing disease severity.26,32 Interestingly, index admission costs were similar between CLI patients with and without diabetes, although analyses of inpatient follow-up cost post-discharge noted higher costs for diabetics.32
Amputation cost
Although evidence suggests that amputation rates may be declining,22 the cost associated with amputation and its elevated morbidity and mortality is still an important component of the overall economic burden of CLI. A few recent studies have quantified amputation-related costs. Hospitalization costs for an amputation may be the easiest to estimate given no requirement for longer follow-up, yet do not appear to have been studied (published) in the CLI population since 2012. Mean or median hospital costs in the 2008/2009 time period have been estimated to range from approximately $10,000 to nearly $30,000 with much of the variability due to the level of amputation required (minor vs major).65,66
Given the challenges in studying patients for periods of time exceeding 1–2 years, especially with the high mortality experienced by this population, the relative lack of contemporary long-term amputation cost studies is understandable. Furthermore, the lone study calculating costs over a 10-year period relies on economic modeling for its cost estimation.66 The authors reported a 10-year expected cost (discounted to net present value) of a primary amputation strategy in Rutherford 5 CLI patients as $78,958.66
The most recent study documenting the costs of amputation used sophisticated statistical modeling of the US MarketScan database (2006–2014) to highlight the economic burden associated with amputation.67 The analysis evaluated the monthly cost per patient after the first CLI diagnosis and compared those costs between patients who underwent a major amputation and those that did not. The authors included a broad array of resources (eg, inpatient, outpatient, labs, etc.) and calculated two incremental costs—one that included all resources except pharmacy and another that included pharmacy costs. The results suggested that CLI patients with a major amputation had costs approximately $5,000 per month higher than those that did not require a major amputation. When adding pharmacy costs, the incremental cost increased to approximately $6,000 per month.67
Documenting the economic burden of amputation contributes to an understanding of the overall cost of CLI. However, drawing definitive conclusions about amputation costs based on the most recently published studies is challenging. Differences in study design and robustness, populations and data sources, medical (and non-medical) resources considered, and other factors all contribute to a wide range of cost estimates.
Discussion
CLI is a serious condition that affects millions of patients globally. Its poor prognosis (in terms of both morbidity and mortality) suggests the impact of the disease is substantial. Furthermore, with increasing PAD prevalence and advancing age in many populations, coupled with the growth in other CLI risk factors such as diabetes (globally)68 and smoking (in many countries),69 there seems little reason for optimism that the number of CLI patients will decrease in the near future. Therefore, a comprehensive understanding of disease burden is warranted.
The clinical consequences of CLI, especially excess mortality and the high risk of amputation, are particularly concerning. That these outcomes appear to have a differential racial and socioeconomic impact only adds to their gravity. Other consequences of CLI include ischemic rest pain, non-healing ulcers, symptomatic gangrene, dependency on caregivers for support, the need for permanent local wound treatment, and the chronic use of pain-relieving medications. All of these factors significantly impair the quality of life for CLI patients by imposing a substantial burden on a patient’s emotional, social, and physical well-being. In addition to its humanistic impact, CLI morbidity and treatment pose significant financial challenges. At a national level, the disease results in expenditures of billions of dollars and euros in the United States and European countries annually. These expenses appear to be driven largely by hospitalization costs and secondarily by the costs of amputation. Extremely high readmission rates only serve to compound the economic burden.
Our review of the literature related to the burden of CLI has several limitations that should be mentioned. First, many studies, although recently published, utilize data from large databases that may report results from over a decade ago. This data lag may reduce the relevance of even contemporarily published research. Second, our review was not intended to evaluate or recommend specific CLI treatment strategies or individual products or devices and, therefore, non-treatment specific studies or large database studies were preferred over individual clinical trials or single-center studies. This preference, however, was relaxed for the assessment of quality of life burden. The examination of the quality of life burden in CLI patients was based primarily on baseline data from CLI interventional studies as there is a scarcity of contemporary quality of life burden of illness studies in CLI patients. These intervention studies were not designed to compare baseline quality of life scores in CLI patients to healthy people in the community but may provide useful information on the magnitude of quality of life burden in CLI patients. Third, a CLI-specific quality of life measure has yet to be developed. Consequently, studies examining the quality of life in CLI patients have used a combination of generic quality of life questionnaires (ie, SF-36) and disease-specific measures (ie, VascuQOL) that have previously been used to measure quality of life in PAD patients. Finally, for a variety of reasons, comprehensive (in terms of both medical and non-medical/indirect costs) and long-term economic analyses are lacking in CLI patients. This necessarily renders our understanding of the economics associated with the disease as incomplete and the economic burden of CLI underestimated.
By necessity, our research effort explicitly focused on summarizing CLI burden yet the search process yielded several observations related to the published literature that should be addressed. For example, despite our comprehensive search strategies, nearly all recent peer-reviewed CLI epidemiologic research is limited to the US perspective. Several abstracts and non-peer-reviewed presentations were identified that described CLI epidemiology in other countries, but these lacked the detail necessary for inclusion in our review. Also, numerous potentially relevant studies discussed trends in utilization of diagnostic modalities and revascularization (or other) treatment approaches, clinical outcomes associated with interventions, or potential demographic/socioeconomic disparities related to the disease without explicit information pertaining to CLI burden. These topics are important areas for continued research and synthesis, warranting a similar comprehensive assessment and literature review.
Conclusion
This literature review is unique in that it provides a broad perspective of the burden of CLI while focusing on the most recent key publications. Our summary documents a sobering assessment of CLI burden—a poor clinical prognosis translating into diminished quality of life and high costs for millions of patients. Continued prevention efforts and improved treatment strategies are the key to ameliorating the substantial morbidity and mortality associated with this disease.
Disclosure
Steve Duff is an employee of Veritas Health Economics Consulting which was contracted to conduct the literature review. Michael S Mafilios is an employee of Health Economics Associates which was contracted to conduct the literature review. Prajakta Bhounsule and James T Hasegawa are employees of Abbott Vascular. The authors report no other conflicts of interest in this work.
References
1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67. doi:10.1016/j.jvs.2006.12.037
2. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126. doi:10.1016/j.jacc.2016.11.007
3. Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62(6):1642–1651.e1643. doi:10.1016/j.jvs.2015.07.065
4. Alabi O, Roos M, Landry G, Moneta G. Quality-of-life assessment as an outcomes measure in critical limb ischemia. J Vasc Surg. 2017;65(2):571–578. doi:10.1016/j.jvs.2016.08.097
5. Alonso A, Garcia LA. The costs of critical limb ischemia. Endovasc Today. 2011;32–36.
6. Barshes NR, Belkin M, MOVIE Study Collaborators. Framework for the evaluation of “value” and cost-effectiveness in the management of critical limb ischemia. J Am Coll Surg. 2011;3(4):552–566. doi:10.1016/j.jamcollsurg.2011.07.011
7. Biancari F. Meta-analysis of the prevalence, incidence and natural history of critical limb ischemia. J Cardiovasc Surg. 2013;54:663–669.
8. Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19(2):91–95. doi:10.1053/j.tvir.2016.04.001
9. Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151(11):1070–1077. doi:10.1001/jamasurg.2016.2018
10. Gulati A, Garcia L, Acharji S, et al. Epidemiology of chronic critical limb ischemia. In: Dieter RS, editor. Critical Limb Ischemia. Switzerland: Springer International Publishing Switzerland; 2017:9–14.
11. Lawall H, Zemmrich C, Bramlage P, Aann B. Health related quality of life in patients with critical limb ischemia. Vasa. 2012;41(2):78–88. doi:10.1024/0301-1526/a000169
12. Monaro S, West S, Gullick J. An integrative review of health-related quality of life in patients with critical limb ischaemia. J Clin Nurs. 2016;26:2826–2844. doi:10.1111/jocn.13623
13. Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol. 2016;68(18):2002–2015. doi:10.1016/j.jacc.2016.04.071
14. Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC, JUVENTAS Study Group; SMART Study Group. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010;52(4):843–849. doi:10.1016/j.jvs.2010.04.057
15. Steunenberg SL, Raats JW, Te Slaa A, de Vries J, van der Laan L. Quality of life in patients suffering from critical limb ischemia. Ann Vasc Surg. 2016;36:310–319. doi:10.1016/j.avsg.2016.05.087
16. Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: current challenges and future prospects. Vasc Health Risk Manag. 2018;14:63–74. doi:10.2147/VHRM.S125065
17. Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60(3):686–695. doi:10.1016/j.jvs.2014.03.290
18. United States Census Bureau, Population Division. Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States: April 1, 2010 to July 1, 2017. Release date June 2018.
19. Baser O, Verpillat P, Gabriel S, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manag. 2013;10(2):26–36.
20. Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc. 2018;7(16):e009724. doi:10.1161/JAHA.118.008528
21. Centers for Medicare and Medicaid Services; Office of the Actuary. CMS Program Data – populations. July 2018 Version. Available from: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/cms-fast-facts/index.html.
22. Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003-2011. J Am Coll Cardiol. 2016;67(16):1901–1913. doi:10.1016/j.jacc.2016.02.040
23. Kolte D, Kennedy KF, Shishehbor MH, et al. Thirty-day readmissions after endovascular or surgical therapy for critical limb ischemia: analysis of the 2013 to 2014 Nationwide Readmissions Databases. Circulation. 2017;136(2):167–176. doi:10.1161/CIRCULATIONAHA.117.027625
24. Luders F, Bunzemeier H, Engelbertz C, et al. CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol. 2016;11(2):216–222. doi:10.2215/CJN.05600515
25. O’Brien-Irr MS, Harris LM, Dosluoglu HH, Dryjski ML. Procedural trends in the treatment of peripheral arterial disease by insurer status in New York State. J Am Coll Surg. 2012;215(3):311–321. doi:10.1016/j.jamcollsurg.2012.05.033
26. Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36(15):932–938. doi:10.1093/eurheartj/ehv006
27. Spreen M, Gremmels H, Teraa M, et al. Decreased limb survival in diabetic patients with critical limb ischemia compared with non-diabetic patients. Cardiovasc Intervent Radiol. 2016;39(3 Supplement 1):S305.
28. Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53(2):330–339.e1. doi:10.1016/j.jvs.2010.08.077
29. Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of readmissions among patients with critical limb ischemia. J Am Coll Cardiol. 2017;69(15):1897–1908. doi:10.1016/j.jacc.2017.02.040
30. Malyar NM, Freisinger E, Meyborg M, et al. Low rates of revascularization and high in-hospital mortality in patients with ischemic lower limb amputation: morbidity and mortality of ischemic amputation. Angiology. 2016;67(9):860–869. doi:10.1177/0003319715626849
31. Klaphake S, de Leur K, Mulder PGH, et al. Mortality after major amputation in elderly patients with critical limb ischemia. Clin Interv Aging. 2017;12:1985–1992. doi:10.2147/CIA.S137570
32. Freisinger E, Malyar NM, Reinecke H, Lawall H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: a large-scaled routine data analysis. Cardiovasc Diabetol. 2017;16(1):41. doi:10.1186/s12933-017-0624-5
33. Steunenberg SL, de Vries J, Raats JW, et al. Quality of life and mortality after endovascular, surgical, or conservative treatment of elderly patients suffering from critical limb ischemia. Ann Vasc Surg. 2018;51:95–105. doi:10.1016/j.avsg.2018.02.044
34. Steunenberg SL, Te Slaa A, Ho GH, Veen EJ, de Groot HG, van der Laan L. Dementia in patients suffering from critical limb ischemia. Ann Vasc Surg. 2017;38:268–273. doi:10.1016/j.avsg.2016.05.136
35. Pisa G, Reinhold T, Obi-Tabot E, Bodoria M, Brüggenjürgen B. Critical limb ischemia and its impact on patient health preferences and quality of life-an international study. Int J Angiol. 2012;21(3):139–146. doi:10.1055/s-0032-1324738
36. Nordanstig J, Wann-Hansson C, Karlsson J, et al. Vascular Quality of Life Questionnaire-6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg. 2014;59(3):700–707. doi:10.1016/j.jvs.2013.08.099
37. Jens S, Conijn AP, Frans FA, et al. Outcomes of infrainguinal revascularizations with endovascular first strategy in critical limb ischemia. Cardiovasc Intervent Radiol. 2015;38(3):552–559. doi:10.1007/s00270-014-0955-5
38. Burt RK, Testori A, Oyama Y, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 2010;45(1):111–116. doi:10.1038/bmt.2009.102
39. Deutschmann HA, Schoellnast H, Temmel W, et al. Endoluminal therapy in patients with peripheral arterial disease: prospective assessment of quality of life in 190 patients. AJR Am J Roentgenol. 2007;188(1):169–175. doi:10.2214/AJR.05.1408
40. Engelhardt M, Bruijnen H, Scharmer C, Wohlgemuth WA, Willy C, Wölfle KD. Prospective 2-years follow-up quality of life study after infrageniculate bypass surgery for limb salvage: lasting improvements only in non-diabetic patients. Eur J Vasc Endovasc Surg. 2008;36(1):63–70. doi:10.1016/j.ejvs.2008.01.026
41. Forbes JF, Adam DJ, Bell J, et al.; BASIL trial Participants. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg. 2010;51(5Suppl):43S–51S. doi:10.1016/j.jvs.2010.01.076
42. Keeling AN, Naughton PA, O’Connell A, Lee MJ. Does percutaneous transluminal angioplasty improve quality of life? J Vasc Interv Radiol. 2008;19(2 Pt 1):169–176. doi:10.1016/j.jvir.2007.09.003
43. Kumar BN, Gambhir RP. Critical limb ischemia-need to look beyond limb salvage. Ann Vasc Surg. 2011;25(7):873–877. doi:10.1016/j.avsg.2011.05.020
44. Landry GJ, Esmonde NO, Lewis JR, et al. Objective measurement of lower extremity function and quality of life after surgical revascularization for critical lower extremity ischemia. J Vasc Surg. 2014;60(1):136–142. doi:10.1016/j.jvs.2014.01.067
45. Peeters Weem SM, Teraa M, Den Ruijter HM, de Borst GJ, Verhaar MC, Moll FL. Quality of life after treatment with autologous bone marrow derived cells in no option severe limb ischemia. Eur J Vasc Endovasc Surg. 2016;51(1):83–89. doi:10.1016/j.ejvs.2015.09.010
46. Shigematsu H, Yasuda K, Iwai T, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17(9):1152–1161. doi:10.1038/gt.2010.51
47. van Hattum ES, Tangelder MJ, Lawson JA, Moll FL, Algra A. The quality of life in patients after peripheral bypass surgery deteriorates at long-term follow-up. J Vasc Surg. 2011;53(3):643–650. doi:10.1016/j.jvs.2010.09.021
48. Wohlgemuth WA, Safonova O, Engelhardt M, Freitag M, Wölfle K, Kirchhof K. Improvement of the quality of life concerning the health of patients with peripheral arterial disease (PAD) after successful bypass surgery. Vasa. 2008;37(4):338–344. doi:10.1024/0301-1526.37.4.338
49. Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. 2012;65(5):497–502. doi:10.1016/j.jclinepi.2011.09.008
50. Bague N, Julia P, Sauguet A, et al. Femoropopliteal in-stent restenosis repair: midterm outcomes after paclitaxel eluting balloon use (PLAISIR Trial). Eur J Vasc Endovasc Surg. 2017;53(1):106–113. doi:10.1016/j.ejvs.2016.10.002
51. Brothers TE. Failure of patients with peripheral arterial disease to accept the recommended treatment results in worse outcomes. Ann Vasc Surg. 2015;29(2):244–259. doi:10.1016/j.avsg.2014.08.006
52. Egberg L, Mattiasson AC, Ljungström KG, Styrud J. Health-related quality of life in patients with peripheral arterial disease undergoing percutaneous transluminal angioplasty: a prospective one-year follow-up. J Vasc Nurs. 2010;28(2):72–77. doi:10.1016/j.jvn.2010.02.001
53. Klepanec A, Mistrik M, Altaner C, et al. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012;21(9):1909–1918. doi:10.3727/096368912X636948
54. Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. EuroQol Group. London, UK: Springer Open; 2014.
55. Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-BREF: preliminary population norms and effect sizes. Soc Indic Res. 2006;77(1):37–59. doi:10.1007/s11205-005-5552-1
56. Corriere MA, Goldman MP, Barnard R, et al. Cumulative number of treatment interventions predicts health-related quality of life in patients with critical limb ischemia. Ann Vasc Surg. 2017;44:41–47.
57. Frans FA, Met R, Koelemay MJ, et al. Changes in functional status after treatment of critical limb ischemia. J Vasc Surg. 2013;58(4):957–965.e1. doi:10.1016/j.jvs.2013.04.034
58. Frans FA, Nieuwkerk PT, Met R, et al. Statistical or clinical improvement? Determining the minimally important difference for the vascular quality of life questionnaire in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2014;47(2):180–186. doi:10.1016/j.ejvs.2013.10.012
59. Murphy MP, Lawson JH, Rapp BM, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53(6):1565–1574. doi:10.1016/j.jvs.2011.01.074
60. Nordanstig J, Pettersson M, Morgan M, Falkenberg M, Kumlien C. Assessment of minimum important difference and substantial clinical benefit with the Vascular Quality of Life Questionnaire-6 when evaluating revascularisation procedures in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2017;54(3):340–347. doi:10.1016/j.ejvs.2017.06.022
61. Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54(4):1021–1031. doi:10.1016/j.jvs.2011.03.281
62. Malyar N, Fürstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. 2013;34(34):2706–2714. doi:10.1093/eurheartj/eht288
63. Dua A, Desai SS, Patel B, et al. Preventable complications driving rising costs in management of patients with critical limb ischemia. Ann Vasc Surg. 2016;33:144–148. doi:10.1016/j.avsg.2015.11.026
64. Martinez RA, Shnayder M, Parreco J, et al. Nationally representative readmission factors in patients with claudication and critical limb ischemia. Ann Vasc Surg. 2018;52:96–107. doi:10.1016/j.avsg.2018.03.011
65. Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005-2008. Prev Chronic Dis. 2011;8(6):A141.
66. Barshes NR, Chambers JD, Cohen J, Belkin M. Model To Optimize Healthcare Value in Ischemic Extremities 1 (MOVIE) Study Collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015–1024. doi:10.1016/j.jvs.2012.02.069
67. Armstrong EJ, Ryan MP, Baker ER, Martinsen BJ, Kotlarz H, Gunnarsson C. Risk of major amputation or death among patients with critical limb ischemia initially treated with endovascular intervention, surgical bypass, minor amputation, or conservative management. J Med Econ. 2017;20(11):1148–1154. doi:10.1080/13696998.2017.1361961
68. World Health Organization. Global report on diabetes 2016. Availbale from: https://www.who.int/diabetes/global-report/en/.
69. World Health Organization Global Health Observatory data repository. Tobacco use: data by country. Available from: http://apps.who.int/gho/data/node.main.65.
70. Reed GW, Raeisi-Giglou P, Kafa R, Malik U. Hospital readmissions following endovascular therapy for critical limb ischemia: associations with wound healing, major adverse limb events, and mortality. J Am Heart Assoc. 2016;5(5):1–9.
71. Jones CE, Richman JS, Chu DI, Gullick AA, Pearce BJ, Morris MS. Readmission rates after lower extremity bypass vary significantly by surgical indication. J Vasc Surg. 2016;64(2):458–464. doi:10.1016/j.jvs.2016.03.422
72. Bodewes TC, Soden PA, Ultee KH, et al. Risk factors for 30-day unplanned readmission following infrainguinal endovascular interventions. J Vasc Surg. 2017;65(2):484–494. doi:10.1016/j.jvs.2016.08.093
73. Masoomi R, Shah Z, Quint C, et al. A nationwide analysis of 30-day readmissions related to critical limb ischemia. Vascular. 2018;26(3):239–249. doi:10.1177/1708538117727955
74. Marston WA, Davies SW, Armstrong B, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108–114. doi:10.1016/j.jvs.2006.03.026
75. Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132(19):1805–1815. doi:10.1161/CIRCULATIONAHA.115.016424
76. Baubeta Fridh E, Andersson M, Thuresson M, et al. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischaemia: a population based study. Eur J Vasc Endovasc Surg. 2017;54:480–486. doi:10.1016/j.ejvs.2017.07.005
77. Soga Y, Iida O, Takahara M, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv. 2014;7(12):1444–1449. doi:10.1016/j.jcin.2014.06.018
78. Melillo E, Micheletti L, Nuti M, et al. Long-term clinical outcomes in critical limb ischemia—a retrospective study of 181 patients. Eur Rev Med Pharmacol Sci. 2016;20:502–508.
79. van Haelst STW, Koopman C, Den Ruijter HM, et al. Cardiovascular and all-cause mortality in patients with intermittent claudication and critical limb ischaemia. BJS. 2018;105:252–261. doi:10.1002/bjs.10657
80. Wohlgemuth WA, Olbricht W, Klarmann S, et al. [Disease-specific Questionnaire for Quality of Life in Patients with Peripheral Arterial Occlusive Disease in the Stage of Critical Ischemia (FLeQKI)–methodical development of a specific measuring instrument and psychometric evaluation of its validity and reliability (Part 1)]. Rofo. 2007;179(12):1251–1257. doi:10.1055/s-2007-963515
Supplementary material
 |
Figure S1 CLI amputation rates. Abbreviations: CLI, critical limb ischemia; OXVASC, Oxford Vascular Study. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
