Back to Archived Journals » Research and Reports in Biochemistry » Volume 5
The botulinum toxin as a therapeutic agent: molecular and pharmacological insights
Received 8 April 2015
Accepted for publication 1 May 2015
Published 8 December 2015 Volume 2015:5 Pages 173—183
DOI https://doi.org/10.2147/RRBC.S60432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Nikolay Dokholyan
Roshan Kukreja,1 Bal Ram Singh2
1Department of Chemistry and Biochemistry, University of Massachusetts, 2Botulinum Research Center, Institute of Advanced Sciences, Dartmouth, MA, USA
Abstract: Botulinum neurotoxins (BoNTs), the most potent toxins known to mankind, are metalloproteases that act on nerve–muscle junctions to block exocytosis through a very specific and exclusive endopeptidase activity against soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins of presynaptic vesicle fusion machinery. This very ability of the toxins to produce flaccid muscle paralysis through chemical denervation has been put to good use, and these potentially lethal toxins have been licensed to treat an ever expanding list of medical disorders and more popularly in the field of esthetic medicine. In most cases, therapeutic BoNT preparations are high-molecular-weight protein complexes consisting of BoNT, complexing proteins, and excipients. There is at least one isolated BoNT, which is free of complexing proteins in the market (Xeomin®). Each commercially available BoNT formulation is unique, differing mainly in molecular size and composition of complexing proteins, biological activity, and antigenicity. BoNT serotype A is marketed as Botox®, Dysport®, and Xeomin®, while BoNT type B is commercially available as Myobloc®. Nerve terminal intoxication by BoNTs is completely reversible, and the duration of therapeutic effects of BoNTs varies for different serotypes. Depending on the target tissue, BoNTs can block the cholinergic neuromuscular or cholinergic autonomic innervation of exocrine glands and smooth muscles. Therapeutic BoNTs exhibit a high safety and very limited adverse effects profile. Despite their established efficacy, the greatest concern with the use of therapeutic BoNTs is their propensity to elicit immunogenic reactions that might render the patient unresponsive to subsequent treatments, particularly in chronic conditions that might lead to long-term treatment and frequent injections.
Keywords: botulinum neurotoxins, therapeutics, esthetics, botox, dysport, xeomin
Therapeutic botulinum toxins: introduction and historical background
Botulinum neurotoxins (BoNTs) produced by anaerobic, spore-forming bacteria of the genus Clostridium are the most toxic proteins known with mouse lethal dose 50% (LD50) values in the range of 0.1–1 ng/kg.1 They are solely responsible for the pathophysiology of botulism, a severe neurological disease characterized by flaccid muscle paralysis, resulting from BoNT-mediated blockage of acetylcholine release at the nerve–muscle junctions.2 BoNTs constitute a family of seven structurally similar but antigenically distinct proteins (types A–G) produced by different strains of Clostridium botulinum. BoNT serotypes share a high degree of sequence homology, but they differ in their toxicity and molecular site of action.2
Botulism was first identified in the early 19th century by Justinus Kerner, a German doctor and poet, when he linked deaths from food intoxication with a poison found in smoked sausages.3 He had even speculated about a variety of potential therapeutic uses of botulinum toxin for movement disorders, hypersecretion of body fluids, ulcers, etc.4 The scientific parameters of the disease were uncovered in 1897 by Emile van Ermengem, who successfully isolated the bacterium and named it Bacillus botulinus,5,6 which was renamed C. botulinum in later years.
In 1928, Snipe and Sommer at the University of California isolated BoNT as a stable acid precipitate for the first time,7 following which, standardized preparations of BoNT and maintenance of rigorous safety standards for its therapeutic use were achieved by Edward J Schantz, Carl Lammana, and colleagues from the Department of Microbiology and Toxicology at the University of Wisconsin, Madison.8–10 The first documented use of BoNT for the treatment of disease was in the 1970s, approximately 150 years after Kerner’s initial observations about the potential use of BoNT as a therapeutic, when Dr Alan Scott, an ophthalmologist, used local injection of minute doses of BoNT to selectively inactivate muscle spasticity in strabismus in monkeys.11 Following the success of a series of clinical studies on humans suffering from strabismus,12 the Food and drug Administration (FDA) in 1989 approved the use of BoNT/A (BOTOX®), manufactured by Allergan pharmaceuticals, for the treatment of strabismus, blepharospasm, and hemifacial spasm. Since then the very lethal botulinum toxins, botulinum types A and B, have been extensively used for the treatment of a myriad of dystonic and nondystonic movement disorders and a host of other medical conditions, including axillary hyperhidrosis, spasticity, tremors, and pain management. The high efficacy of BoNT/A coupled with a good safety profile has prompted its empirical use in a variety of ophthalmological, urological, gastrointestinal, secretory, and dermatological disorders.13 Incredibly, the list of conditions treated with botulinum toxin is expanding at a brisk rate.
The potential use of BoNT/A in esthetics was first demonstrated in 1987 based on the observation that facial wrinkles were diminished on treatment with BoNT/A for blepharospasm.14 Dynamic facial lines and wrinkles are caused by patterns of repetitive muscle contractions or facial expressions. Injection with BoNT temporarily paralyzes the nerve impulses responsible for muscle contraction, resulting in flattened facial skin and improved cosmetic appearance.15 This effect, although temporary, is extremely popular with patients, has a low incidence of side effects, making the use of BoNT/A the most common cosmetic procedure worldwide for facial enhancement.16 Botulinum toxin injections have revolutionized the nonsurgical approach to rejuvenation of an aging face and are now widely used for several esthetic procedures, including treatment of glabella frown lines, forehead furrows, and periorbital wrinkles.17
Molecular structure of BoNTs
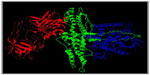
BoNT is produced as a single polypeptide chain with a molecular mass of ~150 kDa that displays low intrinsic activity. This precursor protein is subsequently cleaved by bacterial proteases at an exposed protein-sensitive loop generating a fully active neurotoxin, composed of a 100 kDa heavy chain (HC) and a 50 kDa light chain (LC). The HC and LC remain linked by both noncovalent protein–protein interactions and a conserved interchain disulfide bridge, called the belt, which extends from the HC and wraps around the LC.2 During intoxication process, the interchain bridge is reduced, and this is a necessary prerequisite for the intracellular action of the toxins.18 The three-dimensional structures of BoNTs reveal that they are folded into three distinct domains that are functionally related to their cell intoxication mechanism. The N-terminal domain is the 50 kDa LC, which is a Zn2+-dependent endoprotease. The 100 kDa HC consists of a N-terminal translocation domain and a C-terminal receptor-binding domain2 (Figure 1).
BoNTs are secreted from C. botulinum in the form of multimeric complexes, with a set of nontoxic proteins coded for by genes adjacent to the neurotoxin gene.19 These protein complexes range in size from 300 kDa to 900 kDa. These large protein complexes consist of the 150 kDa neurotoxin moiety and the set of complexing proteins that are made of a nontoxic-nonhemagglutinin protein (or neurotoxin binding protein [NBP]) and several hemagglutinin proteins. These are known as neurotoxin-associated proteins (NAPs) and also as complexing or accessory proteins. Stabilized through noncovalent interactions, NAPs account for ~70% of the total mass.20
The nontoxic NAPs are believed to protect the neurotoxin from degradation during its passage through the low pH environment of the gastrointestinal tract.21 They are also known to assist BoNT translocation across the intestinal mucosal layer.22,23 The association of NAPs with the toxin is pH dependent, and at physiological pH, this complex is reported to rapidly dissociate allowing release of the neurotoxin in the blood stream.24,25 When used for therapeutic purposes, where BoNT/is not delivered orally, the role of these accessory proteins in protection against gastric pH extremes and proteases and in transport across the intestinal epithelium is not clear and not relevant to clinical efficiency.
Mechanism of action of BoNTs
When therapeutic BoNT preparation is injected into the target tissue, it acts as a metalloproteinase that enters peripheral cholinergic nerve terminals and cleaves proteins that are crucial components of the neuroexocytosis apparatus, causing a persistent but reversible inhibition of neurotransmitter release. The exact molecular mechanism of BoNT action still remains to be completely understood but existing experimental evidence suggests that BoNT intoxication occurs through a multistep process involving each of the functional domains of the toxin.26 These steps include binding of the neurotoxin to specific receptors at the presynaptic nerve terminal, internalization of the toxin into the nerve cell and its translocation across the endosomal membrane, and intracellular endoprotease activity against proteins crucial for neurotransmitter release.
BoNTs have a high affinity and specificity for their target cells and use two different coreceptors for binding at the neuronal cell surface. Binding of BoNTs to the neuromuscular junction involves a tight association between its receptor-binding HC domain and complex polysialogangliosides, particularly GT1b and GD1b that are known to be enriched in neurons.27,28 Upon binding to the gangliosides, the membrane-bound ganglioside–toxin complex moves to reach the toxin-specific receptor. Different BoNT serotypes bind to different protein receptors. SV2 (isoforms A–C), a synaptic vesicle glycoprotein, has been identified as a receptor for BoNT/A and BoNT/E.29,30 Synaptotagmin, a synaptic vesicle protein, has been identified as the receptor for BoNT types B and G.31,32
Following binding to neuronal cell surface receptors, BoNT is internalized into cellular compartments by receptor-mediated endocytosis.1 After BoNTs are incorporated within the early endosomes, the acidic environment of the endocytotic vesicles is believed to induce a conformational change in the neurotoxin structure. The HC is inserted into the synaptic vesicle membrane forming a transmembrane protein-conducting channel that translocates the LC into the cytosol.33
Upon internalization into the neuronal cytosol, BoNTs exert their toxic effect by virtue of the metalloprotease activity of the LC, which specifically cleave one of three soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins that are integral to vesicular trafficking and neurotransmitter release.2 The specific SNARE protein targeted and the site of hydrolytic cleavage vary among the seven BoNT serotypes. BoNT serotypes A and E specifically cleave SNAP-25 at a unique peptide bond. BoNT serotypes B, D, F, and G hydrolyze VAMP/synaptobrevin, at different single peptide bonds, and BoNT/C cleaves both syntaxin and SNAP-252 (Figure 2).
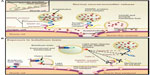  | Figure 2 Schematic model of mode of action of botulinum neurotoxins. |
The remarkable therapeutic utility of botulinum toxin lies in its ability to specifically and potently inhibit involuntary muscle activity for an extended duration. Intoxication of the nerve terminal by BoNTs is fully reversible and does not lead to neurodegeneration.34 Upon synaptic blockade of cholinergic nerve terminals by therapeutic BoNT, the neuron forms new synapses that replace its original ones in a process known as sprouting. As the nerve terminals eventually recover, original synapses are regenerated, the sprouts retreat, and the synaptic contact is reestablished leading to restoration of exocytosis.35
Depending on the target tissue, BoNT can block the cholinergic autonomic innervation of the tear, salivary, and sweat glands or the cholinergic neuromuscular innervation of striated and smooth muscles.36 After intramuscular injection, the dose-dependent paralytic effect of BoNT can be detected within 2–3 days. It reaches its maximal effect in <2 weeks and gradually begins to decline in a few months due to the ongoing turnover of the synapses at the neuromuscular junction.35 The duration of effect lasts somewhere between 3 months and 6 months, and the benefits have been observed to increase with time.37 There has been no evidence of any long-term or permanent degeneration or atrophy of muscles in patients with repeated injections of BoNTs over an extended period.35
Pharmacological aspects of therapeutic BoNTs
Current therapeutic BoNT formulations
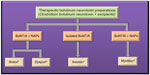
Despite a plethora of research on the molecular action and the medical uses of BoNTs, currently only two serotypes of BoNTs are commercially being used as therapeutics, type A (BoNT/A) and type B (BoNT/B). There are three preparations of BoNT/A that are approved by the FDA, namely, Botox® (onabotulinumtoxinA) manufactured by Allergan Inc., USA; Dysport® (abobotulinumtoxinA) by Ipsen Ltd, UK; and Xeomin® (incobotulinumtoxinA) manufactured by Merz Pharmaceuticals, Germany. BoNT serotype B (MYOBLOC®, rimabotulinumtoxinB; Solstice Neurosciences, USA) was approved by the FDA in year 2000.13 The remarkable therapeutic utility of BoNT lies in its ability to specifically and potently inhibit involuntary muscle activity for an extended duration. The major differences between the botulinum toxin drug preparations include the bacterial strains from which they are produced, their manufacturing processes, composition, and presence of NAPs, and the type and quantity of excipients used in each formulation.
Molecular size and composition
The commercially available BoNT/A preparations consist of different components of complexing proteins/NAPs and, therefore, have different three-dimensional structures and molecular weights.36 Both Botox® and Dysport® contain botulinum toxin type A complexed with NAPs, whereas Xeomin® consists of only the purified 150 kDa neurotoxin responsible for its therapeutic effect and is devoid of the NAPs (Figure 3). The composition of NAPs in BoNT complex is specific to the method of growth and purification.36 The molecular size of the toxin-NAPs complex varies in the three BoNT/A preparations36 but does not have any effect on the biological activity or on the pharmacological properties of these formulations. This may be because BoNT/A dissociates from NAPs almost instantaneously upon reconstitution of the lyophilized or freeze-dried products, before being injected into the target tissue.25 The commercial formulation of Myobloc is a clear injectable solution with a molecular weight of ~700 kDa.38
Manufacturing and stability
All therapeutic BoNT/A preparations are manufactured biologically in accordance with the current Good Manufacturing Practice guidelines. C. botulinum bacteria selected from a special strain are fermented under anaerobic conditions at optimal temperatures where the bacteria multiply and secrete the neurotoxin. The bulk that contains the neurotoxin is subjected to several precipitation and purification steps to isolate the toxin complex from the residual components of the bacteria.35 The purification methods for both Botox® and Dysport® vary, and thus, both preparations possess different physiochemical and clinical characteristics.38 Xeomin® undergoes further chromatographic purification to separate the NAPs resulting in the 150 kDa pure neurotoxin.15 Myobloc® is prepared by fermentation of the C. botulinum type B Bean strain and exists as a complex of 150 kDa neurotoxin associated with NAPs.38
Excipients such as human serum albumin and NaCl or lactose or sucrose are added to the toxin formulations to minimize the risk of product inactivation during long-term storage.15 Thus, each BoNT product is formulated uniquely, lyophilized and packaged for commercial distribution.36 All of the BoNT/A preparations are marketed in the powder form (freeze dried or vacuum dried) and need to be reconstituted with sterile saline prior to application.35 Only Myobloc® is available as a ready to use solution. Most BoNT preparations require special storage temperature regimens except for Xeomin® that can be stored at room temperature. The shelf lives of all therapeutic BoNT/A preparations are similar.36 The prolonged shelf life and less stringent storage conditions required for Xeomin® is remarkable suggesting that NAPs may not play a significant role in BoNT stability.24 Myobloc® is stabilized by reducing the pH value and as a result of which patients injected with this drug have reported intensified injection site pain.35
Biological activity
The biological activity of therapeutic BoNT drugs is measured by an in vivo mouse lethality assay (LD50) and is displayed in mouse units (MU). One unit is defined as the amount of toxin injected intraperitoneally that is required to kill 50% mice. Although one MU is defined by international convention, the activity assays used by manufacturers differ so that the potency labeling of different therapeutic preparations remains proprietary to the manufacturer and cannot be compared directly.39 Approximately 1 MU of Botox® is equivalent to ~3 MU of Dysport®, whereas the activity labeling of Botox and Xeomin® seem to be identical.40 The potency labeling of different BoNT serotypes can also not be compared directly. Therapeutic effects of Botox® and Myobloc® seem to be comparable at a 1:40 ratio.35 Recently, the mouse LD50 assays for the potency determination of therapeutic BoNTs are being replaced by more human cell-based assays with each manufacturer developing their own propriety method. Comparison of the specific biological activity calculated for the three BoNT/A preparations indicates that Xeomin® displays the highest specific biological activity of 227 U/ng followed by Botox® at 137 U/ng and Dysport® at 154 U/ng.36 The foreign protein load delivered per unit of Xeomin® is much lower (0.44 ng/100 U) than that for both Botox® and Dysport®.
Immunogenicity and clinical response
In therapeutic BoNT preparations, the amount of neurotoxin product along with the complexing and residual proteins defines the foreign protein load. As with any therapeutic protein, the host immune system may recognize any part of the neurotoxin as foreign and potentially elicit an immune response, particularly with repeated administration. This may lead to the formation of neutralizing antibodies against BoNT, which may block its biological activity resulting in antibody-induced therapy failure. Complexing proteins/NAPs have no therapeutic role and present a high foreign protein load. Antibodies formed against NAPs do not interfere with the biological activity of BoNTs and are known as nonneutralizing antibodies.35 The formation of neutralizing antibodies in patients receiving therapeutic BoNT/A preparations can render the patient partially or completely unresponsive to further treatments. Several case studies have demonstrated antibody-induced therapy failure in patients receiving treatment for hemifacial spasm, cervical dystonia, and urological disorders.41 Risk factors associated with the development of neutralizing antibodies include product-related factors, such as manufacturing process, antigenic protein load, and specific biological activity, and treatment-related factors, such as an applied toxin dose of >300 U at each injection session and the interval among injection series, booster injections, and prior exposure.42,43
Clinical studies have suggested that higher total protein content might contribute to greater chance of formation of neutralizing antibodies.44 Attempts to overcome the effects of antibody-induced treatment failure have been made in the past. The original Botox® formulation contained 25 ng of neurotoxin complex protein per 100 units. In order to reduce the risk of antibody-induced treatment failure, Allergan, Inc., modified the formulation, and the current Botox® preparation contains only 5 ng of complex protein per 100 units. In a study of patients treated with cervical dystonia with Botox®, neutralizing antibodies were detected in 9.5% of the 42 patients treated with the older formulation, but none of the 119 patients treated exclusively with the current preparation had detectable neutralizing antibodies.45 Analysis of factors, including the influence of age and cumulative dose, suggested that the low risk of antibody formation on treatment with the current Botox® formulation was related to lower protein exposure in patients. However, since the amount of other proteins per unit of active toxin still remain proportionally high even in the current formulation, antibody-induced treatment failure due to the presence of neutralizing antibodies still occurs.46 Although the doses of BoNT preparations used in esthetic applications are lower than those used for therapeutic purposes, neutralizing antibodies production and resulting treatment failure have been observed following low-dose applications of current BoNT/A preparations in esthetics, and reports of secondary treatment failure are also emerging.17
Since immunogenicity is proportional to the foreign protein load, the highly purified formulation of Xeomin® that contains only the neurotoxin can be expected to be less immunogenic, thus resulting in reduced incidence of neutralizing antibody-induced treatment failure compared to the other BoNT/A preparations.15 Preclinical studies have suggested that in contrast to Botox® and Dysport®, Xeomin® did not induce production of neutralizing antibodies following repeated injections in rabbits.47 To date, only one case of antibody-induced treatment failure has been demonstrated with Xeomin®, wherein a patient with progressive hereditary juvenile-onset generalized dystonia, who was pretreated with Dysport® for 15 years, responded with complete therapy failure by the fifth or sixth application of Xeomin®, thus supporting the hypothesis of reduced antigenicity with lower protein load.48
Additional benefits offered by the purified formulation of Xeomin® include improved handling characteristics and greatly reduced oral toxicity compared with other BoNT/A preparations.
Safety profile, risks, and adverse effects
The use of BoNTs as therapeutic agents since late 1970s has enjoyed a remarkable safety profile during this long-term window. Adverse effects resulting from BoNT therapy can be classified as local or systemic. The profile of adverse effects of the available BoNT preparations is shown to be similar,49 except for BoNT/B that displays additional systemic autonomic adverse effects and symptoms due to autonomic dysfunction.50 Local adverse effects result from the diffusion of BoNT from the target tissue into adjacent tissues, whereas systemic effects occur in tissues distant from the injection site by transport of BoNT within the blood circulation.
Diffusion may occur with the passive movement of the neurotoxin beyond its original site. Ptosis of the brow has been reported as an adverse effect following BoNT injection to eliminate frown lines. This could result from the diffusion of neurotoxin into the frontalis muscle causing the eyelids to droop20 and can be avoided by applying digital pressure on the orbital rim to limit diffusion and avoiding a postinjection massage.37 It has been hypothesized that different diffusion characteristics are attributed to protein complex size and pharmacological properties whereby the use of larger molecular weight toxin complex would minimize toxin diffusion from the injection site.51 However, clinical studies have indicated no difference in product diffusion between the use of Botox® (with a higher molecular weight) and Dysport®.52,53 Additional studies comparing the BoNT/A therapeutic formulations of Botox®, Dysport®, and a purified preparation of 150 kDa BoNT/A have suggested no differences in the diffusion patterns from the injection site among the three preparations even when applied at higher doses.54 Localized effects unrelated to the toxin may include bruising at the injection site, local edema, erythema, and transient numbness. Headaches commonly occur within the first 24 hours after BoNT administration but usually become far less common with repeated injections.55
Adverse effects from BoNT application usually occur within a week’s time and last for 1 week or 2 weeks. Severity and duration of the adverse effects depend on the dose applied. Small amounts of BoNT may briefly circulate in the blood after administration, raising concern about the potential for long-term adverse effects. Systemic spread of BoNTs is clinically relevant only when higher doses of BoNT are applied. Such effects where the toxin spreads to more distant muscles has been reported in patients injected with BoNT/A for blepharospasm and cervical dystonia. This was reflected as increased jitter in the limb muscles and changes in the single-fiber EMG.56,57 Transient ptosis, tearing, and dry eye are the most frequently encountered complications with the use of botulinum toxin in the treatment of blepharospasm and hemifacial spasm.20 Dry mouth after BoNT administration may be a systemic effect as small amounts of toxin escape into the bloodstream. The highest rates of dry mouth are reported with the use of Myobloc® followed by Dysport® and Botox®. Myobloc® even when used in low doses frequently produces autonomic adverse effects such as dry mouth, dysphagia, corneal irritation, and irritation of nasal mucosa.58 BoNT/B has a relatively strong effect on the autonomic nervous system and a relatively weak effect on the motor system and hence is used in caution in patients with pre-existing autonomic dysfunction.35 The use of BoNTs in pregnancy or during lactation is contradicted as a precautionary measure due to lack of sufficient clinical evidence of its safety in these conditions.
Contrary to prior belief that the action of BoNTs remains restricted to peripheral synapses and that its effects are confined to the injection site, substantial evidence in recent times have demonstrated the effects BoNT could have on the central nervous system. Several studies on animals indicate that BoNT/A can undergo retrograde axonal transport and transcytosis. In one such study, injection of BoNT/A in the optic tectum of rats led to the appearance of BoNT/A truncated SNAP-25 in retinal synapses suggesting axonal migration of catalytically active BoNT in central and motor neurons followed by transcytosis to afferent neurons.59 Additional studies performed in order to examine the structural and functional consequences of SNAP-25 proteolysis in synapses distant from the site of BoNT injection have provided further insights into the retrograde trafficking mechanism of BoNTs.60,61 Direct evidence of axonal retrograde transport of BoNT/A and BoNT/E away from the site of injection to exert clinical effects in central nervous system has also been demonstrated in spinal cord motor neurons.62 Antinociceptive activity of BoNT/A was studied in a diabetic neuropathy pain model in rats. Bilateral pain reduction after intrathecal BoNT/A injection suggested the involvement of retrograde axonal transport of BoNT/A from the peripheral site of injection to the central nervous system in the antinociceptive action of BoNT in painful diabetic neuropathy.63
In recent clinical studies, therapeutic BoNT/A preparations have demonstrated antidepressive effects in patients along with its cosmetic benefits. One such randomized, double-blind, placebo-controlled trial carried out by Wollmer et al64 revealed positive effects on the mood and a significant improvement in depressive symptoms of the patients being treated with Botox® for glabella frown lines. This prompted the authors to conclude that facial muscles not only express emotions but also play an important role in mood regulation. In another randomized, double-blind trial, when Botox® was injected into the corrugator and procerus muscles of patients being treated for major depressive disorder, significant and sustained anti-depressive effects were observed with a single treatment of BoNT/A.65 The authors have proposed the mechanism of emotional proprioception whereby the brain uses facial muscles to gage the emotional state and mood of an individual. Corrugator muscle contraction may convey distress signals to the brain but injection with BoNT/A could lead to relaxation of these muscles thereby reducing negative signals and influencing mood in a positive way, thus leading to a happy emotional experience in affected individuals.65,66 In a yet another 24-week randomized double-blind placebo-controlled study by Magid et al at the University of Texas Southwestern Medical Center, 30 research subjects with depressive symptoms were randomized to receive botulinum injections into the glabella region or placebo. Following single Botox® injections, significant clinically meaningful decrease in depressive symptoms was observed throughout the study even after the wrinkles reappeared.67
Effects of BoNTs have also been observed on the cognitive abilities of patients treated with therapeutic BoNT preparations. Studies conducted based on the facial feedback hypothesis wherein emotional experience is felt by facial feedback signals generated when we automatically imitate expression on other’s faces, compared Botox (which paralyzes muscles of facial expression) with a control dermal filler that has no effect on facial muscles. The results indicated that the toxin significantly interfered with “embodied emotion cognition”. Participants injected with Botox were found to decipher facial expressions significantly less accurately as compared to the control group and had an overall decrease in the strength of emotional experience.68,69 Effects of BoNTs on emotional cognition were also seen in a study that used BoNT/A injections to temporarily paralyze the frown muscles. Findings indicated that blockage of facial expressions by peripheral denervation of facial musculature resulted in a decrease in the processing of emotional language by the participants. This study supported the facial feedback theories of emotional cognition and demonstrated the potential effects of BoNTs on cognition and emotional reactivity.70
Conclusion and future directions
Therapeutic BoNT preparations are a group of highly potent drugs that have proven to be a powerful tool in medicine. They are widely employed for the treatment of varying indications including neuromuscular, pain, and ophthalmic disorders, but perhaps their most popular application has been in the field of esthetic medicine for the treatment of facial lines and wrinkles. When used judiciously, BoNT therapy can provide physicians with a useful tool for providing patients with symptomatic relief for long periods, thus positively influencing their quality of life.
All BoNT serotypes demonstrate the same mechanism of action but differ in their intracellular targets. The effects of BoNT intoxication and injection are temporary, with results lasting from 3 months to 6 months. BoNT treatments exhibit a high-safety profile with no serious adverse reactions, and they are even extremely rare, particularly at the low doses used for cosmetic purposes. Each commercially available BoNT preparation is unique with the most notable difference among them being the molecular size and structure. While Botox® and Dysport® are formulated neurotoxin protein complexes differing from each other in composition, Xeomin® is a purified formulation consisting of only the 150 kDa neurotoxin. Complexing proteins/NAPs are not known to affect the therapeutic activity of BoNTs but may play a significant role in inducing immunological response after BoNT therapy. They neither enhance the stability nor do they limit the diffusion of therapeutic BoNT preparations. Notably, however, Xeomin®, which does not have NAPs, uses double the amount of human serum albumin compared to BoNT/A products containing NAPs. Given the lack of any pharmacological effect of NAPs for therapeutic and esthetic applications, clinical strategies to reduce or eliminate neutralizing antibody development and secondary treatment failure consider removal of NAPs from the drug product. However, sufficient data on the stabilizing effect and on the long-term host response to toxin71 is not available to recommend against use of the complex forms of the toxins in medical applications.
While tremendous progress has been made in the field of therapeutic uses of BoNTs, many questions still remain unanswered, especially on the molecular and biologicals aspects of these clostridial toxins. There are certain clinical conditions effectively treated with BoNT/A (eg, migraine pain), but our understanding of the underlying mechanism of therapeutic action of BoNTs on these clinical manifestations remains elusive. The mechanisms of effects on BoNTs on depression and cognition are also least understood. Continuation of basic scientific investigation aimed at addressing these issues will enable us to understand their therapeutic effects more precisely. Moreover, unlike previously assumed, latest research findings have suggested that BoNTs may undergo retrograde and anterograde transport inside neurons implying the effects on BoNTs in the central circuits, especially at high doses. It is not yet clear whether these central effects actually contribute to the therapeutic efficacy of BoNT/A. Increased research efforts toward a more detailed understanding of the central actions of BoNT/A will provide valuable information on the present and future uses of this neurotoxin in clinical practice. With the ability of BoNTs to engage in long-range trafficking, novel uses can be envisaged for these toxins as drug delivery vehicles for targeting therapeutics to the central nervous system.
The future of BoNT therapies seems extremely exciting with the development of newer and innovative toxin delivery modalities. RT001 cream, a topical gel form of purified BoNT/A is being manufactured by Revance Therapeutics and is yet to be FDA approved. RT001 uses a proprietary synthetic transport peptide called TransMTS® that enables delivery of BoNT across the skin, eliminating the need of injections. Results from the Phase IIb clinical trials to evaluate RT001 for the treatment of lateral canthal lines (crow’s feet wrinkles) have demonstrated safety and efficacy with a meaningful reduction of the wrinkles. No serious adverse events or systemic safety concerns were reported72 (Revance.com). ANT-1207, botulinum toxin type A lotion (Anterios, Inc.) is yet another topical formulation of BoNT/A being developed for the potential treatment of hyperhidrosis, facial wrinkles and a new clinical indication such as acne. Anterios Inc. uses a proprietary technology that enables local, targeted delivery of BoNT across the skin without the use of needles or other invasive treatments. Initial clinical studies using ANT-1207 for the aforementioned indications have demonstrated safety as well as clinically significant efficacy versus controls. This formulation is yet to be studied further in Phase IIb clinical trials (Anteriosinc.com). Potential implications of such topical formulations are very broad. A topical neurotoxin that can target sweat glands and sebaceous glands could help in the treatment of numerous dermatological conditions, rendering a bright future for BoNTs in medicine.
Improved understanding of the structural and functional basis of BoNTs action has led to the development of novel recombinant proteins with enhanced and extended clinical potential. These novel biomolecules that are engineered from BoNT protein scaffolds wherein the neuron-binding domain is replaced by a new ligand specific for a cell type of interest are known as target secretion inhibitors. They are designed to treat diseases through selective inhibition of cell secretory pathways, thus extending the scope of BoNT’s activity beyond the neuromuscular junction.73,74 The opportunity to expand the therapeutic potential of BoNTs using TSIs has recently been demonstrated in the Phase IIb clinical trials focused on patients with herpetic neuralgia, overactive bladder, and urinary incontinence, wherein the TSIs were specifically targeted to peripheral neurons involved in the transmission of the pain signal.74 In the future, these next-generation novel neurotoxin products will offer the potential for noncytotoxic and prolonged inhibition of cell secretion, thus leading to extended durations of clinical effect, making them an ideal choice for the treatment of chronic ailments such as pain disorders.
Disclosure
The authors report no conflicts of interest in this work.
References
Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:715–760. | |
Singh BR. Botulinum neurotoxin structure, engineering, and novel cellular trafficking and targeting. Neurotox Res. 2006;209:73–92. | |
Erbguth FJ, Nauman M. Historical aspects of botulinum toxin: Justinus Kerner (1786–1862) and the “sausage poison”. Neurology. 1999;53:1850–1853. | |
Ting PT, Freiman A. The story of Clostridium botulinum: from food poisoning to Botox. Clin Med. 2004;4:258–262. | |
van Ermengem E. Classics in infectious diseases. A new anaerobic bacillus and its relation to botulism. Rev Infect Dis. 1979;1:701–719. | |
Cherington M. Clinical spectrum of botulism. Muscle Nerve. 1998;21:701–710. | |
Snipe PT, Sommer H. Studies on botulinus toxin: 3. Acid precipitation of botulinus toxin. J Infect Dis. 1928;43:152–160. | |
Stefanye D, Schantz EJ, Spero L. Amino acid composition of crystalline botulinum toxin, type A. J Bacteriol. 1967;94:277–278. | |
Lamanna C, Spero L, Schantz EJ. Dependence of time to death on molecular size of botulinum toxin. Infect Immun. 1970;1:423–424. | |
Schantz EJ. Some chemical and physical properties of Clostridium botulinum toxins in culture. Jpn J Microbiol. 1967;11:380–383. | |
Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12:924–927. | |
Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–1049. | |
Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins. 2012;4:913–939. | |
Carruthers J, Stubbs HA. Botulinum toxin for benign essential blepharospasm, hemifacial spasm and age-related lower eyelid entropion. Can J Neurol Sci. 1987;14(1):42–45. | |
Lorenc ZP, Kenkel JM, Fsgien S, et al. IncobotulinumtoxinA: background, mechanism of action and manufacturing. Aesthet Surg J. 2013;33:18S–22S. | |
Kane M, Donofrio L, Ascher B, et al. Expanding the use of neurotoxins in facial aesthetics: a consensus panels’ assessment and recommendations. J Drugs Dermatol. 2010;9(Suppl):S7–S22. | |
Sattler G. Current and future botulinum neurotoxin typa A preparations in aesthetics: a literature review. J Drugs Dermatol. 2010;9:1065–1071. | |
Humeau Y, Dossau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release? Biochimie. 2000;82:427–446. | |
Inoue K, Fujinaga Y, Watanabe T, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun. 1996;64:1589–1594. | |
Klein AW. Complications and adverse reactions with the use of botulinum toxin. Dis Mon. 2002;48:336–356. | |
Gu S, Rumpel S, Zhou J, et al. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. 2012;335:977–981. | |
Fujinaga Y, Inoue K, Watarai S, et al. Molecular characterization of binding subcomponents of Clostridium botulinum type C progenitor toxin for intestinal epithelial cells and erythrocytes. Microbiology. 2004;150:1529–1538. | |
Matsumura T, Sugawara Y, Yutani M, et al. Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat Commun. 2015;6:6255. | |
Grein S, Mander GJ, Fink K. Stability of botulinum neurotoxin type A, devoid of complexing proteins. Botulinum J. 2011;2:49–58. | |
Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57:555–565. | |
Montecucco C, Papini C, Schiavo G. Bacterial toxins penetrate cells via a four-step mechanism. FEBS Lett. 1994;346(1):92–98. | |
Nishiki T, Kamata Y, Nemoto Y, et al. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269:10498–10503. | |
Rummel A, Mahrhold S, Bigalke H, Binz T. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol Microbiol. 2004;51:631–643. | |
Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312(5773):595–596. | |
Mahrhold S, Strotmeier J, Garcia-Rodriguez C, et al. Identification of the SV2 protein receptor-binding site of botulinum neurotoxin type E. Biochem J. 2013;453:37–47. | |
Nishiki T, Tokuyama Y, Kamata Y, et al. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996;378:253–257. | |
Rummel A, Eichner T, Weil T, et al. Identification of the receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci U S A. 2007;104:359–364. | |
Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. | |
de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200–3205. | |
Dressler D, Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29:1761–1768. | |
Frevert J. Pharmaceutical, biological and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1–9. | |
Berry MG, Stanek JJ. Botulinum neurotoxin A: a review. J Plast Reconstr Aesthet Surg. 2012;65:1283–1291. | |
Wheeler A, Smith HS. Botulinum toxins: mechanism of action, antinociception and clinical applications. Toxicology. 2013;306:124–146. | |
Hambleton P, Pickett AM. Potency equivalence of botulinum toxin preparations. J R Soc Med. 1994;87:719. | |
Dressler D. Clinical applications of botulinum toxin. Curr Opinon Microbiol. 2012;15:325–336. | |
Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or hindrance. Biologics. 2010;4:325–332. | |
Dressler D. Clinical presentation and management of antibody induced failure of botulinum toxin therapy. Mov Disord. 2004;19(Suppl 8):S92–S100. | |
Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213–217. | |
Jankovic J. Botulinum toxin: clinical implications of antigenicity and immunoresistance. In: Brin MF, Jankovic J, Hallett M, editors. Scientific and Therapeutic Aspects of Botulinum Toxin. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:409–415. | |
Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin cervical dystonia. Neurology. 2003;60:1186–1188. | |
Dressler D. New formulation of Botox: complete antibody-induced therapy failure in hemifacial spasm. J Neurol. 2004;251(3):360. | |
Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystonia. Drugs. 2007;67:669–683. | |
Dressler D. Five-year experience with incobotulinumtoxinA (Xeomin(R)): the first botulinum toxin drug free of complexing proteins. Eur J Neurol. 2012;19:385–389. | |
Bakheit AM. The possible adverse effects of intramuscular botulinum toxin injections and their management. Curr Drug Saf. 2006;1:271–279. | |
Tintner R, Gross R, Winzer UF, Smalky KA, Jankovic J. Autonomic function after botulinum toxin type A or B: a double-blind, randomized trial. Neurology. 2005;65:765–767. | |
De Almeida AT, De Boulle K. Diffusion characteristics of botulinum neurotoxin products and their clinical significance in cosmetic applications. J Cosmet Laser Ther. 2007;9(Suppl 1):17–22. | |
Trindade de Almeida AR, Marques E, de Almeida J, Cunha T, Boraso R. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatol Surg. 2007;33:S37–S43. | |
Hexsel D, Dal’Forno T, Hexsel C, Do Prado DZ, Lima MM. A randomized pilot study comparing the action halos of two commercial preparations of botulinum toxin type A. Dermatol Surg. 2008;34:52–59. | |
Dodd SL, Rowell BA, Vrabas IS, Arrowsmith RJ, Weatherill PJ. A comparison of the spread of three formulations of botulinum neurotoxin A as determined by effects on muscle function. Eur J Neurol. 1998;5:181–186. | |
Carruthers A. Problems with toxins. Body Lang. 2010;35:43–44. | |
Lange DJ, Brin MF, Warner CL, Fahn S, Lovelace RE. Distant effects of local injection of botulinum toxin. Muscle Nerve. 1987;10:552–555. | |
Lange DJ, Rubin M, Greene PE, et al. Distant effects of locally injected botulinum toxin: a doubleblind study of single fiber EMG changes. Muscle Nerve. 1991;14:672–675. | |
Dressler D, Benecke R. Autonomic side effects of botulinum toxin type B treatment of cervical dystonia and hyperhidrosis. Eur Neurol. 2003;49:34–38. | |
Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689–3696. | |
Restani L, Novelli E, Bottari D, et al. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic. 2012;13:1083–1089. | |
Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44–61. | |
Restani L, Giribaldi F, Manich M, et al. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012;8(12):1–19. | |
Bach-Rojecky L, Šalkovic-PetriŠic M, Lackovic Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. Eur J Pharmacol. 2010;633:10–14. | |
Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatry Res. 2012;46:574–581. | |
Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatry Res. 2014;52:1–6. | |
Finzi E. The Face of Emotion: How Botox Affects Our Moods and Relationships. New York: Palgrave Macmillan; 2013. | |
Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837–844. | |
Davis JI, Senghas A, Brandt F, Ochsner KN. The effects of Botox injections on emotional experience. Emotion. 2010;10(3):433–440. | |
Neal DT, Chartrand TL. Embodied emotion perception: amplifying and dampening facial feedback modulates emotion perception accuracy. Soc Psychol Personal Sci. 2011;2(6):673–678. | |
Havas DA, Glenberg AM, Gutowski KA, Lucarelli MJ, Davidson RJ. Cosmetic use of botulinum toxin-A affects processing of emotional language. Psychol Sci. 2010;21(7):895–900. | |
Wang L, Sun Y, Yang W, Lindo P, Singh BR. Type A botulinum neurotoxin complex proteins differentially modulate host response of neuronal cells. Toxicon. 2014;82:52–60. | |
Brandt F, O’Connell C, Cazzaniga A, Wagh JM. Efficacy and safety evaluation of a novel botulinum toxin topical gel for the treatment of moderate to severe lateral canthal lines. Dermatol Surg. 2010;36:2111–2118. | |
Foster KA, Chaddock JA. Targeted secretion inhibitors–innovative protein therapeutics. Toxins. 2010;2:2795–2815. | |
Masuyer G, Chaddock JA, Foster KA, Acharya KR. Engineered botulinum neurotoxins as new therapeutics. Annu Rev Pharmacol Toxicol. 2014;54:27–51. | |
Kukreja R, Singh BR. Botulinum neurotoxins-structure and mechanism of action. In: Proft T, editor. Microbial Toxins: Current Research and Future Trends. Norfolk: Caister Academic Press; 2009:15–40. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.