Back to Journals » Cancer Management and Research » Volume 11
The β-galactoside α2,6-sialyltranferase 1 (ST6GAL1) inhibits the colorectal cancer metastasis by stabilizing intercellular adhesion molecule-1 via sialylation
Authors Zhou L, Zhang S, Zou X, Lu J, Yang X, Xu Z, Shan A, Jia W, Liu F, Yan X, Su H, Liang T, Zheng M, Zhang Y , Feng B
Received 15 March 2019
Accepted for publication 9 May 2019
Published 4 July 2019 Volume 2019:11 Pages 6185—6199
DOI https://doi.org/10.2147/CMAR.S208631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Leqi Zhou,*,1,2 Sen Zhang,*,1,2 Xia Zou,3 Jishun Lu,3 Xiao Yang,1,2 Zhijue Xu,3 Aidong Shan,3 Wenjuan Jia,3 Feng Liu,3 Xialin Yan,1,2 Hao Su,1,2 Tao Liang,3 Minhua Zheng,1,2 Yan Zhang,3 Bo Feng1,2
1Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Shanghai Minimally Invasive Surgery Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Ministry of Education Key Laboratory of Systems Biomedicine, Shanghai Center for Systems Biomedicine (SCSB), Shanghai Jiao Tong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Background: Colorectal cancer (CRC) is one of the most frequent malignancies of the digestive system. Elevated expression of β-galactoside α2,6-sialyltranferase 1 (ST6GAL1) has been observed in multiple cancers. But the mechanism of how ST6GAL1 might affect cancer cells remains to be clarified. Our previous study recognized intercellular adhesion molecule-1(ICAM-1) as a probable substrate of ST6GAL1 through mass spectrometry (MS) analysis. ICAM-1 is related to tumor metastasis in various cancers.
Methods: First, ST6GAL1 was overexpressed and knocked down to perform transwell and wound healing assays, and the results were further confirmed in vivo. Based on the results of MS, GO and KEGG analysis were applied to reveal the connection between ST6GAL1 and ICAM-1. Immunoblot and tissue microarrays were administered to investigate the expression of ICAM-1 in different stages of CRC. Next, PCR, lectin precipitation and cycloheximide (CHX) were used to demonstrate the mechanism of ST6GAL1 on ICAM-1. Moreover, we investigated the sialylation on soluble ICAM in serum and its connection to tumor staging.
Results: Overexpression of ST6GAL1 inhibited the migratory ability, while knockdown of ST6GAL1 cells had the reverse effect. Moreover, nude mice injected with ST6GAL1-knockdown cells harvested more liver metastases. Based on the GO and KEGG analysis, data from TCGA database showed a positive correlation between ST6GAL1 and ICAM-1. ICAM-1 also demonstrated a significant decrease in stage III/IV compared with stage I/II tumors. Our results revealed that ST6GAL1 could increase the stability of ICAM-1 through sialylation but had little influence on transcriptional level. Additionally, results of serum lectin precipitation revealed a correlation between the level of sialylation on soluble ICAM and CRC staging.
Conclusion: This study illustrated that ST6GAL1 inhibited the metastatic ability of CRC by stabilizing ICAM-1 via sialylation and demonstrated a correlation between CRC staging and the sialylation on soluble ICAM-1 in serum.
Keywords: colorectal cancer, β-galactoside α2, 6-sialyltranferase 1, ST6GAL1, intercellular adhesion molecule-1, ICAM-1, sialylation, metastasis
Introduction
Colorectal cancer (CRC) is one of the most frequent malignancies of the digestive system.1 Although great improvements have been made in the diagnosis and treatment of CRC, the mortality rate caused by CRC is still rather high, ranking third in terms of incidence but second in terms of mortality.1,2 The main cause of mortality is recurrence and metastasis of the tumor, even after a successful surgical resection and postoperative therapy. Some 60% of patients suffer from metastasis during the course of CRC.3,4 Consequently, it is necessary to fully understand the mechanism of metastasis or develop an effective biomarker for early diagnosis.
Changes in glycosylation are reported to be associated with oncogenic transformation. Tumor cells display a wide range of glycosylation alterations compared with their non-transformed counterparts.5 Among these, sialylation is an important modification in cellular glycosylation, as sialylated carbohydrates have essential roles in cellular recognition, adhesion, and signaling.5 The aberrant level of sialylation mainly results from the altered expression of sialyltransferases.6 Recently, β-galactoside α2,6-sialyltranferase 1 (ST6GAL1), which adds α2,6-linked sialic acids on N-glycans, has gained much attention due to the observation of elevated expression of ST6GAL1 in various carcinomas like colorectal, cervical, and hepatocellular carcinomas.7–10 This dysregulated expression of ST6GAL1 in tumor may elicit multiple physiological and pathological outcomes.11 For examples, ST6GAL1 could promote cell motility by activating the PI3K/AKT signaling pathway,7,12 and it could directly facilitate metastatic tumor growth by catalyzing a significant increase of sialylated carbohydrate.13 Furthermore, knockdown of ST6GAL1 significantly inhibits the cell metastasis in diverse carcinomas,14–16 as forced expression of ST6Gal-I in human mammary tumor cells and ovarian carcinoma cells leads to reduced cell–cell adhesion and enhanced capacity for invasion.15,17 The upregulation of ST6GAL1 was first described in colon cancer,10 but the comprehensive mechanism of how ST6GAL1 affects tumor cells remains to be clarified.
In the previous study, our group revealed a dynamic expression of ST6GAL1 with the progression of CRC: a significant decrease in stage III/IV compared with stage I/II tumors.Lymphatic and distant metastasis are recognized as a key feature of stage III/IV tumors. The mass spectrometry (MS) analysis screened out over 300 glycoproteins as changed proteins after overexpression of ST6GAL1, most of which are cellular movement-related proteins, including intercellular adhesion molecule-1 (ICAM-1).18 ICAM-1 is a structurally related transmembrane glycoprotein of the immunoglobulin supergene family and is the ligand for β2-integrin molecules present on leukocytes.19,20 Generally, ICAM-1 appears to play a critical role in the development of the nervous system, in immune and inflammatory responses.21 A recent study suggests that ICAM-1 can act as a tumor suppressor that sensitizes metastatic tumor cells to cytotoxic T lymphocyte-mediated killing by interfering with activation of the PI3K/AKT pathway.22 It has also been proposed that ICAM-1 may be involved in the process of cancer metastases by promoting the spread of metastatic cancer cells to secondary sites.23–26 Apart from the ICAM-1 expressed on CRC cells, there exists a soluble form of ICAM-1 (sICAM-1) in serum, which is also reported to be connected with CRC progression.27 There are also studies that recommend sICAM-1 as a biomarker for CRC.28,29
Our study revealed that the expression of ICAM-1 in CRC tissues had the same pattern as ST6GAL1 and was relevant to patients’ relapse-free survival. By overexpressing ST6GAL1 in the SW480 cell line and knocking it down in the SW620 cell line, a corresponding change of ICAM-1 yet a reverse metastatic phenotype were observed. And further studies confirmed that ST6GAL1 could inhibit CRC metastasis by stabilizing ICAM-1 through sialylation, both in vitro and in vivo. Moreover, our results also revealed a novel correspondence between the sialylation on sICAM-1 in serum and CRC staging.
Materials and methods
Tissue specimen and blood sample
From May 2009 to June 2012, a total of 62 patients were recruited from Ruijin hospital (Shanghai, China) with pathological stage ranging from stage I (n=19), II (n=20), III (n=17), to IV (n=6). All participants gave written, informed consent and no participants had received any medication prior to sample collection under the guidelines of the Ethics Committee of Ruijin Hospital. The pathological stage was determined according to the criteria of the Union for International Cancer Control (UICC). The tumor tissues and paired normal colonic tissues located ~10 cm from the distal edge of the tumor were stored in liquid nitrogen for further analysis. The protein extraction, the standard for follow-up, and the inclusion and exclusion criteria of patients were performed as described elsewhere.30 For blood sample, peripheral venous blood samples were collected preoperatively and then drawn into sterile tubes. Serum samples were allowed to coagulate at room temperature for 30 min, and then centrifuged at 2000 g for 10 min. The serum was separated, aliquoted and stored at −80 °C until assay. The patients were followed up for 90 months or until death. The time of death and recurrence of tumor were recorded under the approval of Ethics Committee of Ruijin Hospital.
Cell lines and cell culture
The HEK293T and SW620, SW480 cell lines were purchased from ATCC (Manassas, VA, USA). The HEK293T cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS at 37 °C; SW480 and SW620 cells were maintained in Leibovitz’s L15 medium (HyClone, Logan, UT, USA) with 10% FBS under a humidified atmosphere at 37 °C without CO2.
Establishment of ST6GAL1 overexpression and knockdown cell lines
The cDNAs of human ST6GAL1 (kindly provided by Dr. H. Narimatsu from the National Institute of Advanced Industrial Science and Technology, Japan) were inserted into pENTR1A tagged with 3xFLAG at the C-terminus using the in-fusion method (Takara Bio). The inserted sequence in entry vectors was confirmed by DNA sequencing. The CSII-CMV-RfA lentiviral vector was gifted from RIKEN BRC DNA BANK. Using LR clonase (Invitrogen, Carlsbad, CA, USA), the subcloned cDNAs in entry vectors were transferred into the CSII-CMV-RfA for ST6GAL1 overexpression. The obtained lentiviral vectors were transfected into 293T cells with packaging plasmids by the calcium phosphate for the preparation of viruses. The obtained viruses were then incubated with SW480 cell lines for 72 h. The infected cells were selected by SNA lectin using FACS Aria II (BD Bioscience, Bedford, MA, USA).
To establish the ST6GAL1-knockdown cells, the shRNA sequences specifically targeting ST6GAL1 (5ʹ AATTCAAAAACCCAGAAGAGATTCAGCCAAATCTGACAGGAAGTTTGGCTGAATCTCT-3ʹ (sh1) and 5ʹ- AATTCAAAAACGTGTGCTACTACTACCAGTCTGACAGGAAGCTGGTAGTAGTAGCACACGG-3ʹ (sh2) were inserted into the pmiRZip vector. The plasmids were transfected into HEK293T cells using a Lipofectamine 2000 transfection reagent; 48–72 h later, the culture medium was collected, filtered and then used to infect SW620 cells. ST6GAL1 knockdown cells were obtained by antibiotic selection (puromycin 6 ug/mL).
Data acquisition and processing
Clinical and transcriptomic data of colon adenocarcinoma (COAD) and rectal carcinoma (READ) were collected from the GDC data portal of TCGA database. In order to identify significantly enriched pathways regarding ST6GAL1, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis was performed by utilizing cluster-Profiler R package. Corrplot R package was used for correlation analysis of ST6GAL1 and ICAM-1 and adjustment of relative clinical and demographic parameters, and the results were shown with ggstatsplot R package. p-value was determined by Pearson test.
Immunoblot
Cells were washed with PBS and then lysed with lysis buffer (10 mM Tris-HCl, 1% Triton X-100, 150 mM NaCl) containing protease and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). Insoluble materials were removed by centrifugation at 15,000 g for 15 min at 4 °C. The concentration of the collected protein was measured by BCA assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein were separated using 10% SDS-PAGE, transferred to nitrocellulose membrane, blocked with 5% non-fat milk at room temperature for 1 h, and immunoblotted with primary antibodies: polyclonal antibody against ST6GAL1 (R&D, Minneapolis, MN, USA), polyclonal antibodies against ICAM-1 and anti-GAPDH antibody (Proteintech, Rosemont, IL, USA). Immunoreactive bands were visualized using an ECL kit (Amersham Biosciences, Piscataway, NJ, USA). All experiments were repeated three times independently.
Lectin precipitation
For lectin precipitation of cell lysate, the cell lysates (1 mg of protein) were incubated overnight at 4 °C with rotation using 30 μL SNA agarose beads (Vector, Burlingame, CA, USA), which specifically recognizes α2,6 sialylation. After washing three times with PBS, the lectin precipitates were subjected to 10% SDS-PAGE, and the separated proteins were transferred to a nitrocellulose membrane. The membrane was incubated with anti-ICAM-1 antibody for immunoblot analysis. For lectin precipitation of serum, 10 μL of serum from each patient was diluted with 990 μL diluent (PBS and 10% SDS). The mixture was first added with 30 μL Protein G agarose beads (Roche Diagnostics, Mannheim, Germany) at 4 °C for 1 h with rotation to remove the proteins of high abundance. Then the supernatants were mixed with 30 μL SNA agarose beads overnight at 4 °C with rotation. After washing three times with PBS, the precipitated glycoproteins were subjected to 10% SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was blocked with 5% non-fat milk at room temperature for 1 h, and then incubated with anti-ICAM-1 antibody for immunoblot analysis. Immunoreactive bands were visualized by chemiluminescence. The total intensities of the bands were semi-quantified using Quantity One software (Bio-Rad, Hercules, CA, USA) and normalized to the intensity of GAPDH band as the internal control.
Immunohistochemical staining
The tumor and paired normal tissue sections (4 μm) were deparaffinized and dehydrated, and then treated with 3% H2O2 at room temperature for 10 min to block endogenous peroxidase activity. Next, the tissue sections were incubated with citrate buffer for the retrieval of the antigen. Then the tissues were blocked with 3% BSA at room temperature for 30 min, followed by incubating with anti-ST6GAL1 antibody and anti-ICAM-1 antibody (1: 100) at 4 °C overnight. Finally, the tissue slides were counterstained with hematoxylin and eosin (H&E).
Quantitative real-time PCR analysis
Total RNA was extracted from cells by Iso-RNA lysate reagent (Takara, Shiga, Japan). cDNA was obtained from the reverse-transcription of 1 ug total RNA after treated with a PrimeScript RT reagent Kit to erase gDNA (Takara). The sequences of primers for qPCR were: ST6GAL1 (5ʹ- AAAAGTTCAGCTGCTGCGTC-3ʹand 5ʹ- TGGCCAATTTCCCCAGACTC-3ʹ), ICAM-1 (5ʹ- GAGCACTCAAGGGGAGGTC-3ʹand 5ʹ- GGCTGCTACCACAGTGATGA-3ʹ) and GAPDH (5ʹ-TTCAACAGCAACTCCCACTCTT-3ʹand 5ʹ-TGGTCCAGGGTTTCTTACTCC-3ʹ). All experiments were repeated three times independently.
Lectin binding and flow cytometry analysis
SW480 and SW620 cells were grown to ~90% confluency and detached using trypsin at 37 °C, and washed three times with cold PBS. Then, cells were incubated with 10 μg/mL SNA-biotin (EY Laboratories, San Mateo, CA, USA) for 60 min on ice, followed by strepavidin-680 incubation for 60 min. Finally, cells were washed three times with PBS and detected by flow cytometry (BD Biosciences, San Jose, CA, USA). Data were analyzed with FlowJo 7.6 software. All experiments were repeated three times independently.
Wound healing and cell migration assay
For wound healing assay, the SW480 and SW620 cells were seeded into 12-well plates until the cells reached >95% confluence, and then wounded with pipette tips. The floating cells were removed using fresh medium and cultured at 37 °C without CO2 addition. The wound closing was photographed every 8 h. The cell migration assays were performed using 8-μm transwell chambers (Corning, New York, USA) with 24-well plates. The migration assays were conducted using transwell chambers precoated with 1% collagen I, 2×105 of SW620 cells and SW480 cells were suspended in 500 μL serum-free medium and seeded into the upper chamber; and medium with 10% FBS was added into the lower chamber. After incubation for 24 h, cells were fixed with 4% paraformaldehyde at room temperature for 30 min, and then stained with 0.1% crystal violet at 37 °C for 1 h. The stained cells were counted in three fields with random choice. All experiments were repeated three times independently.
Determination of ICAM-1 stability
SW480 and SW620 cells were treated with 100 μg/mL cycloheximide (CHX) (Sigma, St. Louis, MO, USA). Cells were harvested at the indicated time points and the whole lysates were subjected to SDS-PAGE and blotted with anti-ICAM-1 or anti-GAPDH antibodies.
Animal studies
For intrasplenic injections to examine the liver metastasis, the spleen was exteriorized via an abdominal midline incision after the application of general anesthesia, and 1×106 of SW620 cells were injected slowly into the spleen through a 30G needle. After 8 weeks, mice were sacrificed and livers were harvested, fixed over-ight in 10% buffered formalin, embedded in paraffin, and sectioned for H&E and IHC.
Statistical analysis
Statistical analyses were performed using either a Student’s t-test or one-way analysis of variance (ANOVA), using GraphPad Prism6.
Results
ST6GAL1 suppressed tumor metastasis of CRC in vitro and in vivo
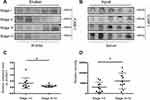
To investigate the potential effect of ST6GAL1 on CRC metastasis in vivo and in vitro, two CRC cell lines, SW480 and SW620, were chosen for further experiments. As immunoblot assay showed a relative higher level of ST6GAL1 in SW620 cells (Figure 1A), we decided to generate ST6GAL1-OE SW480 cells and ST6GAL1-KD SW620 cells. The effects of overexpression and knockdown of ST6GAL1 was confirmed by immunoblot (Figure 1B and C) and flow cytometry analysis (Figure 1D and E). To determine whether ST6GAL1 had an impact on CRC metastasis, wound healing and transwell assays were conducted and the results showed that overexpression of ST6GAL1 in SW480 cells inhibited the cell migratory ability while knockdown of ST6GAL1 in SW620 cells significantly facilitated cell migration (Figure 1F–I). Next, to verify the in vitro observations, we generated a liver metastasis model in nude mice by performing intrasplenic transplantations of ST6GAL1-NC and ST6GAL1-KD cells, respectively. At necropsy, more liver metastases were found in mice transplanted with ST6GAL1-KD cells (Figure 1J). This was further confirmed by HE staining and IHC of tissue sections (Figure 1K). In addition, patients with a high expression of ST6GAL1 had a significantly better relapse-free survival (RFS) than those with low expression of ST6GAL1 (Figure S1A). Collectively, these results suggested that ST6GAL1 suppressed invasion of CRC both in vitro and in vivo and may represent a prognostic factor.
The potential substrate of ST6GAL1
Our findings implied the connection of ST6GAL1 and CRC metastasis, but the molecular mechanism involved remained unknown. In our previous study,18 the MS analysis screened out a total of 318 glycoproteins that were affected after the overexpression of ST6GAL1 in SW480. To evaluate the role of ST6GAL1 in CRC metastasis, GO analysis was administered to explore the function of these proteins. Figure 2A–C showed the top 20 perturbed functions upon the overexpression of ST6GAL1 in terms of cellular component, biological process, and molecular function, respectively. As is shown, most of the perturbed functions were related to cellular movement. Moreover, the KEGG pathway analysis (Figure 2D) indicated that ST6GAL1 had a potential interaction with cell adhesion molecules (CAMs).
Stage-dependent expression of ICAM-1 in CRC progression
To experimentally confirm these observed changes, we chose ICAM-1, which showed great difference in the extent of the change (Supplementary Figure 1B) to investigate the mechanistic relation between ST6GAL1 and CRC. We first examined the expression of ICAM-1 with Western blot assay in 62 CRC specimens ranging from stage I to stage IV as well as the paired normal tissues. The results showed that the expression of ICAM-1 was significantly higher in tumor tissues than in normal tissues (Figure 3A). Moreover, in subgroup analysis, ICAM-1 showed a declining trend from stage I and II CRC patients to stage III and IV cases, which suggested an important role of ICAM-1 in CRC metastasis (Figure 3B). These results were then validated by tissue microarrays of 71 paired CRC tissues (Figure 3D and E). Moreover, among 62 enrolled patients, those with high expression of ICAM-1 had a better prognosis (Figure 3F and G) in terms of RFS. These results indicated that ICAM-1 is a potent metastasis suppressor of CRC. To verify our hypothesis that ST6GAL1 may exert the function of suppressing CRC metastasis via modulation ICAM-1, the correlation analysis of these two genes were performed with the transcriptome profiling data extracted from the TCGA database. Consistently, the results suggested ICAM-1 was positively correlated with ST6GAL1 (Figure 3G), which suggested that ST6GAL1 could mediate the expression of ICAM-1.
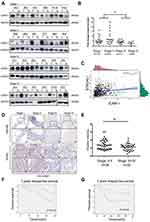 |
Figure 3 Stage-dependent expression of ICAM-1 in CRC progression. (A) Representative images of the immunoblot for ICAM-1 in primary colorectal tumors at different stages. For the rest of our immunoblot images, please refer to Figure S2; (B) ICAM-1 bands detected from the immunoblot were quantified by densitometric scanning and protein intensity values were normalized by GAPDH. The normalized ICAM-1 intensity in tumors was compared with that in its pair-matched normal tissues; (C) Correlation analysis of ST6GAL1 and ICAM-1 from the TCGA database suggested ICAM-1 was positively correlated with ST6GAL1; (D and E) Representative images of ICAM-1 expression in different stage of CRC tissue microarrays and the statistical analysis of the histochemistry score of the CRC tissue microarrays; (F and G) 3-year and 5-year relapse-free survival (RFS) of ICAM-1 high and low expression group. The cut-off value of ICAM-1 expression (Ratiotumor/normal =1.58) was based on Youden’s index from the ROC curve. *P<0.05 by paired t-test or Mann–Whitney U test). Abbreviations: CRC, colorectal cancer; ICAM-1,intercellular adhesion molecule-1. |
ST6GAL1 upregulated ICAM-1 by enhancing its stability
To confirm the relation between ST6GAL1 and ICAM-1 in CRC, we performed PCR and Western blot assay to determine the modulation of ST6GAL1 to ICAM-1. As expected, overexpression of ST6GAL1 leaded to an increased level of ICAM-1 while knockdown of ST6GAL1 resulted in the opposite (Figure 4A and B.). However, negligible changes were observed in PCR (Figure 4C and D). These results indicated that ST6GAL1 might regulate ICAM-1 in a post-transcriptional way. Given the accumulating evidence for the role of glycosylation in protein stability,31,32 we first checked the sialylation pattern of ICAM-1 through immunoprecipitation. The results revealed that overexpression of ST6GAL1 clearly increased the sialylation on ICAM-1, while knockdown of it led to the opposite (Figure 4E and F). Then we used CHX to explore the stability of ICAM-1 after ST6GAL1 was overexpressed or knocked down. CHX could interact directly with the translocase enzyme, interfering with the translocation step, and thus interfere the synthesis of proteins in eukaryotes. Protein was extracted at the indicated time after the addition of CHX and then analyzed by immunoblot. ICAM-1 was still detectable in ST6GAL1-OE cells after 24 h of CHX addition, but hardly detectable in ST6GAL1-mock cells (Figure 4G and H). In contrast, in ST6GAL1-KD cells, ICAM-1 showed a significant decrease after 8 h treatment of CHX but little change of ICAM-1 was observed in ST6GAL1-NC cells (Figure 4I and J). These results indicated that ST6GAL-1 significantly prolonged the half-life of ICAM-1, probably through inducing sialylation of ICAM-1 protein.
Sialylation of soluble ICAM-1 in serum correlates with CRC staging
A number of studies have reported that protein level of soluble ICAM-1 in serum was associated with progress and metastasis of CRC33–35 and was recommended as probable biomarker in CRC,28,29,36 whereas sICAM-1 had limited application owing to the relative low specificity, precluding its use for screening strategies and diagnostic potential.37 Furthermore, most of the typical clinically utilized serological biomarkers for cancer diagnosis and monitoring of malignant progression, as well as prognostic biomarkers of disease recurrence, are glycoproteins.38 To investigate the potential clinical value of ICAM-1, we examined the sialylation on sICAM-1 in different stages of CRC with lectin precipitation. The lectin precipitation confirmed the existence of sialyation (Figure 5A and B) and the statistical analysis showed the sialylation on sICAM-1 in stage I/II is significantly higher than those in stage III/IV (Figure 5C) though the protein level revealed an elevation (Figure 5B and D).
Discussion
Altered expression of ST6GAL1 has been observed in multiple types of carncer, among which, colorectal cancer was first reported.10 Plenty of studies have discovered the connection between the elevated expression of ST6GAL1 and tumor progression.13,16,29 However, there is also evidence that ST6GAL1 negatively correlates with the malignancy in some types of carcinoma, like glioma.16,39,40 Our previous work discovered a dynamic expression of ST6GAL1 in CRC progression, and an apparent decrease in stage III/IV cases compared with stage I/II,18 suggesting that the reduction of ST6GAL1 might be related to the metastasis of CRC. In this study, our results showed that knockdown of ST6GAL1 dramatically enhances the migratory ability in SW620 cells (Figure 1G and I) while overexpression of ST6GAL1 has the opposite effect in SW480 cells (Figure 1F and H). The experiment in vivo further confirmed our hypothesis that nude mice injected with ST6GAL1-KD cells harvested more liver metastases compared to the control groups (Figure 1J and K). In regard to this paradoxical role of ST6GAL1 in cancer, we hypothesized that these discrepancies could be partially due to the different expression of sialylated proteins in distinct cells or types of cancer. For example, some important receptors on the cell membrane like Met and EGFR have been considered as axiomatic in determining tumor malignancy. However, interestingly, ST6GAL1 elicits the opposite effects: knockdown of ST6GAL1 greatly suppresses the stability as well as the phosphorylation level of Met,41 while depletion of ST6GAL1 increases the phosphorylation level of EGFR and augments its downstream signaling.16,39,42
Besides the discovery of the negative correlation between ST6GAL1 and tumor progression, combined with our former MS analysis,18 we also screened out one specific substrate of ST6GAL1-ICAM-1, a suppressor of metastasis in CRC.23–26 In our study the immunoblot analysis of CRC tissues of ICAM-1 exhibited similar expression pattern to that of ST6GAL1, a significant decrease in stage III/IV versus I/II (Figure 3A–B and D–E), and the expression of ICAM-1 was also relevant to patients’ relapse-free survival (Figure 3F–G), all of which led to the supposition that ST6GAL1 could mediate tumor metastasis by regulating the level of ICAM-1. Therefore, we concluded that ST6GAL1 might inhibit tumor metastasis by upregulating the expression of ICAM-1. ICAM-1 has been implicated in cancer metastasis. Previous reports have investigated the expression of ICAM-1 in lung cancer samples and have reported a correlation between the level of ICAM-1 expression in tumor samples with advanced stages of lung cancer and metastasis.23 In one particular study of CRC, ICAM-1 expression in primary tumors from stage III and IV colon cancer patients was significantly decreased compared with that of primary lesions from stage I and II colon cancer patients, which is consistent with our findings. And by restraining efferocytosis of apoptotic tumor cells, ICAM-1 could block M2 macrophage polarization through regulation of PI3K/AKT activation, which leads to prevention of tumor metastasis.43
To illustrate the mechanism by which ST6GAL1 regulated the expression of ICAM-1, we first examined ICAM-1 mRNA but little change was observed either in ST6GAL1-OE cells or in ST6GAL1-KD cells (Figure 4C and D). Then immunoprecipitation analysis showed that ICAM-1 had a high level of sialylation after overexpression of ST6GAL1 yet knockdown of ST6GAL1 had a reverse influence on ICAM-1 (Figure 4E and F). Moreover, ICAM-1 demonstrated a prolonged half-life after ST6GAL1-induced sialylation (Figure 4G and H). Given the increasing evidence for the role of glycosylation in maintaining protein stability,31,32 these results suggest that ST6GAL1 might sustain the stability of ICAM-1 through the catalysis of sialylation and consequently affect the metastasis of CRC. Although not completely understood, the function of sialylation mediated by ST6GAL1 probably involves interference with the structure of the attached glycans or the carrier proteins. Previous studies showed that the addition of sialylation to the terminal N-glycans shielded the galectin recognition sites for binding of β-galactoses, which in turn switched off the galectin functions including adhesion, migration, and apoptosis.10,11,44 In contrast to the inhibitory effect on glycan–galectin binding, sialylation has also been reported to bind specifically to the siglec-2 family of lectins. Since siglec-2 are mainly expressed on immune cells, and the changes in tumor cell sialylation could affect the activity of siglec-expressing immune cells, and consequently modulate the antitumor immune response.45 On the other hand, sialylation have direct effects on the structure/function of specific sialylated glycoproteins. Sialylation has been shown to alter conformation of the β1 integrin,46 clustering of the CD45,47 EGFR42and PECAM,48 cell surface retention of PECAM,48 and Fas death receptor.49 Taking all these together, we envisioned that the sialylation on ICAM-1 modulated by ST6GAL1 might participate in multiple aspects in ICAM-1-related tumor cell biology such as cell recognition, cell signaling, and lymphocyte activation. Clearly, further research is warranted to explore this hypothesis.
Apart from the ICAM-1 expressed on CRC cells, there exists a soluble form of ICAM-1 in serum, which is also reported to be connected with CRC progression. SICAM-1 is produced by diverse cell types, including endothelial cells, carcinoma cells, keratinocytes, and astrocytes,50 but the mechanism of release and structure of sICAM-1 is poorly understood. A previous study showed that the tumor concentration of sICAM-1 seemed to increase during tumorigenesis, whereas tumor sICAM-1 seemed to be lost and shed into circulation in association with disease progression, especially at an advanced stage.37 The serum level of sICAM-1 had a significant increase in stage III/IV and was involved in cytotoxicity,27,36 and was suggested as a prognostic biomarker for CRC.15,28 However, as a biomarker, sICAM-1 still lacks sensitivity and specificity, which leads to a rather high false positivity regarding inflammation and other diseases.51 Intriguingly, the sialylation level of sICAM-1 showed a dramatic decrease with tumor progression (Figure 5A and C), although the protein level revealed an elevation (Figure 5B and D). Nevertheless, these findings were only preliminary results. The role that sICAM-1 plays in the progression of CRC remains mysterious. Due to the limitation of samples and the lack of large-scale testing methods, whether the sialylation of sICAM-1 could actually be a biomarker needs further exploration.
To conclusion, increasing evidences implies that aberrant sialylation participates in diverse pathological conditions. Here we revealed that sialyltransferase ST6GAL1 could inhibit the metastatic feature of CRC by increasing the stability of ICAM-1 through sialylation. Additionally, it also modulates the metastatic features of CRC through regulating the expression of ICAM by stability.
Conclusion
Increasing evidences implies that aberrant sialylation participates in diverse pathological conditions. Here we revealed that sialyltransferase ST6GAL1 could inhibit the metastatic feature of CRC by increasing the stability of ICAM-1 through sialylation. Additionally, there also existed a novel probability that the sialylation on sICAM-1 could be a favorable biomarker for CRC diagnosis.
Abbreviation list
CRC, colorectal cancer; ST6GAL1, β-galactoside α2,6-sialyltranferase 1; ICAM-1, intercellular adhesion molecule-1; qPCR, quantitative RT-PCR; NC, negative control; sICAM-1, soluble ICAM-1.
Ethics approval and consent to participate
All participants provided written, informed consent and no participants had received any medication prior to sample collection under the guidelines of Ethics Committee of Ruijin Hospital, which approved this study. This study was conducted in accordance with the Declaration of Helsinki. The animal protocols were approved by Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University. All experiments were performed in accordance with the official recommendations of the Chinese Zoological Society, and animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals.”
Acknowledgments
The authors thank all members of the Shanghai Minimally Invasive Surgery Center and Shanghai Center for Systems Biomedicine for assistance in various aspects of this work. This study has been supported by the Natural Science Foundation of Shanghai (18ZR1424100), National Science and Technology Major Project of China (2018ZX10302205), National Natural Science Foundation of China (31600643, 31770850), and Shanghai Translational Medicine Collaborative Innovation Center Program (TM201701).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-310912
3. Kallini JR, Gabr A, Abouchaleh N, et al. New developments in interventional oncology: liver metastases from colorectal cancer. Cancer J. 2016;22(6):373–380. doi:10.1097/PPO.0000000000000226
4. Gruber-Rouh T, Marko C, Thalhammer A, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol. 2016;89:20151060. doi:10.1259/bjr.20151060
5. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi:10.1038/nrc3982
6. Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14(5):569–576.
7. Zhao Y, Li Y, Ma H, et al. Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol Cell Proteomics. 2014;13(2):520–536. doi:10.1074/mcp.M113.034025
8. Recchi MA, Hebbar M, Hornez L, Harduin-Lepers A, Peyrat JP, Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58(18):4066–4070.
9. Wang PH, Li YF, Juang CM, et al. Altered mRNA expression of sialyltransferase in squamous cell carcinomas of the cervix. Gynecol Oncol. 2001;83(1):121–127. doi:10.1006/gyno.2001.6358
10. Dall’Olio F, Malagolini N, Di Stefano G, Minni F, Marrano D, Serafini-Cessi F. Increased CMP-NeuAc: Galbeta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity in human colorectal cancer tissues. Int J Cancer. 1989;44(3):434–439.
11. Lu J, Gu J. Significance of beta-Galactoside alpha2,6 Sialyltranferase 1 in Cancers. Molecules. 2015;20(5):7509–7527. doi:10.3390/molecules20057509
12. Isaji T, Im S, Gu W, et al. An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J Biol Chem. 2014;289(30):20694–20705. doi:10.1074/jbc.M113.542688
13. Vierbuchen MJ, Fruechtnicht W, Brackrock S, Krause KT, Zienkiewicz TJ. Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features. Cancer. 1995;76(5):727–735.
14. Zhang Z, Sun J, Hao L, Liu C, Ma H, Jia L. Modification of glycosylation mediates the invasive properties of murine hepatocarcinoma cell lines to lymph nodes. PLoS One. 2013;8(6):e65218. doi:10.1371/journal.pone.0065218
15. Lin S, Kemmner W, Grigull S, Schlag PM. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp Cell Res. 2002;276(1):101–110. doi:10.1006/excr.2002.5521
16. Zhu Y, Srivatana U, Ullah A, Gagneja H, Berenson CS, Lance P. Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochim Biophys Acta. 2001;1536(2–3):148–160.
17. Christie DR, Shaikh FM, Lucas J, Lucas JA
18. Zhang S, Lu J, Xu Z, et al. Differential expression of ST6GAL1 in the tumor progression of colorectal cancer. Biochem Biophys Res Commun. 2017;486(4):1090–1096. doi:10.1016/j.bbrc.2017.03.167
19. Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52(6):925–933.
20. Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28(9):1379–1386.
21. Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. doi:10.1038/346425a0
22. Hamai A, Meslin F, Benlalam H, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with PI3K/AKT pathway. Cancer Res. 2008;68(23):9854–9864. doi:10.1158/0008-5472.CAN-08-0719
23. Lin YC, Shun CT, Wu MS, Chen CC. A novel anticancer effect of thalidomide: inhibition of intercellular adhesion molecule-1-mediated cell invasion and metastasis through suppression of nuclear factor-kappaB. Clin Cancer Res. 2006;12(23):7165–7173. doi:10.1158/1078-0432.CCR-06-1393
24. Brooks KJ, Coleman EJ, Vitetta ES. The antitumor activity of an anti-CD54 antibody in SCID mice xenografted with human breast, prostate, non-small cell lung, and pancreatic tumor cell lines. Int J Cancer. 2008;123(10):2438–2445. doi:10.1002/ijc.23793
25. Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng. 2008;36(4):661–671. doi:10.1007/s10439-008-9445-8
26. Wang S, Coleman EJ, Pop LM, Brooks KJ, Vitetta ES, Niederkorn JY. Effect of an anti-CD54 (ICAM-1) monoclonal antibody (UV3) on the growth of human uveal melanoma cells transplanted heterotopically and orthotopically in SCID mice. Int J Cancer. 2006;118(4):932–941. doi:10.1002/ijc.21289
27. Becker JC, Dummer R, Hartmann AA, Burg G, Schmidt RE. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991;147(12):4398–4401.
28. Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer. 2001;37(18):2392–2397.
29. Toiyama Y, Miki C, Inoue Y, et al. Soluble intercellular adhesion molecule-1 as a prognostic marker for stage II colorectal cancer patients. Ann Surg Oncol. 2008;15(6):1617–1624. doi:10.1245/s10434-008-9874-5
30. Zou X, Feng B, Dong T, et al. Up-regulation of type I collagen during tumorigenesis of colorectal cancer revealed by quantitative proteomic analysis. J Proteomics. 2013;94:473–485. doi:10.1016/j.jprot.2013.10.020
31. Zhang L, Syed ZA, van Dijk Hard I, Lim JM, Wells L, Ten Hagen KG. O-glycosylation regulates polarized secretion by modulating Tango1 stability. Proc Natl Acad Sci U S A. 2014;111(20):7296–7301. doi:10.1073/pnas.1322264111
32. Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing - deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820(12):2079–2094. doi:10.1016/j.bbagen.2012.09.014
33. Becker JC, Termeer C, Schmidt RE, Brocker EB. Soluble intercellular adhesion molecule-1 inhibits MHC-restricted specific T cell/tumor interaction. J Immunol. 1993;151(12):7224–7232.
34. Makgoba MW, Sanders ME, Ginther Luce GE, et al. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988;18(4):637–640. doi:10.1002/eji.1830180423
35. Chong AS, Boussy IA, Jiang XL, Lamas M, Graf LH
36. Sanchez-Rovira P, Jimenez E, Carracedo J, Barneto IC, Ramirez R, Aranda E. Serum levels of intercellular adhesion molecule 1 (ICAM-1) in patients with colorectal cancer: inhibitory effect on cytotoxicity. Eur J Cancer. 1998;34(3):394–398.
37. Araki T, Miki C, Kusunoki M. Biological implications of circulating soluble intercellular adhesion molecule-1 in colorectal cancer patients. Scand J Gastroenterol. 2001;36(4):399–404.
38. Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol. 2010;63(4):322–329. doi:10.1136/jcp.2009.071035
39. Park JJ, Yi JY, Jin YB, et al. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem Pharmacol. 2012;83(7):849–857. doi:10.1016/j.bcp.2012.01.007
40. Basoglu M, Atamanalp SS, Yildirgan MI, et al. Correlation between the serum values of soluble intercellular adhesion molecule-1 and total sialic acid levels in patients with breast cancer. Eur Surg Res. 2007;39(3):136–140. doi:10.1159/000100110
41. Qian J, Zhu CH, Tang S, et al. alpha2,6-hyposialylation of c-Met abolishes cell motility of ST6Gal-I-knockdown HCT116 cells. Acta Pharmacol Sin. 2009;30(7):1039–1045. doi:10.1038/aps.2009.84
42. Yen HY, Liu YC, Chen NY, et al. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc Natl Acad Sci U S A. 2015;112(22):6955–6960. doi:10.1073/pnas.1507329112
43. Yang M, Liu J, Piao C, Shao J, Du J. ICAM-1 suppresses tumor metastasis by inhibiting macrophage M2 polarization through blockade of efferocytosis. Cell Death Dis. 2015;6:e1780. doi:10.1038/cddis.2015.144
44. Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572(2–3):285–293.
45. Kimura N, Ohmori K, Miyazaki K, et al. Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. J Biol Chem. 2007;282(44):32200–32207. doi:10.1074/jbc.M702341200
46. Woodard-Grice AV, McBrayer AC, Wakefield JK, Zhuo Y, Bellis SL. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J Biol Chem. 2008;283(39):26364–26373. doi:10.1074/jbc.M800836200
47. Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278(9):7469–7475. doi:10.1074/jbc.M209595200
48. Kitazume S, Imamaki R, Ogawa K, et al. Alpha2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J Biol Chem. 2010;285(9):6515–6521. doi:10.1074/jbc.M109.073106
49. Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286(26):22982–22990. doi:10.1074/jbc.M110.211375
50. Erturk K, Tastekin D, Bilgin E, Serilmez M, Bozbey HU, Sakar B. Serum activated leukocyte cell adhesion molecule and intercellular adhesion molecule-1 in patients with gastric cancer: can they be used as biomarkers? Biomed Pharmacother. 2016;77:86–91. doi:10.1016/j.biopha.2015.12.006
51. Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15(2):91–98.
Supplementary materials
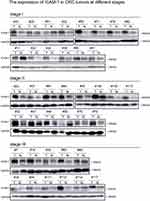 |
Figure S2 The expression of ICAM-1 in CRC tumors at different stages.Abbreviations: ICAM-1, intercellular adhesion molecule-1; CRC, colorectal cancer. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.